Introduction
With the description of the current mineral and discounting ‘girdite’ and ‘oboyerite’, which are now discredited (Kampf et al., Reference Kampf, Mills and Rumsey2017; Missen et al., Reference Missen, Kampf, Mills, Housley, Spratt, Welch, Coolbaugh, Marty, Chorazewicz and Ferraris2019), the Tombstone mining district has now yielded 12 new minerals (Table 1). All of these minerals, except cryptomelane, are tellurium oxysalts. Of the 11 tellurium oxysalts, four are tellurites with Te only in the 4+ oxidation state, six are tellurates with Te only in the 6+ oxidation state, and one is a mixed tellurite–tellurate, with Te in both the 4+ and 6+ oxidation states. The mixed tellurite–tellurate is the new mineral described herein and given the name tombstoneite.
Table 1. Mineral species first described from the Tombstone mining district.

Tombstoneite is named for the Tombstone mining district and the nearby town of Tombstone, Arizona, USA. The new mineral and name (symbol Tbs) were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2021–053, Kampf et al., Reference Kampf, Mills, Housley, Ma and Thorne2021). The description is based on one holotype specimen deposited in the collections of the Natural History Museum of Los Angeles County, Los Angeles, California, USA; catalogue number 76195.
Occurrence
Tombstoneite occurs at the Grand Central mine (31.70250, –110.06194) in the Tombstone district, Cochise County, Arizona, USA, ~1 km south of the town of Tombstone. The type specimen was originally collected by Sidney A. Williams and was obtained by one of the authors (BT) from Excalibur Minerals. The crystals were flagged as a probable new mineral first by Raman spectroscopy and then by SEM–EDS analysis at Caltech. The Grand Central mine exploits a Ag–Au–Pb–Cu–Zn deposit in which the ore, consisting principally of oxidised Ag- and Au-rich galena, occurs in faulted and fractured portions of a large dyke hosted by the Bisbee Group limestone. A good description of the history, geology and mineralogy of the Tombstone district has been provided by Williams (Reference Williams1980b). Tombstoneite occurs in cavities in quartz matrix in association with rodalquilarite and jarosite (Fig. 1).

Fig. 1. Tombstoneite (green) on quartz with rodalquilarite (yellow green) and jarosite (beige to orange); field of view 0.56 mm across. Natural History Museum of Los Angeles County catalogue number 76195.
Physical and optical properties
Tombstoneite crystals are trigonal (pseudohexagonal) tablets, up to 100 μm across and up to 20 μm thick, that grow in subparallel stacks (Fig. 1). Tablets are flattened on {001}, exhibit the forms {100}, {001} and {101} (Fig. 2) and are twinned, probably by reflection on {001}. The mineral is green and transparent with adamantine lustre. The streak is pale green. No fluorescence was observed in either longwave or shortwave ultraviolet illumination. The Mohs hardness is ~2½ based upon scratch tests. Crystals are brittle with irregular fracture. There is one perfect cleavage on {001}. The calculated density based on the empirical formula and unit-cell parameters obtained from single-crystal X-ray diffraction data is 5.680 g cm–3 and that for the ideal formula is 5.668 g cm–3. The density could not be measured because it exceeds that of available density liquids and there is an insufficient quantity for physical measurement. At room temperature, tombstoneite is soluble in dilute HCl.

Fig. 2. Crystal drawing of tombstoneite, clinographic projection.
Optically, tombstoneite is uniaxial (–). Due to the very small amount of material and the high indices of refraction, it was impractical to measure the indices of refraction. The Gladstone–Dale relationship (Mandarino, Reference Mandarino2007) predicts an average index of refraction of 2.002 using the empirical formula. The mineral is pleochroic: O = green, E = light yellow green; O > E.
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS using a 532 nm diode laser, 100 μm slit, 1800 gr/mm diffraction grating and a 100× (0.9 NA) objective. The spectrum from 4000 to 60 cm–1 recorded perpendicular to {001} are shown in Fig. 3. Note that the spectrum recorded parallel to {001} is essentially the same. The wavenumbers of the principal Raman bands are labelled in the figure.

Fig. 3. The Raman spectrum of tombstoneite recorded with a 532 nm laser.
The weak band at 1092 cm–1 is attributable to v 3 SO4 antisymmetric stretching and the moderately strong band at 971 cm–1 to v 1 SO4 symmetric stretching. The broad very weak band at ~870 cm–1 probably corresponds to the v 1 symmetric stretching mode for SeO3. The v 3 SeO3 antisymmetric stretching bands, typically occurring at ~800 to 750 cm–1 (see Mills et al., Reference Mills, Kampf, Christy, Housley, Thorne, Chen and Steele2014), are presumably hidden under the very strong band at 755 cm–1, which can reasonably be assigned to v 1 Te4+O3 symmetric stretching. The weaker band at 705 cm–1 may be due to v 1 Te6+O6 symmetric stretching and the band at 680 cm–1 is most likely due to v 3 Te4+O3 antisymmetric stretching (see Missen et al., Reference Missen, Weil, Mills, Libowitzky, Kolitsch and Stöger2020). We do not feel confident in assigning modes to specific bands in the 600 to 300 cm–1 range; however, these all are likely to be due to various stretching and/or bending modes of Cu2+O5 and Te6+O6, as well as bending modes of SO4, Te4+O3 and Se4+O3. Bands at lower wavenumbers are mostly due to lattice modes.
Chemical composition
Analyses (4 points) were performed at Caltech on a JEOL 8200 electron microprobe in wavelength dispersive spectroscopy mode. Analytical conditions were 15 kV accelerating voltage, 10 nA beam current and 5 μm beam diameter. Insufficient material is available for CHN analysis; however, the fully ordered structure unambiguously established the quantitative content of H2O. The crystals did not take a good polish, which accounts for the low analytical total. Analytical data are given in Table 2.
Table 2. Chemical composition (in wt.%) for tombstoneite.

* Allocated in accord with the structure.
** Based upon the crystal structure with Te+Se+S = 12 and O = 41 apfu.
The empirical formula based on Te + Se + S = 12 and O = 41 apfu is Ca0.51Pb3.49Cu2+5.85Te6+2.00Te4+6.47Se4+1.39S2.14O41H6.01 or assigned according to the structure (Ca0.51Pb0.49)Σ1.00Pb3.00Cu2+5.85Te6+2.00O6(Te4+1.00O3)6(Se4+0.69Te4+0.24S0.07O3)2(S1.00O4)2⋅3H2O.
The simplified formula is (Ca,Pb)Pb3Cu2+6Te6+2O6(Te4+O3)6(Se4+O3)2(SO4)2⋅3H2O. Note that the Pb1 site in the structure appears to require the presence of approximately equal amounts of Pb and Ca for bond-valence balance at the site. For this reason, we provide an ideal formula with Ca0.5Pb0.5 at the Pb1 site. The ideal formula is (Ca0.5Pb0.5)Pb3Cu2+6Te6+2O6(Te4+O3)6(Se4+O3)2(SO4)2⋅3H2O, which requires CaO 0.92, PbO 25.77, CuO 15.74, TeO2 31.59, TeO3 11.59, SeO2 7.32, SO3 5.28, H2O 1.78, total 100 wt.%.
X-ray crystallography and structure refinement
Powder X-ray diffraction was done using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer, with monochromatised MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample and observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc.). The powder data are presented in Table 3.
Table 3. Powder X-ray diffraction data (d in Å) for tombstoneite.*

*Only calculated lines with I > 1.5 are listed. The eight strongest lines are given in bold.
Single-crystal X-ray studies were done on the same diffractometer and with the same radiation. The Rigaku CrystalClear software package was used for processing structure data, including the application of an empirical multi-scan absorption correction using ABSCOR (Higashi, Reference Higashi2001). The structure was solved using SHELXT (Sheldrick, Reference Sheldrick2015a). Refinement proceeded by full-matrix least-squares on F 2 using SHELXL-2016 (Sheldrick, Reference Sheldrick2015b). All crystals are intergrowths of individuals stacked subparallel to {001}, some of which are apparently twinned by reflection on {001}. The crystal chosen for structure data collection consists of one principal individual and numerous smaller misaligned crystals. The data were of sufficient quality to allow the solution and refinement of the structure with anisotropic displacement parameters, although the presence of some reflection overlaps could not be avoided or properly accounted for, resulting in most of the O atoms exhibiting significantly oblate ellipsoids. The Pb1 site was refined with joint occupancy by Pb and Ca and the Se site was refined with joint occupancy by Se and Te. All non-hydrogen sites were refined with anisotropic displacement parameters. Difference-Fourier synthesis did not suggest a possible location for the H atom. Data collection and refinement details are given in Table 4, atom coordinates and displacement parameters in Table 5, selected bond distances in Table 6 and bond-valence sums (BVS) in Table 7. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Data collection and structure refinement details for tombstoneite.

*R int = Σ|F o 2–F o 2(mean)|/Σ[F o 2]. GoF = S = {Σ[w(F o 2–F c 2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o 2–F c 2)2]/Σ[w(F o 2)2]}½; w = 1/[σ2(F o 2) + (aP)2 + bP] where a is 0, b is 24 and P is [2F c 2 + Max(F o 2,0)]/3.
Table 5. Atom coordinates and displacement parameters (Å2) for tombstoneite.

*Occupancies: Pb1 = Pb0.497Ca0.503(12); Cu = Cu0.984(13); Se = Se0.84Te0.16(3)
Table 6. Selected bond distances (Å) in tombstoneite.

Table 7. Bond-valence sums for tombstoneite. Values are expressed in valence units (vu).

Multiplicity is indicated by ×→↓. Bond valences related to the Pb1 and Se sites are based on refined occupancies. Te6+–O bond valence parameters are from Mills and Christy (Reference Mills and Christy2013). All others are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). Hydrogen-bond valences are based on O–O bond lengths from Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988). Negative values indicate donated bond valence.
Description and discussion of the structure
The asymmetric unit of the structure of tombstoneite (Fig. 4) includes one SO4 sulfate tetrahedron, one Jahn-Teller distorted Cu2+O5 tetragonal pyramid [with four equatorial O atoms (Oeq) and one apical O atom (Oap)], one regular Te16+O6 tellurate octahedron, one Te24+O3 tellurite trigonal pyramid, one Se4+O3 selenite trigonal pyramid, two Pb sites (Pb1 and Pb2) and one H2O site (O8). Two Cu2+O5 pyramids link to one another by sharing an Oeq–Oap edge to form a Cu2O8 dimer. The Se4+O3 pyramid shares each of its basal O vertices with Cu2O8 dimers. The Se4+ at the apical vertex of the pyramid forms long bonds to O5 atoms within the Te4+O3 pyramid. The Te4+O3 pyramid shares two of its basal O vertices with Cu2O8 dimers and the third with a Te6+O6 octahedron. The Te6+O6 octahedron shares three vertices with Cu2O8 dimers and three vertices with Te4+O3 pyramids. The Te16+O6 octahedron and the Te24+O3 pyramids linked to it form a finite Te6+O3(Te4+O3)3 cluster with a pinwheel-like configuration (Fig. 5).

Fig. 4. The structure of tombstoneite viewed down [110] with [001] vertical. The unit cell outline is shown with dashed lines.

Fig. 5. The pinwheel-like Te6+O3(Te4+O3)3 cluster in the structure of tombstoneite, viewed down c.
The linkages noted above create a thick heteropolyhedral layer containing pockets, which host the Pb2 cations. The Pb22+ cation is eight coordinated to surrounding O atoms in the heteropolyhedral layer. The Pb2–O bonds cover a narrow range (2.570 to 2.673 Å); hence, the Pb22+ 6s2 lone-pair electrons are not stereoactive. Also of note, the Se4+ at the apical vertex of the Se4+O3 pyramid forms long bonds to O5 atoms within the same heteropolyhedral layer.
The SO4 tetrahedron, the Pb1 site and the O8 H2O site are located in the interlayer region of the structure. The O8 bonds only to Pb1. The three O2 atoms of the SO4 tetrahedron each form two long bonds with Te24+ cations in the same heteropolyhedral layer and the O1 atom forms three long bonds to Te24+ cations in the adjacent layer. The Pb1 site is nine coordinated, forming three 2.709 Å bonds to O6 sites in one heteropolyhedral layer, three 2.709 Å bonds to O6 sites in the next layer and three 2.38 Å bonds to O8 H2O sites in the interlayer. The Pb1 site is half occupied by Pb2+ and half occupied by Ca2+. The joint occupancy appears to be necessary for bond-valence balance at the site. If only Pb2+ occupies the site, the BVS of the site is 2.66 vu, but with Pb0.5Ca0.5 occupancy, the BVS is 2.23 vu. Note that the distribution of bonds to the Pb1 site (see Fig. 6) suggests the possibility that the Pb12+ 6s2 lone-pair electrons may be stereoactive in two opposing directions, oriented 50% along +c and 50% along –c. Considering that Pb occupies only half of this site, the repulsive effect on the O6 atoms in each direction would only be 25% of that normally provided by lone pairs. It is also worth noting that the second highest electron density residual (1.96 e/A3) is located 0.79 Å from the Pb1 site along the c axis in both directions, approximately where one would expect electron density due to lone-pair electrons (see Fig. 6).
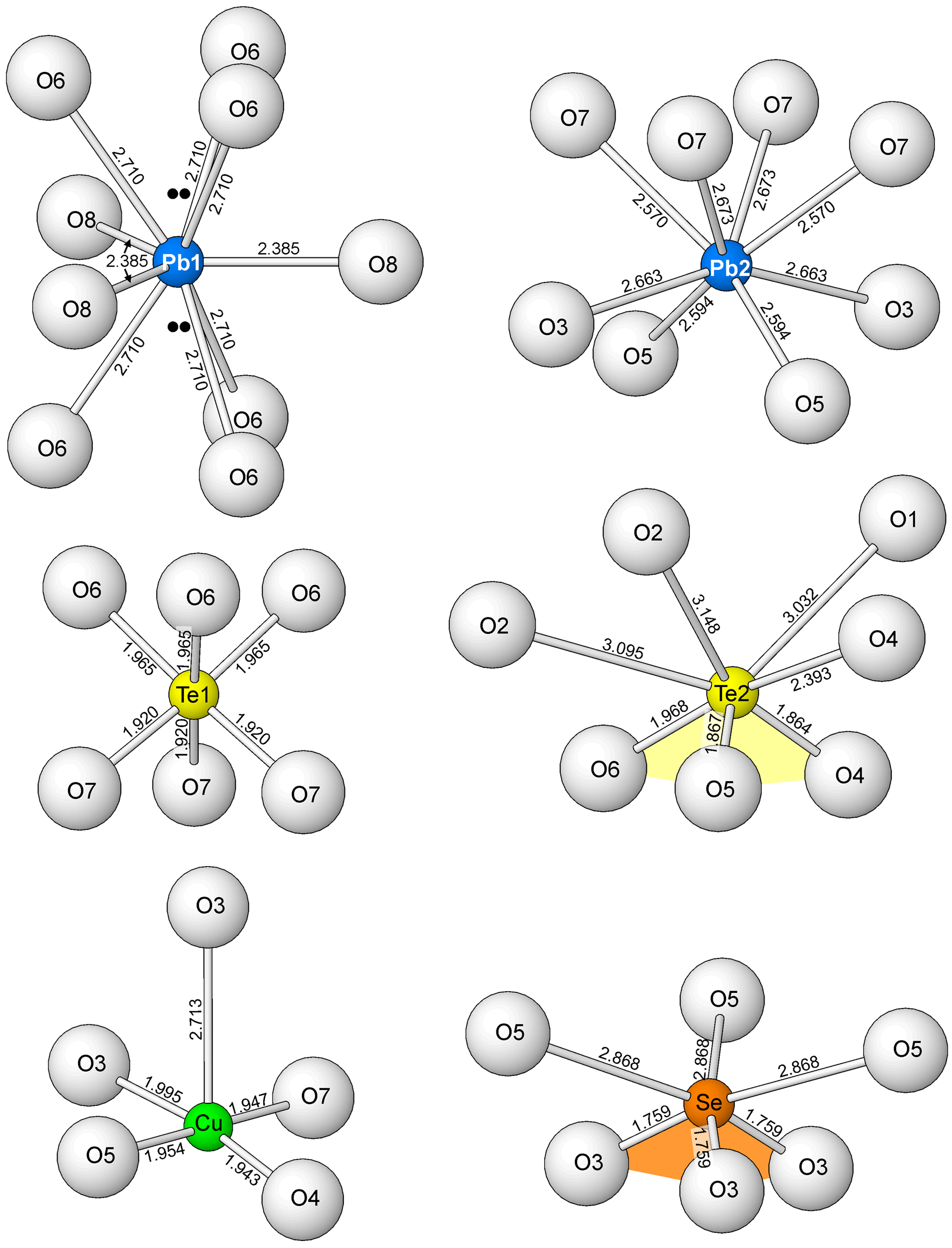
Fig. 6. The complete coordinations of all of the cations (except S6+) in the structure of tombstoneite. The location of the electron density residuals possibly corresponding to the location of Pb1 lone-pair electrons are shown with double black dots. Note that, for the Te2 and Se coordinations, the three short Te2–O and three short Se–O bonds define the Te4+O3 (yellow) and Se4+O3 (orange) pyramids, respectively.
It is noteworthy that three different cations in tombstoneite have lone-pair electrons: Pb2+, Te4+ and Se4+. The lone-pair electrons of Te4+ and Se4+ are clearly stereoactive, resulting in their distinctive trigonal pyramidal coordinations; however, the Pb22+ lone-pair electrons are not stereoactive and it is not entirely clear whether the Pb12+ lone-pair electrons are. The complete coordinations of all of the cations (except S6+) are shown in Fig. 6.
In the structural classification of Te oxycompounds of Christy et al. (Reference Christy, Mills and Kampf2016), tombstoneite is a mixed-valence Te oxysalt, with both Te4+ and Te6+. Christy et al. state that “It is noteworthy that there are no structures in which Te4+ and Te6+ polyhedra are linked together into a finite Te–O complex.” In fact, considering only the linkages between the Te24+O3 pyramids and the Te16+O6 octahedron in the structure of tombstoneite, it is the first known structure (natural or synthetic) with a finite complex that includes both Te4+ and Te6+ polyhedra. The only other mineral crystal structures that include both Te4+ and Te6+ polyhedra are: tlapallite, (Ca,Pb)3CaCu6[Te4+3Te6+O12]2(Te4+O3)2(SO4)2⋅3H2O, (mixed-valence phyllotellurate anion [Te4+3Te6+O12]12−; Missen et al., Reference Missen, Kampf, Mills, Housley, Spratt, Welch, Coolbaugh, Marty, Chorazewicz and Ferraris2019) and carlfriesite, CaTe4+2Te6+O8 (nanoporous [Te4+2Te6+O8]2– framework; Missen et al., Reference Missen, Kampf, Mills, Housley, Spratt, Welch, Coolbaugh, Marty, Chorazewicz and Ferraris2019), which both have their own new unique configurations.
Acknowledgements
Structures Editor and reviewers Oleg Siidra and Nicolas Meisser are thanked for their constructive comments on the manuscript. Preliminary Raman spectroscopy and EDS analyses at Caltech were funded by a grant from the Northern California Mineralogical Association. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.98
Competing interests
The authors declare none.















