Introduction
Flowering plants have evolved a great diversity of breeding systems, such as hermaphroditism, gynodioecy – populations with females and hermaphrodite plants, androdioecy – populations where hermaphrodites coexist with males (Pannell, Reference Pannell2000), trioecy – populations with females, males, and a few hermaphrodite plants (Cruden and Lloyd, Reference Cruden and Lloyd1995), and dioecy – populations with separate male and female plants (Sakai et al., Reference Sakai, Weller, Culley, Campbell, Dunbar-Wallis and Andres2008). Dioecy evolution is possible in two opposite directions, from and to hermaphroditism (Delph, Reference Delph2009). In the natural evolution of hermaphroditism to dioecy, the genetic variation in sex allocation among individuals within a population must exist, and selection will favor individuals whose sex allocation differs from the mean, depending on how the frequency of the coexisting unisexual morphophysiological changes (Delph, Reference Delph2009). For the evolution of dioecy to occur, a trade-off between the sex functions should exist, such that an increase in one causes a decrease in the other (Crowley et al., Reference Crowley, Harris and Korn2017; Schärer et al., Reference Schärer, Sandner and Michiels2004).
Secondary sexual dimorphism (SSD) in flowering plants is expressed in sexual differences of characters that are not directly related to gamete production (Barrett and Hough, Reference Barrett and Hough2013; Sakai and Weller, Reference Sakai, Weller, Geber, Dawson and Delph1999). SSD is seen as differences between sexes in a wide variety of morphological and physiological traits (Dawson and Geber, Reference Dawson, Geber, Geber, Dawson and Delph1999), which could vary over life history, especially in perennial species (Rakocevic et al., Reference Rakocevic, Costes and Assad2011, Reference Rakocevic, Medrado, Martim and Assad2009). SSD in plants is not simple to detect, impaired by the fact that they can modify sexual expression throughout their life (Vega-Frutis et al., Reference Vega-Frutis, Macías-Ordóñez, Guevara and Fromhage2014). In animals, males tend to be larger than females in larger species, whereas females tend to be larger in smaller species (Cassini, Reference Cassini2020; Lindenfors et al., Reference Lindenfors, Gittleman, Jones, Blackenhorn, Fairbairn and Székely2007). In parallel, woody plants could therefore be compared to large animals, with possibly larger males of higher reproductive success, increasing their progeny (Obeso, Reference Obeso2004). In herbaceous plants, larger size females have an advantage related to higher fecundity, when smaller size males might permit earlier maturation and a demographic advantage for precocious reproduction (Oñate and Munné-Bosch, Reference Oñate and Munné-Bosch2009).
Many sexually dimorphic characteristics, including both vegetative and flowering traits, are associated with differences in costs of reproduction, which are usually greater in females, particularly in long-lived species (Barrett and Hough, Reference Barrett and Hough2013). These differences can influence the frequency and distribution of females and males across resource gradients and within heterogeneous environments, causing niche differences and spatial segregation of the sexes. This means that sexual dimorphism and the associated physiological characteristics can act as an alternative to differential habitat selection by male and female plants (Slate et al., Reference Slate, Rosenstiel and Eppley2017). Ecologically, dioecy is associated with land cover, altitude, species richness, and mean annual temperature (Lin et al., Reference Lin, Tseng, Hsieh and Hu2020). Therefore, it would be expected that plants from different altitudes express different frequencies of sexual dimorphism.
If females require a higher cost of reproduction, this cost should be measurable as a lower vegetative growth rate, less frequent flowering, or reduced longevity when compared to males (Popp and Reinartz, Reference Popp and Reinartz1988). In a review considering about 100 dimorphic species, female plants invest proportionately more in reproduction than males, regardless of their size (Obeso, Reference Obeso2004). If female and male reproductive functions draw on different resources, then they will display additional different allocation strategies as they grow. For instance, Mercurialis annua females allocate less to reproduction at early stages, but this trend is reversed at later stages, while males allocate proportionally more of their biomass toward root development at later stages (Vilas and Pennell, Reference Vilas and Pannell2011).
Yerba mate (Ilex paraguariensis A. St.-Hil.) is an evergreen dioecious woody species, native to South America. Its natural habitat is a subtropical humid forest with Araucaria angustifolia, where it occupies the medium-upper layer, reaching 15 m in height. It has rhythmic growth with two annual growth units (GU), one in spring and one in autumn (Guédon et al., Reference Guédon, Costes and Rakocevic2018; Matsunaga et al., Reference Matsunaga, Rakocevic and Brancher2014; Rakocevic et al., Reference Rakocevic, Janssens, Scherer, Bezerra and Ferreira2012), and two annual growth pauses, one in summer (total or partial) and another in winter (total) (Rakocevic and Martim, Reference Rakocevic and Martim2011).
Yerba-mate pruning is carried out during the growth pauses, every 18 or 24 months. In the first pruning, a cut at the height of 40 cm is performed, while in the posterior harvests, the plants are pruned at the height of about 1–1.15 m to obtain and maintain a bushy form (Santin et al., Reference Santin, Benedetti, Barros, Fontes, Almeida, Neves and Wendling2017). The pruned leaves and fine twigs are used for beverage preparations. A great variety of yerba-mate forms are observed, from plant architecture (Rakocevic et al., Reference Rakocevic, Costes and Assad2011, Reference Rakocevic, Janssens, Scherer, Bezerra and Ferreira2012) to different leaf shapes and colors (Rakocevic et al., Reference Rakocevic, Janssens, Scherer, Bezerra and Ferreira2012), defining six morphotypes (Duarte et al., Reference Duarte, Gabira, Tomas, Amano, Nogueira and Wendling2022; Reissmann et al., Reference Reissmann, Dünisch, Boeger and Hüttl2003; Wendling et al., Reference Wendling, Sturion, Reis, Stuepp and Peña2016). Yerba-mate morphotypes are defined from the leaf traits, as yellow, gray, sassafras, dark green, dully green, and furry.
In several dioecious species, females can compensate for additional reproductive investment by regulating photosynthesis rates (Obeso, Reference Obeso2004). Leaves of yerba-mate female plants show SSD with a greater photosynthetic rate, which is expressed only during vegetative stages before flowering and fruit ripening (Rakocevic et al., Reference Rakocevic, Costes and Assad2011, Reference Rakocevic, Medrado, Martim and Assad2009), without differing from males in growth rates (Guédon et al., Reference Guédon, Costes and Rakocevic2018). Plants that are pruned to harvest the leaves, set flowers, and the females bear fruits during the following years (Rakocevic et al., Reference Rakocevic, Medrado, Martim and Assad2009). Fruit production induces higher reproductive costs in females than in males (Obeso, Reference Obeso2002). If physiological regularization is not achieved (through greater photosynthetic rate), females compensate by breeding less frequently than males, or their greater reproductive efforts may result in reduced growth, lower biomass production, or higher mortality, as it has been seen in Ocotea tenera (Wheelwright and Logan, Reference Wheelwright and Logan2004).
Knowledge of yerba-mate SSD initiated with gender segregation in leaf gas exchanges (Rakocevic et al., Reference Rakocevic, Medrado and Lavoranti2007a, Reference Rakocevic, Medrado, Martim and Assad2009), leaf chemical composition (Pauli et al., Reference Pauli, Scheel, Delaroza, Rakocevic, Bruns and Scarminio2019; Rakocevic et al., Reference Rakocevic, Medrado, Lavoranti and Valduga2007b; Tormena et al., Reference Tormena, Pauli, Marcheafave, Scheel, Rakocevic, Bruns and Scarminio2020), beverage sensorial quality (Rakocevic et al., Reference Rakocevic, Medrado, Lucambio and Valduga2008a), plant growth, and architecture (Guédon et al., Reference Guédon, Costes and Rakocevic2018; Matsunaga et al., Reference Matsunaga, Rakocevic and Brancher2014; Rakocevic et al., Reference Rakocevic, Costes and Assad2011; Rakocevic et al., Reference Rakocevic, Silva, Assad and Megeto2008b; Silva and Rakocevic, Reference Silva and Rakocevic2010). Biomass production does not differ between two genders (Rakocevic et al., Reference Rakocevic, Medrado, Lavoranti and Valduga2007b), regardless of the system of yerba-mate cultivation, monoculture (full sunlight cultivation), or agroforestry production under shaded conditions (Matsunaga et al., Reference Matsunaga, Rakocevic and Brancher2014). Under simulated abiotic stress, such as low temperatures or low precipitation during the last two GU formations, male plants attain higher biomass production than female plants (Matsunaga et al., Reference Matsunaga, Rakocevic and Brancher2014).
It was hypothesized that: (1) during the early years of plant life, two yerba-mate genders would segregate in biomass production at a lower frequency than in later years, due to differences in allocation strategies throughout life; (2) in the case of sexual segregation, higher biomass production in female plants than in male plants would be more frequent with advancing plant age, due to the possible female compensation for additional reproductive investment; (3) more SSD in biomass productivity could appear in morphotypes that present leaf adaptations to full sunlight cultivation; and (4) progenies originated from provenances at higher altitudes should show higher sexual segregation when considering biomass production.
Materials and Methods
Experimental design
For evaluation of yerba-mate half-sibling progenies (seedlings originating from the same mother tree) from six provenances, an experiment was installed in monoculture (full sunlight cultivation). The criteria for initial progeny selection were high plant biomass production and resistance to pests. The experiment was installed in Ivaí, Paraná, Brazil (see coordinates below), where all seedlings were produced and planted in November 1997. The planting arrangement was 3 m row distance and 2 m plant-to-plant row distance. The Ivaí climate type is Cfa (Köppen-Geiger’s classification), corresponding to humid subtropical climate with hot summers, infrequent frosts, and the tendency of rains concentrated in the summer months, without a defined dry season. Minimum, average, and maximum annual temperatures are 12.7, 17.8, and 24.9 °C, respectively. Mean annual rainfall is 1588 mm, and mean annual relative humidity is 79.7%. The soil had not been fertilized, nor plants protected against pests.
Five provenances were from Paraná state (PR), and one from Rio Grande do Sul state (RS), denominated in analyses as: (Iv) Ivaí-PR, 25° 01' 39" S, 50° 51' 32" W, altitude 748 m; (Co) Colombo-PR, 25° 17' 30" S, 49° 13' 27" W, altitude 893 to 1201 m; (BC) Barão de Cotegipe-RS, 27° 37' 15" S, 52° 22' 48", altitude 765 m; (QI) Quedas do Iguaçú-PR, 25° 27' S 52° 54' 28", altitude 630 m; (Pi) Pinhão-PR, 25° 41' 45" S, 51° 39' 36", altitude 1041 m; (Ca) Cascavel-PR, 24° 57' 20'' S, 53° 27' 19'' W, altitude 782 m (Figure 1). Altitude was presented as the % of the highest altitude, that is, average 1047 m for Colombo-PR (Figure 1a).
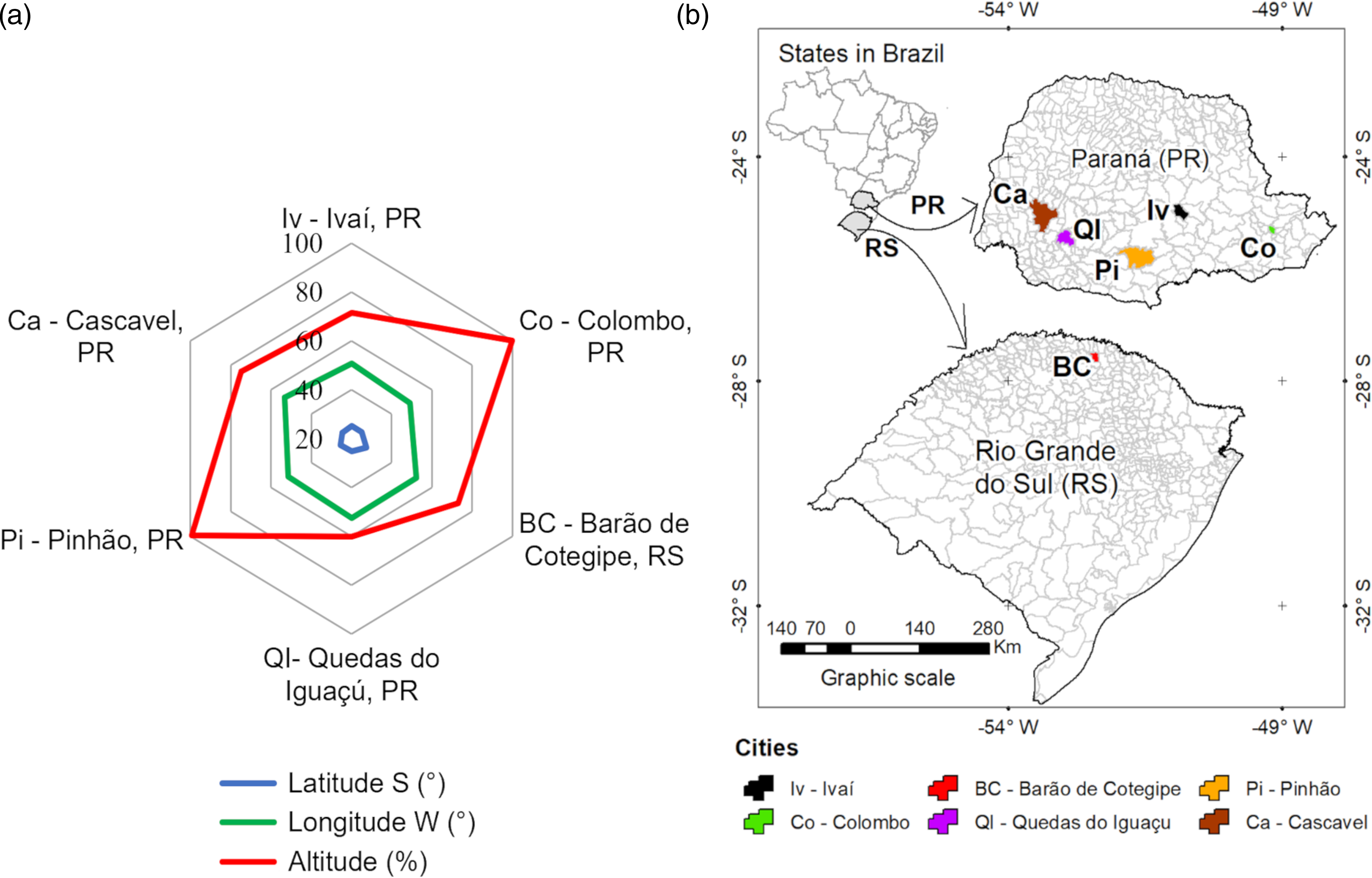
Figure 1. Schematic representation of geographical characteristics (latitude, longitude, altitude, and location) of six yerba-mate provenances: (a) altitude presented as the % of the highest altitude (1047 m, average for Colombo-PR, Brazil) and (b) location of respective municipalities.
We used the progeny denomination for the yerba-mate breeding program that followed an ordered numerical sequence (1, 2, 3, ….) with up to 25 progenies from each provenance (Wendling et al., Reference Wendling, Sturion, Reis, Stuepp and Peña2016). For example, progenies 1–25 from provenance Iv, 26–50 from provenance Co, …, and progenies 150–175 from provenance Ca (Supplementary Table S1). Not every provenance was represented by 25 progenies, nor every provenance from a breeding program was included in our analyses, as was the case for one provenance represented by only five progenies, occupying the numerical sequence from 126 to 150. In the year of the breeding experiment establishment, 60 plants of each of 135 progenies were randomly distributed in the experimental field, summing 8100 seedlings. Pruning was carried out in the winter period (July–August), with an interval of 2 years, when the commercial biomass production (70% of green leaves of every plant and fine branches up to 7 mm of thickness) was recorded (kg plant−1), with a precision of two decimals. Recorded pruning on all surviving trees occurred in 1999, 2001, 2003, and 2015.
Tree gender was determined in 2004 and confirmed in 2021, without the success of sex identification of all existing trees. If incoherence in plant sex determination was found (sex not determined for that plant or different classifications in the two evaluation years), mortality or replanting occurred. Those plants were excluded from the data set used for statistical analyses. Besides the exclusion of plants without coherent sex classification or without biomass records, only progenies represented by at least three plants per each gender (3–21 plants per gender) were used. For those reasons, the number of plants whose production was used for SSD analyses was 5135 (1999), 4894 (2001), 4636 (2003), and 4970 (2015), due to the minimum requirement of three plants per gender for the Levene’s homogeneity test necessary to choose the type of t-test to be applied in the SSD investigation. Female trees represented approximately 51% of the total number of plants evaluated, while the males corresponded to 49%. This sexual ratio was maintained throughout the period of the experiment.
Association of SSD with biomass production within progenies throughout life
The expression of sexual dimorphism in yerba-mate production was first verified by comparison of the means and the variability of biomass production per plant for each gender, during the harvest years evaluated here (1999, 2001, 2003, 2015), within each progeny. The comparison of mean plant production among years was not carried out, because of the clear increase in biomass production, due to the constant growth of the trees (Supplementary Tables S2 and S3).
SSD frequency and increasing superiority of mean biomass in female plants throughout life
The SSD frequencies (%) for each harvest year were computed by dividing the number of progenies presenting SSD in each year by the respective number of progenies tested for SSD, without directly accounting to their provenances. Statistical methods for contingency table analysis were employed to investigate the association between SSD frequency and plant age. Similar methods were used to assess the influence of plant age on the frequency of superiority of mean biomass in female plants (F > M).
SSD in biomass production within provenances and yerba-mate leaf morphotypes
The expression of SSD was additionally evaluated within the six yerba-mate leaf morphotypes, for each provenance and harvest year. The morphotypes were (1) yellow (Y) with light-toned leaves and yellowish main and secondary veins; (2) gray (G) with greenish-gray leaves and not so yellowish veins; (3) sassafras (S) with dark green leaves, lighter veins, and a shiny appearance of the leaf blade on the adaxial face; (4) dark green (DaG) with intense green leaves and lack of a shiny appearance on the adaxial surface; (5) dully green (DuG) with opaque and waxy green leaves and a lack of a shiny appearance on the adaxial surface; and (6) furry (Fu) with hairy leaves on the adaxial face (Reissmann et al., Reference Reissmann, Dünisch, Boeger and Hüttl2003; Welding et al., Reference Wendling, Sturion, Reis, Stuepp and Peña2016). These morphotypes were identified in 2015, and the identification was retrospectively attributed to harvested plants in the previous years. The fact that more than one morphotype appeared within a particular progeny, or within a specific gender of the same progeny, indicated the variability in half-siblings as descendants of unknown fathers.
The morphotype identification was not attributed to every plant but to approximately 55.8–58.4% of the plants used in our sexual segregation study within progenies or provenances, resulting in a highly unbalanced data set regarding the absolute frequencies of plants by morphotype (Table 1). In 2015, for example, the following order was observed: Y (0) < Fu (11) < G (71) < S (138) < DaG (902) > DuG (1642). The relative frequency of plants with identified morphotypes for each gender was well balanced, with 52–53% of female plants for morphotypes characterizing elevated plant number (DuG, DaG, and S) and varying from 38 to 55% for the less frequent morphotypes (Fu and G). SSD in yerba-mate production was assessed by the comparison of female and male mean biomass, for each harvest year, provenance, and morphotype.
Table 1. Number of identified and analyzed yerba-mate plants * according to morphotype, gender (female: F and male: M), and harvest year in Ivaí, PR, Brazil
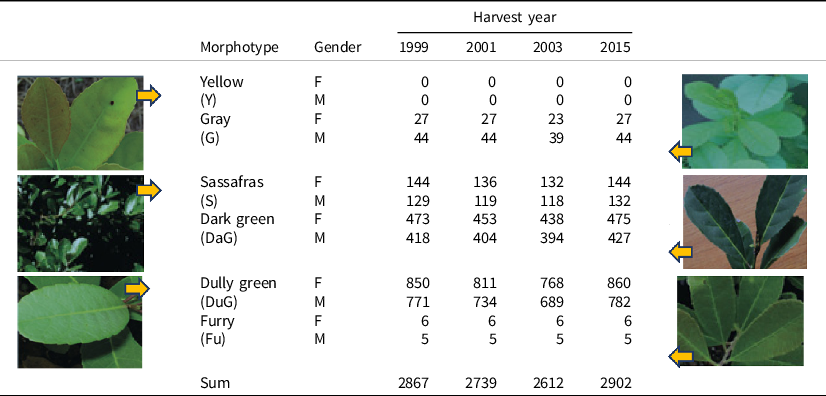
* The set of analyzed plants corresponds to the set of identified plants after the exclusion of plants without production records and the cases with less than three plants of each gender within the combinations: year × provenance × morphotype.
Association of provenance altitude with biomass production SSD
Firstly, cluster analyses were performed to examine the similarity among the mean biomass production of plants from different provenances by gender. The resulting dendrogram indicated if the biomass production could be related to provenance altitude.
Additionally, the overall relative frequency of progenies presenting SSD cases in each of the six provenances was calculated by comparing all the respective cases of progenies tested for SSD, using a t-test. The frequencies of SSD and no-SSD cases (Yes, No) by provenance were arranged in a 6 × 2 contingency table. Subsequently, a more detailed analysis was performed to investigate the association between altitude (< 1000 and > 1000) and SSD occurrence, also assessing methods for contingency table analysis.
Statistical procedures and software
The expression of SSD in yerba-mate production was investigated for each harvest year within two classification factors: progenies and plant leaf morphotypes within provenances. In both cases, the hypothesis of SSD occurrence was assessed by comparing mean biomass production between genders using bilateral t-tests for independent samples. The type of t-test to be used (assuming equal or different variances) was chosen according to the respective results of Levene’s test for variance homogeneity.
The association between the studied factors and SSD in biomass production or female biomass superiority (F > M) was assessed by Fisher’s exact test for unilateral or bilateral alternative hypotheses (Andrés and Tejedor, Reference Andrés and Tejedor1995) in the following cases: (A) SSD frequency throughout harvest years; (B) F > M frequency comparing early and late plant ages; (C) SSD versus plant leaf morphotypes; (D) SSD versus groups of morphotypes (DuG and others); (E) leaf morphotypes versus F > M; (F) SSD versus provenance location; and (G) SSD versus provenances groups based on altitude.
In the cluster analyses, the Euclidean distance was used as a metric to determine the separation between points or clusters and Ward’s minimum variance as the clustering method (Randriamihamison et al., Reference Randriamihamison, Vialaneix and Neuvial2021).
Statistical analyses were performed using specific procedures of the statistical software SAS/STAT® (SAS Institute Inc, 2018): (1) the ‘TTEST procedure’ to compare gender biomass variability (Levene’s test) and assess SSD within progenies or morphotypes; (2) the ‘FREQ procedure’ for assessing the influence of plant age or geographic factors of provenance location on SSD or female biomass superiority (F > M) frequencies (%), via Fisher’s exact test; (3) the ‘CLUSTER procedure’ to perform analysis of similarities among different provenances production profiles (cluster analysis); and (4) the ‘TREE procedure’ for dendrogram construction using the results generated by the respective cluster analysis. The significance level adopted for statistical inference in this study was 0.10, because of the high interplant variability within progenies.
Results
Association of SSD with biomass production within progenies throughout life
In the first evaluated harvest year (1999), when the pruning of formation was performed, only 10 progenies showed a relationship between SSD and biomass production (Figure 2a). This frequency of segregation was followed by 13 progenies in 2001 and 12 in 2003 and 2015 (Figure 2b, 2c and 2d). The segregation between genders within different years not always occurred in the same progenies, nor there was a same predominant gender in terms of biomass production. Comparing the segregation over harvest years, differences in SSD frequencies throughout the life were not found to be significant (Fisher’s exact test p = 0.8937, Table 2). The lowest significant SSD segregation in biomass production was observed in progenies originated from Quedas do Iguaçú (QI), where only progeny 77 expressed a productive SSD in 2003 (Figure 2c) and progeny 89 in 2015 (Figure 2d).
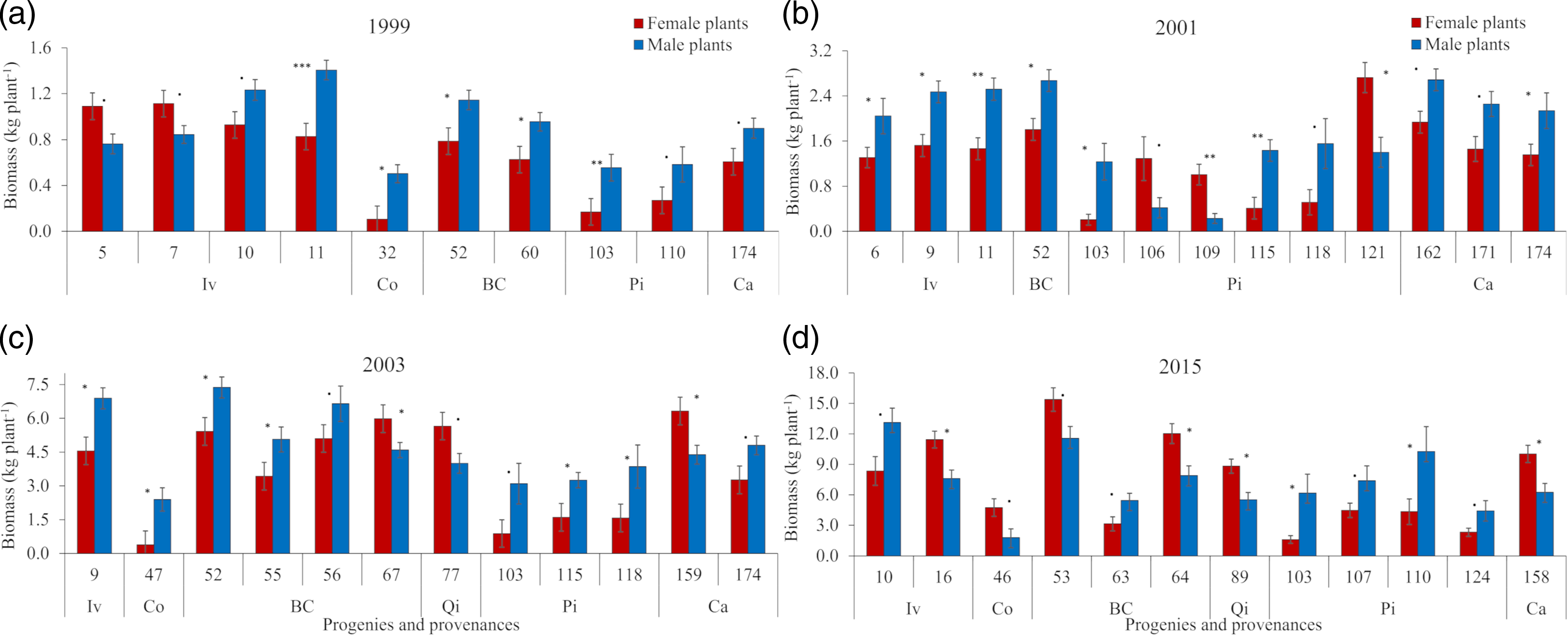
Figure 2. Dynamics in average biomass production (kg plant−1) for male and female plants during four harvest years: (a) 1999; (b) 2001; (c) 2003; and (d) 2015, considering only progenies (untitled 1–174) for which the difference between female and male mean biomass was significant. Provenances (Iv, Co, BC, QI, Pi, and Ca, see Figure 1) and significance of the t-test: ‘***’<0.001, ‘**’<0.01, ‘*’<0.05, ‘.’ <0.1 were also indicated.
Table 2. Absolute number (n) and relative frequencies (%) of progenies or cases expressing secondary sexual dimorphism (SSD) or female mean biomass superiority (F > M) according to the investigated factor (harvest year, plant age, morphotype, morphotype group, provenance, or group of provenances based on altitude)
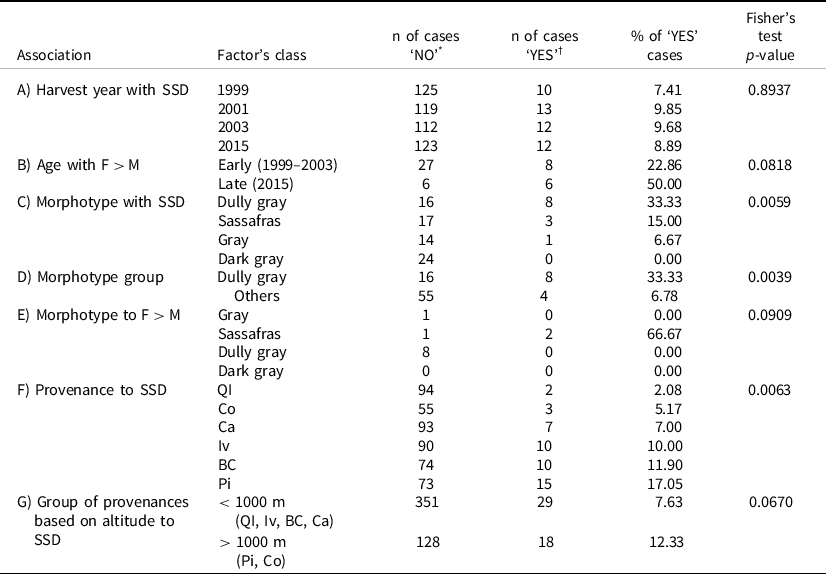
* Cases not presenting SSD or cases with M > F mean biomass production (M > F).
† Cases presenting SSD or cases with F > M mean biomass.
The mean coefficient of variation (CV) of yerba-mate biomass production in four harvest years revealed high variability among plants, varying from 69% (male plants in 2003) to 93% (female plants in 2001) (Supplementary Table S2). In all harvest years, the highest interplant variability in biomass production was observed for provenances of the highest altitudes, Colombo and Pinhão, showing the highest CV mean values: 172% (M) and 103% (F), respectively, in 2001 (Supplementary Table S3). Despite the absolute mean biomass values naturally increasing with the growth of the trees, the relative variability (CV) did not increase with age, when comparing 2001 and 2015 (Supplementary Figures S1b and S1d) because both the mean and standard deviation increased with time in the same proportion. The respective values of mean biomass production were 0.73 kg plant−1 in 1999, increasing to 1.58 kg plant−1 in 2001, 4.62 kg plant−1 in 2003, and 7.35 kg plant−1 in 2015 (Supplementary Table S3).
SSD frequency and increasing superiority of mean biomass in female plants throughout life
In 1999, males were more productive than females in eight progenies, compared to two progenies for which the opposite trend was observed (Figure 2a). Ten progenies with higher biomass production in male plants versus three progenies with higher biomass production in female plants were observed in 2001 (Figure 2b). Nine progenies with higher production in males versus three with higher biomass production in females were observed in 2003 (Figure 2c). Males were more productive in only six progenies compared to an equal number of progenies where females were more productive in 2015 (Figure 2d). An increased frequency of more productive female plants than male plants throughout the life was found to be statistically significant when using a left-sided Fisher’s test (p-value 0.0818), comparing the first three harvests (1999, 2001, and 2003) to the final harvest (2015) (Table 2).
SSD in biomass production within provenances and yerba-mate leaf morphotypes
Among the six morphotypes studied here (Table 1), an association between SSD and biomass production was observed only in dully green, sassafras, and gray morphotypes (Figure 3). The association between morphotypes and gender biomass production increased with increasing age, with only two progenies with segregated biomass production in 1999 (both DuG), three in 2001 (all DuG), three in 2003 (DuG and S), and four in 2015 (DuG, S, and G). Despite the DaG being the second most frequent yerba-mate leaf morphotype (Table 1), it had not expressed the SSD in biomass (Figure 3). The dully green morphotype segregated male and female individuals in relation to biomass production for all four harvests, for provenances QI and Ca in 1999, BC, Pi, and Ca in 2001, Pi and Ca in 2003, and Pi in 2015. Only in the latter harvests, SSD was expressed in the sassafras morphotype for provenance QI in 2003, and Co and QI in 2015. In the gray morphotype, represented by a modest number of plants (Table 1), SSD was expressed only in 2015 (Figure 3). Generally, the frequency of SSD in relation to biomass production differed significantly (Fisher’s exact test, p = 0.0059) among DuG, S, G, and DaG morphotypes (Table 2). DuG expressed a significantly higher frequency of SSD in relation to biomass production when compared to all other morphotypes (Fisher’s exact test, p = 0.0039, Table 2).
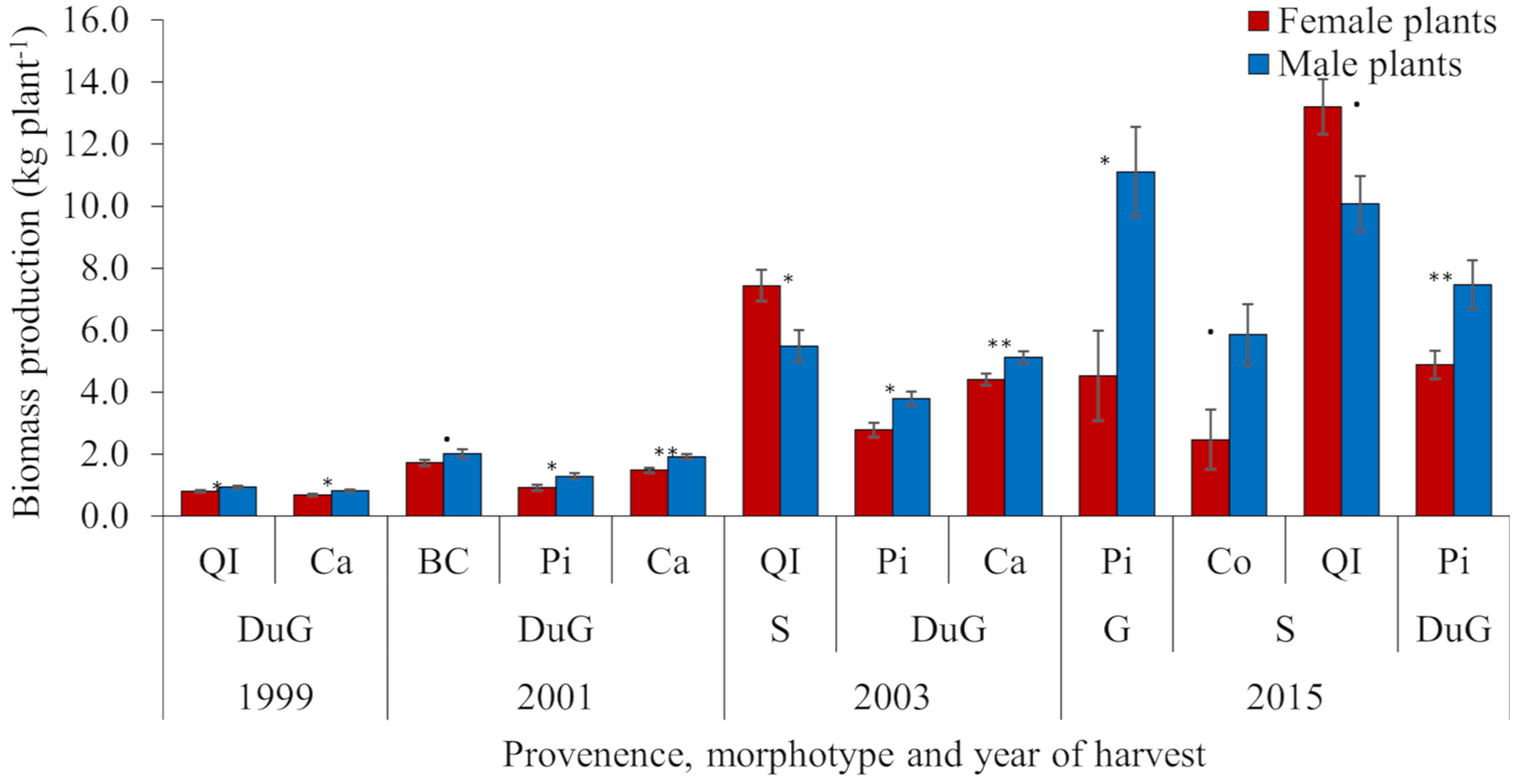
Figure 3. Mean biomass production (kg plant−1) per female (F) and male (M) plants from various provenances (Co, BC, QI, Pi, and Ca, see Figure 1), classified according to leaf plant morphotypes (DuG – dully green, S – sassafras, G – gray), during four harvest years (1999, 2001, 2003, and 2015). The significance of the t-test was indicated as ‘***’<0.001, ‘**’<0.01, ‘*’<0.05, ‘.’ <0.1.
The frequency of F > M expression differed significantly among DuG, S, G, and DaG morphotypes (p-value = 0.0909, Table 2). DuG males were more productive than females regardless of harvest year or provenance, while females were more productive than males only in sassafras morphotype and QI provenance in 2003 and 2015 (Figure 3).
Association of provenance altitude with biomass production SSD
As a first step, the study of provenance impact on biomass production via an exploratory cluster analysis included all progenies, those with and without SSD expression (Figure 4). The cluster analysis showed provenance segregation into two groups. Group 1 (Ca, Iv, BC, and QI) was represented by the highest mean biomass production, while group 2 (Pi and Co) corresponded to the lowest biomass production. Overall, higher female than male biomass production was observed in Ca, Iv, and Co provenances, while the opposite trend was found in BC, QI, and Pi.
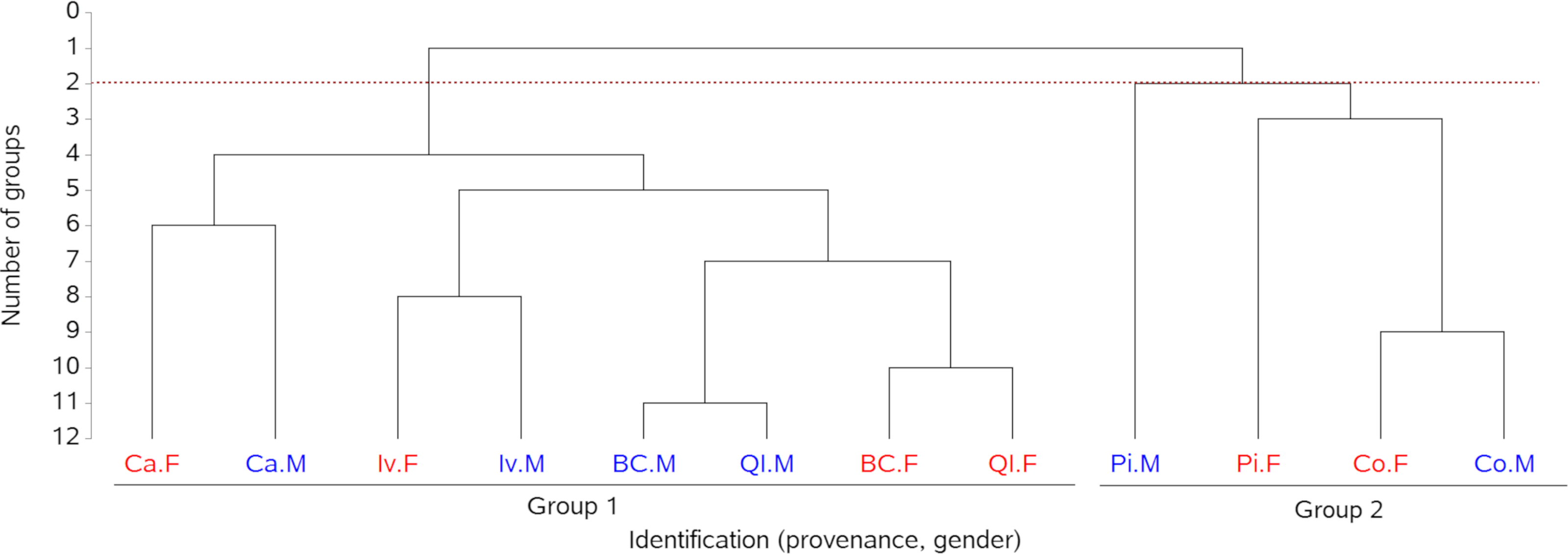
Figure 4. Dendrogram of similarity among the mean productivity profiles of male and female plants of progenies from six provenances (Co, BC, QI, Pi, and Ca, see Figure 1), considering all harvest years (1999, 2001, 2003, and 2015).
The second step of the analysis considered the association between biomass production and sexual segregation. The significant p-value of the Fisher’s test (0.0063) calculated for the frequency of SSD in relation to biomass production ranked provenances in the following order: Pi > BC > Iv > Ca > Co > QI (Table 2).
In the third step, all provenances were classified into two classes of altitude: higher than 1000 m (Co, Pi) and lower than 1000 m (QI, Ca, Iv, BC) (Table 2). On average, SSD segregation in biomass production occurred with greater frequency at higher than in lower altitude provenances.
Discussion
Dioecy is a rare breeding system in flowering plants. The global incidence of dioecy among angiosperms is estimated to be about 6% (Renner and Ricklefs, Reference Renner and Ricklefs1995), and in most of them, sex can be recognized only when the plants start flowering and set fruits (Al-Obaidi et al., Reference Al-Obaidi, Halabi, Al-Khalifah, Asanar, Al-Soqeer and Attia2017). Flowering in yerba mate starts 2–3 years after transplanting in the field. The slightly superior female (51%) to male plant frequency in our experiment could reflect a longer female reproduction period, facilitating female tree identification.
We succeeded in detecting yerba-mate SSD biomass production, by using the statistical tools that were able to differentiate between the high levels of variation in progeny sexual responses following four prunings. High variability among progenies, adaptability, and even interplant variability observed in dioic species (Galfrascoli and Calviño, Reference Galfrascoli and Calviño2020) are obstacles in the estimation of SSD. This is the case for a study developed by Wendling et al. (Reference Wendling, Sturion, Reis, Stuepp and Peña2016) in Ivaí (Paraná, Brazil), using only the 2015 data from the same experimental field site that was studied here. Their data analysis did not provide enough evidence of gender segregation because the ANOVA evaluated gender segregation within each provenance, not discriminating the progenies nor considering the possibility of interaction between progeny and gender that could artificially mask the evidence of SSD by pooling progenies from the same provenance.
The increasing variability and adaptation that occur with aging, related to repetitive stressful conditions (Pérez-Llorca and Munné-Bosch, Reference Pérez-Llorca and Munné-Bosch2021), lead us to hypothesize that aging could promote an increased frequency of biomass production SSD in dioic species. However, the results did not confirm this hypothesis. On the other hand, interplant variability seems crucial for detecting sexual differences and may also have important ecological and evolutionary implications (Galfrascoli and Calviño, Reference Galfrascoli and Calviño2020). In five yerba-mate provenances, the genetic divergence detected using RAPD markers shows great intrapopulational variation in caffeine and theobromine content in leaves, higher within (92%) than among (8%) different populations (Friedrich et al., Reference Friedrich, Gonela, Vidigal, Vidigal Filho, Sturion and Cardozo Junior2017), supporting our results of continuously elevated interplant biomass variability observed during all four harvest years.
Stressful conditions and leaf life strategy play a crucial role, for example, in gender dimorphism of Pistacia spp., both in physiological and morphological responses (Seyedi et al., Reference Seyedi, Costa, Maguas and Correia2019). For instance, P. lentiscus females display higher leaf mass per area than males, higher C/N ratio, and lower carbon isotope composition (δ13C), which is related to higher stomatal conductance during photosynthesis. In yerba mate, no differences in N content were found between genders (Rakocevic et al., Reference Rakocevic, Medrado, Lavoranti and Valduga2007b), but the higher reproductive investment of females might be compensated by higher leaf photosynthesis than males in the vegetative stages that precede flowering and fruit ripening (Rakocevic et al., Reference Rakocevic, Medrado, Martim and Assad2009). Males produced higher biomass than females during the initial harvest years. As a result of adaptive responses to repetitive stressful conditions, an increased frequency of more productive yerba-mate females appeared in 2015, resulting in a near-equal frequency of female and male plants in each biomass production class of segregated progenies, confirming our second hypothesis.
Gender segregation in biomass production was observed only in some of the yerba-mate morphotypes, dully green, sassafras, and gray. Those three leaf morphotypes could be related to the elevated presence of various cuticular wax structures, which can generally act to protect the plant from elevated levels of UV radiation through elevated scattering and reflection of UV bands as seen in various species (Grant et al., Reference Grant, Heisler, Gao and Jenks2003) and mediate nonstomatal water loss under full sun or drought conditions (Busta et al., Reference Busta, Schmitz, Kosma, Schnable and Cahoon2021). The upregulation of UV-reflecting pigments or structures may be a strategy to protect leaves at elevated altitudes and in full sunlight cultivation (Valenta et al., Reference Valenta, Dimac-Stohl, Baines, Smith, Piotrowski, Hill, Kuppler and Nevo2020), indicating that increased SSD in yerba-mate biomass production could be associated with morphotypes adapted to full sunlight cultivation and thus confirm our third hypothesis.
Our study showed, for the first time to our knowledge, that SSD in biomass production was related to the altitude of progenies provenance in one tree species. The spatial variations of dioecy are more common in the tropics and subtropics (Lin et al., Reference Lin, Tseng, Hsieh and Hu2020) or in Siberian biomes (Godin, Reference Godin2017) than in temperate zones, especially at lower altitudes (Lin et al., Reference Lin, Tseng, Hsieh and Hu2020), but this variation has not been analyzed in terms of SSD, until the present study. Yerba-mate progenies originating from lower altitudes (provenances QI, Iv, BC, and Ca) showed a lower frequency of SSD in biomass production when compared to progenies from higher altitudes (provenances Pi and Co). This could be attributed to the greater interplant variability in biomass production observed in progenies from the highest altitude provenances, Pi and Co. Those results confirmed, our fourth hypothesis, that the progenies originating from higher altitude provenances would show a greater frequency of SSD in biomass production when compared to those from lower altitudes.
As a final consideration, we emphasize the importance of using adequate, non-conventional statistical methods, such as categorical data analysis, including the nonparametric Fisher’s exact test in its left- or right-sided versions, which were more efficient than the bilateral tests, especially when a unilateral alternative hypothesis was being investigated, as in the case of increasing biomass female superiority in relation to plant age. The costs associated with yerba-mate reproduction could be most easily met in biomass production gender differentiation, showing a general tendency of more vigorous female survivors in advanced years. Additionally, it can be deduced that a greater physiological investment in the photosynthesis of yerba-mate female trees than in the male ones (Rakocevic et al., Reference Rakocevic, Costes and Assad2011, Reference Rakocevic, Medrado, Martim and Assad2009), compensated for their higher reproductive costs, particularly after a long life (18 years in 2015), results in SSD biomass production. This influenced the increasing frequency of progenies with female biomass superiority as the plants age, which was additionally related to progeny from lower altitudes.
Conclusions
The occurrence of sexual dimorphism in biomass accumulation is particularly important when selecting clones for breeding. The frequency of SSD in biomass production did not change throughout the plant’s life. On the other hand, because of adaptive responses to stressful conditions and elevated interplant variability, the frequency of more productive yerba-mate females increased throughout plant life. In the last observed harvest year, an equal percentage of segregated female and male progenies was observed, confirming our second hypothesis. This might be supported by the increased success of the physiological efforts of yerba-mate female trees, compensating for their greater cost of reproduction. An increased sexual segregation was observed in some morphotypes (dull green, sassafras, and gray) adapted to elevated irradiance and UV, confirming our third hypothesis. As a result of adaptative responses to stressful conditions and elevated interplant variability, SSD in biomass production of progenies originating from higher altitudes segregated with higher frequency when compared to lower altitudes, confirming our fourth hypothesis.
Supplementary Material
For supplementary material for this article, please visit https://doi.org/10.1017/S0014479722000552
Acknowledgements
The authors are grateful to ‘Chimarrão Bitumirim Indústria e Comércio de erva-mate Ltda’ for all the support on conducting the experiment and field evaluations.
Competing Interests
The authors declare no competing interests.
Financial Support
MR and MMD were supported by the National Council for Scientific and Technological Development (CNPq, Brazil), MR as a Visiting Researcher (Proc. 350509/2020-4), and MMD as an Industrial Researcher (Proc. 382360/2020-4). This work was funded by Brazilian Agricultural Research Corporation (20.18.01.002.00.00, phase III).









