Introduction
Neotropical freshwater catfishes from the genus Rhamdia are found throughout the Americas, from Mexico to Argentina. Rhamdia is taxonomically complex and no consensus exists on the total number of species that the genus contains, with hypotheses spanning a huge range, from 11 to 16 species (Silfvergrip, Reference Silfvergrip1996; Perdices et al., Reference Perdices, Bermingham, Montilla and Doadrio2002; Hernández et al., Reference Hernández, Ortega-Lara, Sanchez-Garces and Alford2015; Arroyave & De La Cruz-Fernández, Reference Arroyave and De La Cruz-Fernández2021). There is agreement, however, that the genus originated in South America and expanded northwards by colonizing Central America after the rise of the Isthmus of Panama – and stopped at the Isthmus of Tehuantepec in Mexico, the northernmost distribution limit for several Neotropical organisms. This region between Tehuantepec and Panama corresponds to Tropical Middle America sensu Choudhury et al. (Reference Choudhury, García-Varela and Pérez-Ponce de León2017), which is inhabited by two species of Rhamdia supported by morphometric and phylogenetic analyses, the pale catfish or ‘juil’, Rhamdia guatemalensis; and the filespine ‘chulín’, Rhamdia laticauda (Perdices et al., Reference Perdices, Bermingham, Montilla and Doadrio2002; Arroyave & De La Cruz-Fernández, Reference Arroyave and De La Cruz-Fernández2021).
The distinction of these two species is supported by the studies by Perdices et al. (Reference Perdices, Bermingham, Montilla and Doadrio2002), in which the mtDNA analyses place the trans-Andean ‘Rhamdia quelen’ of Central America in a different clade from the typical cis-Andean R. quelen of South America (Hernández et al., Reference Hernández, Ortega-Lara, Sanchez-Garces and Alford2015). Rhamdia quelen found in South America is known to be a complex of cryptic species, containing at least seven allopatric molecular operational taxonomic units associated to different hydrological basins (Ribolli et al., Reference Ribolli, Zaniboni-Filho, Soares-Scaranto, Akio-Shibatta and Barros-Machado2021), which could become separate species if shown to be independent evolutionary lineages.
Although clearly understudied in the Neotropics (Mendoza-Palmero et al., Reference Mendoza-Palmero, Blasco-Costa and Scholz2015; García-Prieto et al., Reference García-Prieto, Dáttilo, Rubio-Godoy and Pérez-Ponce de León2022), some monogenean parasites from the speciose Gyrodactylidae have been described infecting catfishes (Siluriformes). Members of Scleroductus (Jara & Cone, Reference Jara and Cone1989) were identified from this group of fish: Scleroductus yuncensi Jara & Cone, Reference Jara and Cone1989 from Pimelodella yuncensis, Steindachner in Peru (Jara & Cone, Reference Jara and Cone1989); Scleroductus lyrocleithrum Kritsky, Boeger, Mendoza-Franco & Vianna, Reference Kritsky, Boeger, Mendoza-Franco and Viann2013, from R. guatemalensis in Mexico; Scleroductus angularis Kritsky, Boeger, Mendoza-Franco & Vianna, Reference Kritsky, Boeger, Mendoza-Franco and Viann2013 from barred surobim Pseudoplatystoma fasciatum in Brazil; and undescribed Scleroductus sp. from R. quelen and other wild heptaterid and pimelodid catfishes in Brazil (Kritsky et al., Reference Kritsky, Boeger, Mendoza-Franco and Viann2013). A few species of Gyrodactylus have also been described from siluriformes in Brazil: Gyrodactylus anysopharynx Popazoglo & Boeger, Reference Popazoglo and Boeger2000, Gyrodactylus samirae Popazoglo & Boeger, Reference Popazoglo and Boeger2000 and Gyrodactylus superbus (Szidat, 1973) Popazoglo & Boeger, Reference Popazoglo and Boeger2000, all three from armored catfishes Corydoras paleatus and Corydoras ehrhardti (Popazoglo & Boeger, Reference Popazoglo and Boeger2000); Gyrodactylus bueni Bueno-Silva & Boeger, Reference Bueno-Silva and Boeger2014, Gyrodactylus major Bueno-Silva & Boeger, Reference Bueno-Silva and Boeger2014 and Gyrodactylus scleromistaci Bueno-Silva & Boeger, Reference Bueno-Silva and Boeger2014, all from sailfin corydoras, Scleromystax macropterus and banded corydoras, Scleromystax barbatus (Bueno-Silva & Boeger, Reference Bueno-Silva and Boeger2014); and Gyrodactylus lilianae Razzolini, Murari, Baldisserotto & Boeger, Reference Razzolini, Levay-Maurari, Baldisserotto and Boeger2019 from R. quelen (Razzolini et al., Reference Razzolini, Levay-Maurari, Baldisserotto and Boeger2019). Finally, Gyrodactylus sp. have been recorded from both R. guatemalensis and R. laticauda in Mexico (Salgado-Maldonado et al., Reference Salgado-Maldonado, Aguilar-Aguilar, Cabañas-Carranza, Soto-Galera and Mendoza-Palmero2005, Reference Salgado-Maldonado, Caspeta-Mandujano, Moravec, Soto-Galera, Rodiles-Hernández, Cabañas-Carranza and Montoya-Mendoza2011; Mendoza-Garfias et al., Reference Mendoza-Garfias, Garcia-Prieto and Pérez-Ponce de León2017).
In the present study, two new species of Gyrodactylus from R. guatemalensis and R. laticauda collected in Mexico are described using morphological and molecular analyses.
Materials and methods
Sample collection and preparation
Surveys were conducted between 2013 and 2018 to collect two species of heptapterids in southern Mexico: pale catfish or ‘juil’ Rhamdia guatemalensis (Günther, 1864); and ‘filespine chulín’ R. laticauda (Kner, 1858). Fish were collected by electrofishing from Río La Antigua, Apazapan, Veracruz, and Río Chiquito, Cuicatlán, Oaxaca, respectively. Fish were anaesthetized with an overdose of 2-phenoxyethanol (Sigma-Aldrich, St. Louis, Missouri) prior to their preservation in 95% ethanol. Monogenean parasites were removed using surgical needles and processed individually. Two lots of worms were processed following different techniques to visualize their morphology: (a) Grey & Wess was used to examine internal structures such as the male copulatory organ (MCO) and the pharynx – for this, whole worms were cleared and mounted in a drop of Grey & Wess solution, coverslipped and incubated overnight at 60°C (Vidal-Martínez et al., Reference Vidal-Martínez, Aguirre-Macedo, Scholz, González-Solís and Mendoza-Franco2001); and (b) proteinase-K based digestion was employed to better visualize haptoral hook morphology – for this, haptors were excised using a scalpel, and a partial proteolytic digestion was completed to remove tissue enclosing the haptoral armature (Rubio-Godoy et al., Reference Rubio-Godoy, Paladini, Freeman, García-Vásquez and Shinn2012). Digestion was arrested by the addition of a 50:50 formaldehyde–glycerine solution, and specimens were then coverslipped and sealed with nail varnish. For further molecular analyses, the bodies whose haptors had been excised were preserved in 95% ethanol and stored individually at −20°C in 1.5 ml Eppendorf tubes.
Morphological analysis
To study the digested haptoral hard parts, these were placed on a Leica DM750 compound microscope (magnification of 10 × 100 for the hamuli with oil immersion 100 objective lens for the marginal hooks, in phase contrast). Photographs were taken using imaging analysis software LAS V4.12 V4 in a Leica ICC50 HD camera, attached to a Leica DM 750 compound microscope (magnification of 10 × 100 with oil immersion lens). Using the ImageJ 1.46r software, attachment hook measurements were taken from images. A total of 29 point-to-point measurements detailed by García-Vásquez et al. (Reference García-Vásquez, Razo-Mendivil and Rubio-Godoy2015) were obtained from each specimen (see table 1). All measurements are given in micrometres, showing average ± standard deviation, minima and maxima in parentheses. Micrographs of the following Gyrodactylus specimens (pers. comm. Walter A. Boeger 2022) were used to compare the marginal hook morphology of previously described species with those of the current study: G. anisopharynx; G. bueni; G. corydori; G. lilianae; G. major; G. samirae; G. scleromystaci; and G. superbus.
Table 1. Morphological measurements of the new Gyrodactylus species collected from Cuicatlán, Oaxaca and Apazapan, Veracruz, Mexico infecting ‘chulín’, and Rhamdia laticauda ‘juil’ Rhamdia guatemalensis, respectively.

HTL, hamulus total length; HA, hamulus aperture; HPSW, hamulus point shaft width; HPL, hamulus point length; HDSW, hamulus distal shaft width; HSL, hamulus shaft length; HICL, hamulus inner curve length; HAA°, hamulus aperture angle; HPCA°, hamulus point curve angle; IHAA°, inner hamulus aperture angle; HRL, hamulus root length; VBL, ventral bar length; VBW, ventral bar width; VBPML, ventral bar process to mid length; VBML, ventral bar median length; VBPL, ventral bar process length; VBMemL, ventral bar membrane length, DBL, dorsal bar length; DBW, dorsal bar width; DBAPTL, dorsal bar attachment point length; MHTL, marginal hook total length; MHSL, marginal hook shaft length; MHSiL, marginal hook sickle length; MHSiPW, marginal hook sickle point width; MHToeL, marginal hook toe length; MHSiDW, marginal hook sickle distal width; MHA, marginal hook aperture; MHAA, marginal hook aperture angle; MHI/AH, marginal hook instep/arch height; and MHFL, marginal hook filament loop.
Molecular analysis
DNA extraction, amplification and sequencing
Total genomic DNA was isolated from eight gyrodactylids fixed in 96% ethanol. Genomic DNA of each individual was extracted (following two protocols) using the DNeasy® Blood and Tissue Kit (QIAGEN, Valencia, California) according to the manufacturer's protocol (for specimens collected from Veracruz) and DNAzol Molecular Research Center, INC. (for specimens collected from Oaxaca). Individual worms were digested overnight at 56°C in a solution containing 10 μl of 100 mm Tris-hydrochloride (pH = 7.6), 10 μl of 200 mm sodium chloride, 20 μl of 0.5 M ethylenediaminetetraacetic acid (pH = 8.0), 10 μl of 10% Sarkosyl, 1.4 μl of 20 mg/μl proteinase K and 48.6 μl of sterile distilled water. Following digestion, DNA was isolated from the supernatant using DNAzol reagent (Molecular Research Center, Cincinnati, Ohio, USA) according to the manufacturer's instructions. Three fragments of DNA were amplified: the mitochondrial cytochrome oxidase subunit II gene (COII) using the primer COII F1 (5′-TACATAYCGCCCGTCAATYT-3′) and COII R1 (5′-TCARTAYCACTGDCGDCCYA-3′) (Xavier et al., Reference Xavier, Faria, Paladini, Van Oosterhout, Johnson and Cable2015); the ribosomal region spanning the 3′ end of the 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2, and the 5′ end of the 28S rRNA gene (ITS rDNA) using the primers BD1 (5′-GTCGTAACAAGGTTTCCGTA-3′) and BD2 (5′-ATCTAGACCGGACTAGGCTGTG-3′) (Bowles & McManus, Reference Bowles and McManus1993; Bowles et al., Reference Bowles, Blair and McManus1995); and the D1 + D2 region of the 28S rDNA using the primers C1 (5′-ACCCGCTGAATTTAAGCAT-3′) and D2 (5′-TGGTCCGTGTTTCAAGAC-3′) (Wu et al., Reference Wu, Chilton, Zhu, Xie and Li2005; Mandeng et al., Reference Mandeng, Bilong, Pariselle, Vanhove, Nyom and Agnèse2015). Polymerase chain reaction (PCR) amplifications were carried out in 25 μl reactions, containing 1 μl of each primer (10 μM), 2.5 μl of 10× buffer (Promega, Madison, WI, USA), 1.5 μl of MgCl2 (25 mm), 0.5 μl of dNTP mixture (10 mm), 0.125 μl of Platinum Taq DNA polymerase (5 U/μl) (Invitrogen Corporation, São Paulo, Brazil) and 2 μl of diluted template DNA. The following amplification profiles for COII/ITS rDNA/28S rDNA were used, respectively: denaturation at 95/94/94°C for 1.5/1/2 min, 40/35/40 cycles of 95/94/93°C for 0.5/1/0.5 min; annealing at 50/50/56°C for 1.5/1/0.5 min; extension at 72°C for 1.5/1/1 min; and a final extension of 72/75/72°C for 7/10/10 min. Unincorporated nucleotides and primers of each PCR amplicon were removed using ExoSap-IT (USB Corporation, Ohio). PCR amplicons for COII, ITS rDNA and 28S rDNA were cycle sequenced from both strands using the PCR primers. Sequencing reactions were performed in a final volume of 10 μl, using 1 μl of sequencing buffer 2.5×, 0.8 μl of the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, California), 2.5 μl of purified amplicons, 1 μl of primer (3 pmol) used in the amplification, and 4.7 μl of sterile distilled water. Sequencing products were purified by filtration with Sephadex™ G50 (Sigma-Aldrich, St. Louis, MO) and analysed on an ABI PRISM 310 genetic analyser (Applied Biosystems). Contigs were assembled and base-calling differences resolved using Geneious 8.1.8 (https://www.geneious.com). Sequences were deposited in the GenBank data set.
Alignment and phylogenetic analyses
Sequences of COII, ITS rDNA obtained in this study were aligned with sequences of other Gyrodactylus spp. available in the GenBank database (see table 2). Very few 28S rDNA sequences are available for Gyrodactylus, so no phylogenetic analyses are presented for the sequences generated in this study. All the sequences generated for ITS rDNA and COII were run in the Basic Local Alignment Search Tool, and species with the highest percentage of identity were included in the alignment, for example, G. lilianae with the maximum score. Data of the two genes were aligned separately using the software ClustalW (Thompson et al., Reference Thompson, Higgins and Gibson1994) with default parameters implemented in MEGA version 7.0 (Kumar et al., Reference Kumar, Stecher and Tamura2016). COII sequences were adjusted manually and checked for correct amino acid translation. Nucleotide substitution models were selected for each molecular marker using jModeltest 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) and applying Akaike's information criterion for each dataset (COII = GTR + I + G; ITS rDNA = TIM2 + I + G). Individual data sets were analysed using Bayesian inference (BI). Trees under BI analyses were inferred with MrBayes v3.2 (Ronquist et al., Reference Ronquist, Teslenko and Van Der Mark2012), where Metropolis-coupled Markov chain Monte Carlo (MC3) simulations were run for 10 million generations and sampled every 1000 generations, and the first 2500 samples were discarded as burn–in (25%). To search for the convergence of different parameters, to estimate the approximate number of generations at which log likelihood values stabilized, to recognize the effective sample size (EES > 200) for each parameter, and to estimate the magnitude of model parameters in individual and combined runs, the outputs were examined with Tracer v1.4 (Rambaut & Drummond, Reference Rambaut and Drummond2007). The initial 25% of MC3 was verified to include all the generations before stationarity was achieved. We obtained posterior probabilities of clades from the 50% majority rule consensus of sampled trees after we excluded the initial 25% as burn-in for MrBayes. The genetic divergence among species of Gyrodactylus was estimated using uncorrected ‘p’ distances with MEGA 7.0 (Kumar et al., Reference Kumar, Stecher and Tamura2016).
Table 2. Newly generated sequences and sequences available on GenBank for Gyrodactylus spp.

Results
Two new species of Gyrodactylus were identified from two States in Mexico: Gyrodactylus chulini n. sp. was found infecting R. laticauda in Río Chiquito, Cuicatlán, Oaxaca; and Gyrodactylus juili n. sp. was found on R. guatemalensis in Río La Antigua, Apazapan, Veracruz. These are the first two species of Gyrodactylus described from Rhamdia spp. in Mexico. Morphological descriptions of each new species are presented in alphabetical order and their measurements are shown in table 1 (average ± standard deviation, and minima and maxima in parentheses).
Nomenclatural acts: This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the International Code of Zoological Nomenclature. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix ‘http:/zoobank.org/’. The LSID for this publication is: urn:lsid:zoobank.org:pub:86AA1B2C-B9A3-4DD7-8B2C-E6CD4DC0B570.
Gyrodactylus chulini n. sp. (figs 1a and 2, table 1)
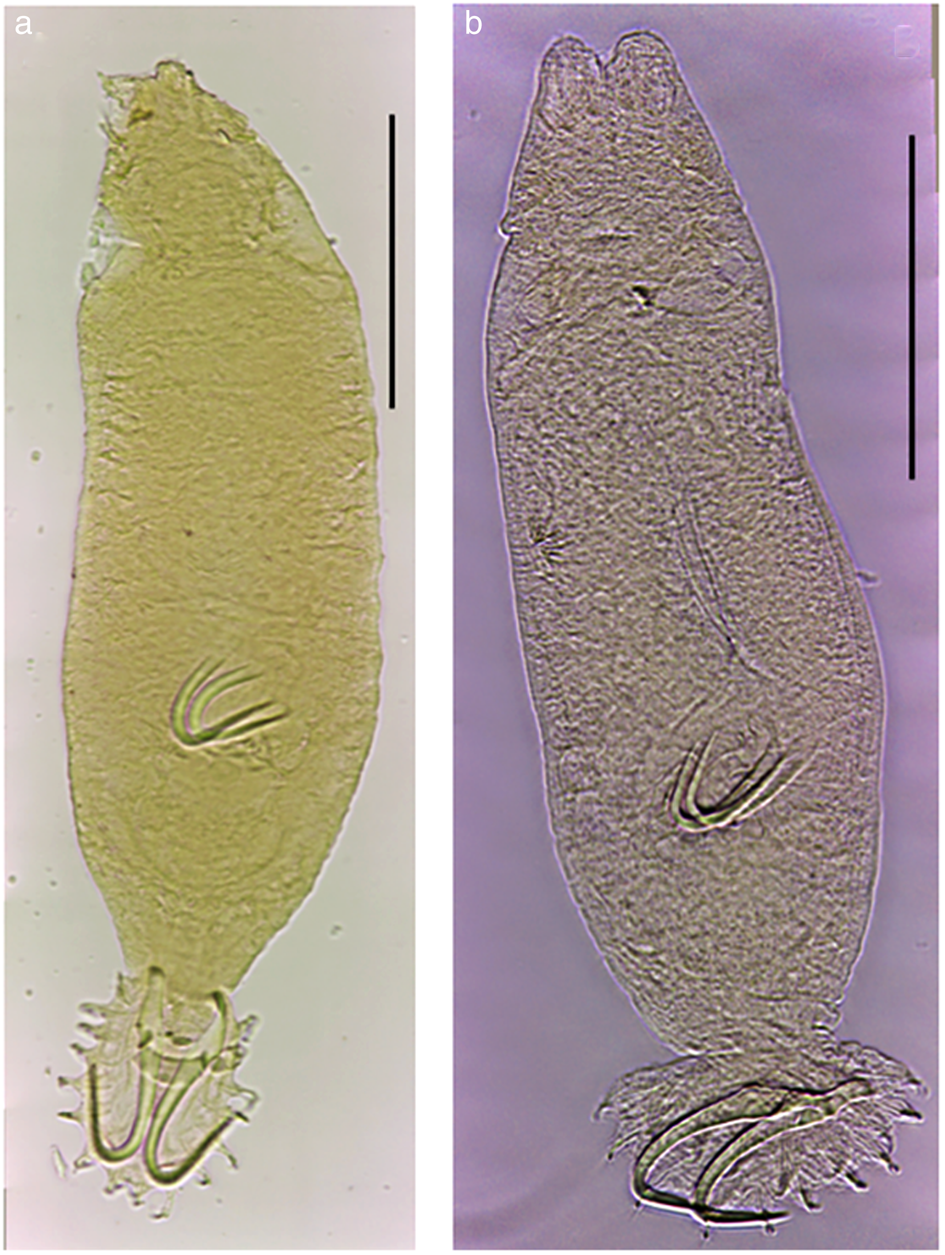
Fig. 1. Light micrographs of Gyrodactylus species described from Rhamdia guatemalensis and Rhamdia laticauda in Mexico: (a) Gyrodactylus chulini n. sp. at a glance; (b) Gyrodactylus juili n. sp. at a glance. Scale bars: 100 μm.

Fig. 2. Light micrographs and drawings of Gyrodactylus chulini n. sp. described from ‘chulín’, Rhamdia laticauda from Río Chiquito, Oaxaca, Mexico: (a) hamuli complex; (b, k) ventral bar; (c, j) dorsal bar; (d, l) marginal hook at a glance; (e, f, m) marginal hook sickle; (g, h) micrographs and drawing of male copulatory organ showing the principal hook (asterisk) and the spines (1–7 in clockwise direction); (i) hamulus. Scales bars: (a) 20 μm; (b, d, g–i, k, l) 10 μm; (c, e, f, j, m) 5 μm.
LISD urn:lsid:zoobank.org:act:0EEEDD35-2EB5-4C20-A2BF-3FD7DE5ADE82
Type host: Rhamdia laticauda (Kner, 1858)
Site of infection: Fins.
Type locality: Río Chiquito, Cuicatlán, Oaxaca, Mexico (17°48′41.7′′ N, 96°57′38.7′′ W)
Type material: Holotype (accession nos. CNHE11703), two paratypes (accession nos. CNHE11704, CNHE11705) and three vouchers (accession nos. CNHE11706, CNHE11707, CNHE11708) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City.
DNA reference sequences: Sequences obtained from five individuals are deposited in GenBank (ITS accession nos. OP023860-OP023864; COII accession nos. OP009445-47; and 28S accession nos. OP023895, OP023896).
Etymology: This species is named after its type host, R. laticauda, commonly known in Central America as ‘chulín’.
Description (average (range), in micrometers): Morphological description based on 18 specimens, ten whole worms cleared in Grey & Wess solution, and eight haptors proteolytically digested. Body 411 (352–463) long; 108 (89–133) wide at uterus level. Pharynx (n = 8) 28 (22–33) long × 29 (24–32) wide (fig. 1a). Haptor 68 (63–85) length × 67 (48–82) width. Hamuli 53 (51–66) long, 3 (3–5) wide; almost the same thickness throughout the hook, except at the dorsal bar attachment point, which becomes wider; shaft 32 (30–40) long; slim point 28 (25–36) long, comprising more than half of the shaft length, slightly curved outward at the point proper; proximal shaft width 7 (6–8) wide; slightly curved shaft; hamulus aperture distance 15 (13–18) long; hamulus aperture angle 29° (24°–33°) long; hamulus root 20.2 (17–26) long (fig. 2b, i). Ventral bar 19 (16–24) wide with pointed edges, 20 (18–23) long; rounded, short ventral bar processes 2 (1–3) long; trapezoidal ventral bar median portion 6 (6–8) long, short triangular ventral bar membrane 11 (9–13) long, acute edge slightly pointed (fig. 2b, k). Dorsal bar straight 15 (10–21) wide, slightly elongated in the middle 2 (1–2) long, oval attachment points 7 (6–8) long (fig. 2c, j). Marginal hook 20 (17–25) long; shaft 14 (10–17) long (fig. 2d, l). Marginal hook sickle 6 (6–7) long, tilted forward, when reaching the point is barely curved (fig. 2e, f, m). Marginal hook distal width 3 (2–4), long and slim. Blunt toe 2 (2–3) long, sickle base wavy. Heel pointed. Marginal hook shaft entirely curved and angled towards the toe, short point ending well forward toe level. Short and curved bridge. Marginal hook aperture 6 (5–7) 1ong. Marginal hook instep height almost straight 0.3 (0.2–0.4) deep (fig. 2d–f). Filament loop 11 (10–14) long, almost the same length of shaft. MCO visible and completely developed in three specimens. Globular 17 (15–18) long × 14 (12–15) wide, consisting in one large principal hook 6 (5–7) long, seven spines distributed next to each other forming a circle, points facing to the principal hook point, as follows (clockwise direction): (a) slender and long spine 1 (0.7–2) long, which is closer to the principal hook point, with a narrow base; (b) big spine 4 (3–4) long, with a wide base and long slim point; (c) big spine 3.5 (3.8–4.6) long, with a wider base than the second spine, point being the thickest of all spines; (d) resembling the second spine, but with shorter point and slightly wider base, 4 (3–5) long; (e) wider base and short point, being the biggest of all spines 4 (4–5) long; (f) almost identical to the second spine, but thicker point 4 (2–4) long; and (g) small and narrow base with a long point 2 (1–3) long (fig. 2g, h).
Remarks:
The marginal hook morphology of G. chulini n. sp. roughly resembles those of G. juili n. sp. (see below), G. lilianae and G. superbus (fig. 3). However, these species can be discriminated. Although similar, in G. juili n. sp. the sickle base has a more compact, pointed toe; and the sickle shaft is more upright than in G. chulini n. sp. Both G. lilianae and G. superbus are generally similar to G. chulini n. sp., but the sickle base is slightly stouter and rounded at the toe, in the new species; and the sickle shaft allows to differentiate the species, as this structure is clearly more robust and tilted forward in G. lilianae (fig. 3r), and at a more upright angle in G. superbus (fig. 3z).
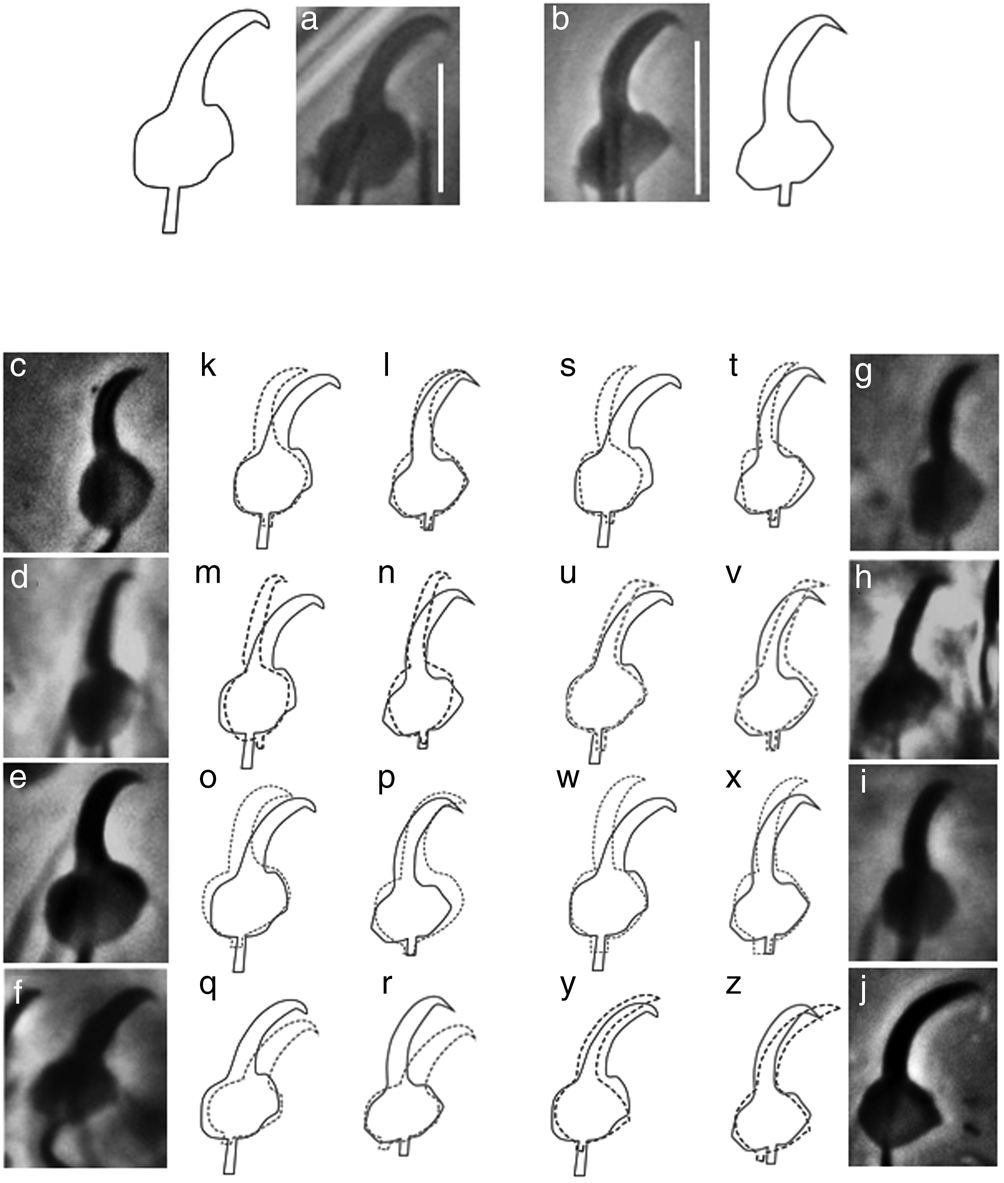
Fig. 3. Micrographs and drawings of the marginal hooks of the new Gyrodactylus species collected in Mexico from Rhamdia spp., compared with those of gyrodactylids described from Silurid hosts in Brazil (micrographs kindly provided by Dr Walter A. Boeger, Universidade Federal do Paraná): (a) marginal hook sickle of Gyrodactylus chulini n. sp. from Rhamdia laticauda; (b) marginal hook sickle of Gyrodactylus juili n. sp. from Rhamdia guatemalensis; (c) marginal hook sickle of Gyrodactylus anisopharynx from Corydoras paleatus; (d) marginal hook sickle of Gyrodactylus bueni from Scleromystax macropterus; (e) marginal hook sickle of. Gyrodactylus corydori from Corydoras ehrhardt; (f) marginal hook sickle of Gyrodactylus lilianae from Rhamdia quelen; (g) marginal hook sickle of Gyrodactylus major from S. macropterus; (h) marginal hook sickle of Gyrodactylus samirae from C. ehrhardti; (i) marginal hook sickle of Gyrodactylus scleromystaci from Scleromystax barbatus; (j) marginal hook sickle of Gyrodactylus superbus from C. ehrhardti; (k) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. anisopharynx (dotted line); (l) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. anisopharynx (dotted line); (m) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. bueni (dotted line); (n) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. bueni (dotted line); (o) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. corydori (dotted line); (p) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. corydori (dotted line); (q) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. lilianae (dotted line); (r) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. lilianae (dotted line); (s) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. major (dotted line); (t) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. major (dotted line); (u) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. samirae (dotted line); (v) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. samirae (dotted line); (w) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. scleromystaci (dotted line); (x) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. scleromystaci (dotted line); (y) overlay of the marginal hook sickle of G. chulini n. sp. (solid line) with that of G. superbus (dotted line); and (z) overlay of the marginal hook sickle of G. juili n. sp. (solid line) with that of G. superbus (dotted line). Scale bars:5 μm.
Gyrodactylus juili n. sp. (figs 1b and 4, table 1)

Fig. 4. Micrographs of Gyrodactylus juili n. sp. described from ‘juil’, Rhamdia guatemalensis from Río La Antigua, Veracruz, Mexico: (a) hamuli complex; (b, i) ventral bar; (c, j) dorsal bar; (d, k) marginal hook at a glance; (e, l) marginal hook sickle; (f, g) male copulatory organ showing the principal hook (asterisk) and the spines (1–7 in clockwise order); and (h) hamulus. Scale bars: (a, h) 20 μm; (b–d, f, g, i–k) 10 μm; (e, l) 5 μm;
LSID urn:lsid:zoobank.org:act:8764053B-4862-45A7-B9D6-03A75183C9D1
Type host: Rhamdia guatemalensis (Günther, 1864).
Site of infection: Fins.
Type locality: Río La Antigua, Apazapan, Veracruz, Mexico (19°19′31.49′′ N, 96°43′31.57′′ W)
Type material: Holotype (accession no. CNHE10254), three paratypes (accession nos. CNHE10255, CNHE11701, CCNHE11702) and two vouchers (accession nos. CNHE10256, CNHE11700) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City.
DNA reference sequences: Sequences obtained from three individuals are deposited in GenBank (accession nos. OP023865-OP023867, for ITS; COII accession nos. OP009448, OP009449; and 28S accession nos. OP023897, OP023898).
Etymology: This species is named after its type host, Rhamdia guatemalensis, commonly known in Mexico as ‘juil’. The word probably comes from the Aztec ‘xohuilin’, meaning ‘spotted, trout-like fish which lays many eggs’.
Description (average (range), in micrometers): Morphological description based on 17 specimens, 11 whole worms cleared in Grey & Wess solution, and six haptors proteolytically digested. Body (n = 6) Body 454 (271–554) long; 106 (73–157) wide at uterus level (fig. 1b). Pharynx (n = 11) 26 (20–36) long × 27 (20–36) width (fig. 1b). Haptor (n = 6) 66 (61–75) length × 57 (37–72) width. Hamuli 50 (44–55) long, 3 (3–4) wide, being thicker in the ventral bar attachment point; shaft 31 (27–35) long; slim point 25 (21–27) long, comprising almost the same shaft length; proximal shaft width 6 (6–7) wide; slightly curved shaft; hamulus aperture distance 17 (14–20) long; hamulus aperture angle 35° (30°–45°) long and wide; hamulus root 19 (17–21) long, ends pointed and facing to hamulus point (fig. 4a). Ventral bar 17 (15–19) wide, 19 (17–22) long; triangular ventral bar processes 2 (1–3) long, facing upwards to the hamuli roots; ventral bar median portion 5 (4–7) long, slightly curved on the edges, triangle-shaped middle notch, ventral bar membrane 12 (9–15) long, V-shaped with a stretch mark in the middle (fig. 4b). Dorsal bar 15 (11–24) wide, 2 (1–2) long and straight, oval attachment points 6 (6–7) long, (fig. 4c). Marginal hook 17 (14–19) long; shaft 12 (9–13) long, barely curved at the end (fig. 4d). Marginal hook sickle 6 (5–6) long, upright shaft bending distally ending in short point facing sickle base, just after the toe level. Marginal hook distal width 3.3 (2.6–3.8) long and slim. Toe 2 (1–2) long. Marginal hook aperture 5 (4–6) 1ong. Heel ovate. Marginal hook bridge short and pointed. Marginal hook instep height barely noticeable 0.4 (0.2–0.6). Filament loop 10 (9–11) long, almost same length of the shaft (fig. 4d, e). MCO (n = 3) globular to ovoid 12 (10–14) long × 12 (10–13) wide. Consisting of one large principal hook 5 (5–6) long (fig. 2f, g), two tiny and thin spines, five robust triangular spines almost same length, all distributed around the principal hook (clockwise direction): two tiny spines (1: 1 and 7: 1), located to each side of the base point of the principal hook (only visible on one specimen) (fig. 3g); two slim spines 2: 4 (3–4) long, 6: 3 (3–4) long, positioned to each side of the principal hook, points facing the centre of MCO; three thick base spines: 3: 5 (4–5) long; 4: 4 (4–5); and 5: 4.3 (4.2–4.4) long, gradually narrowing into an acute point when reaching the centre (fig. 4f, g).
Remarks:
In G. juili n. sp., the marginal hook morphology closely resembles that of gyrodactylids infecting catfishes (Callichthydae) in southern Brazil: G. anisopharynx; G. bueni and G. corydori (fig. 3). Nonetheless, these species can be discriminated. Compared to G. anisopharynx, G. juili n. sp. has a more angular marginal hook base, and the sickle shaft although upright, begins lower and is comparatively tilted forward (fig. 3k). The marginal hook base of G. juili n. sp. is clearly more angular than in G. bueni, and the sickle shafts markedly differ in width and their distal end (fig. 3m). The marginal hook of G. corydori is the most similar, but this structure is overall more rounded and robust in this species, enabling discrimination from G. juili n. sp. (fig. 3o).
Molecular characterization and genetic divergence
The ITS rDNA sequences were similarly long in the two new species: G. juili n. sp. (1341 bp) and G. chulini n. sp. (1391 base pairs (bp)) showed an ITS1 of 536 and 588 bp, respectively, whereas ITS2 was 649 and 646 bp long, respectively. The length of the 5.8S rDNA gene in G. juili n. sp. was of 156 bp while in G. chulini n. sp. it was of 157 bp. With respect to COII, the partial length of the sequences was of 262 bp. The partial length of the 28S rDNA was of 752 bp. The genetic divergence estimated with the three molecular markers for G. chulini n. sp. and G. juili n. sp. ranged from 14.59 to 14.58% for ITS rDNA, from 10.92 to 11.55% for COII, and from 3 to 3.5% for 28S rDNA; and between G. lilianae and the two new species, ITS rDNA and COII divergence ranged from 23.28 to 30.65% and from 17.20 to 19.78%, respectively. Intraspecific genetic divergence in G. juili n. sp. and G. chulini n. sp. was of 1.8% and 0% for ITS rDNA, 3.3% and 0% for COII, respectively, and null for 28S rDNA (table 3).
Table 3. Pairwise differences (p-distances) of internal transcribed spacers (ITS1-5.8S–ITS2) rDNA (above the diagonal) and cytochrome oxidase II (COII) mtDNA (below the diagonal) among new species of Gyrodactylus.

Genetic divergence values are expressed as a percentage. Values in the diagonal (grey fill) represent the intraspecific genetic divergence (COII/ITS).
Phylogenetic analyses
We obtained sequence data from three loci (ITS rDNA, COII and 28S rDNA) for G. juili n. sp. (n = 3) from Veracruz and G. chulini n. sp. (n = 5) from Oaxaca, Mexico. Phylogenetic analyses of the sequences of the ITS rDNA and COII gene fragments support the erection of two new gyrodactylid species, each forming independent clades with high posterior probability support (see fig. 5). Gyrodactylus chulini n. sp. and G. juili n. sp. were found to be reciprocally monophyletic in all analyses, and to be related as sister species; both with strong nodal support (≥0.96). These two gyrodactylids are recovered as sister species to G. lilianae (fig. 5).

Fig. 5. Phylogenetic hypothesis inferred from internal transcribed spacer 1, 5.8S rRNA, internal transcribed spacer 2 and cytochrome oxidase II for two new species of Gyrodactylus infecting native fishes from Oaxaca and Veracruz, Mexico. Numbers near internal nodes show the posterior probability clade frequencies. Scale bars indicate the number of substitutions per site. For new species described in the present study, the clades are dashed in green (Gyrodactylus juili n. sp.), and red (Gyrodactylus chulini n. sp.).
Discussion
The Amazon basin is one of the most diverse biomes for fish and parasite species in the world (Luque & Poulin, Reference Luque and Poulin2007; Nelson et al., Reference Nelson, Grande and Wilson2016), and a place where evolutionary novelties/oddities occurred in the Monogenea. For instance, continental waters in Brazil are the only place where oviparous gyrodactylids have been recorded, with 23 species described from armoured (Loricariidae) and long-whiskered (Pimelodidae) catfishes; and it has recently been suggested that Oogyrodactylidae diversified following the Gondwana breakup and the separation of Africa and South America (Boeger et al., Reference Boeger, Kritsky, Patella and Bueno-Silva2020). Also, the speciose family Dactylogyridae, which primarily includes gill-infecting species and has undergone a marked evolutionary radiation in Africa (e.g. Tanganyika) (Vanhove et al., Reference Vanhove, Pariselle and Van Steenberge2015), occasionally evolved in the Neotropics to an endoparasitic habit more commonly associated to Polystomatids, as a handful of monogeneans inhabit the urinary bladder and ureters of their fish hosts – examples include: Kristkyia moraveci Kohn, Reference Kohn1990, infecting R. quelen in Brazil (Kohn, Reference Kohn1990); Kristkyia annakohnae Boeger, Tanaka & Pavanelli, Reference Boeger, Tanaka and Pavanelli2001 recorded from piranhas Serrasalmus marginatus and Serrasalmus spilopleura in Brazil (Boeger et al., Reference Boeger, Tanaka and Pavanelli2001); Kristkyia boegeri Takemoto, Lizama & Pavanelli, Reference Takemoto, Lizama and Pavanelli2002 from streaked prochilod, Prochilodus lineatus in Brazil (Takemoto et al., Reference Takemoto, Lizama and Pavanelli2002); Acolpenteron australe Viozzi & Brugni, Reference Viozzi and Brugni2003 from Creole perch, Percichthys trucha in Patagonia, Argentina (Viozzi & Brugni, Reference Viozzi and Brugni2003); or Philureter trigoniopsis Viozzi & Gutiérrez, Reference Viozzi and Gutiérrez2001 infecting common galaxias, Galaxias maculatus, also in Patagonia, Argentina (Viozzi & Gutiérrez, Reference Viozzi and Gutiérrez2001) – although this is not unique to the Neotropics, as a few species with this habit are known from Cameroon, Russia and the United States. Considering these examples, it is generally interesting to further characterize the species-rich and relatively understudied Monogenea infecting Neotropical fishes. Furthermore, scrutinizing parasites of fishes in the Americas also sheds light on the validity of the ‘Great American Biotic Interchange’ as a bidirectional exchange of Neotropical and Nearctic faunae, a concept that has recently been challenged regarding freshwater fishes and their associated parasites, as many more fish host–parasite associations seem to have travelled northward from the Neotropics than in the other direction, southwards from the Nearctic (Choudhury et al., Reference Choudhury, García-Varela and Pérez-Ponce de León2017).
The two new species of Gyrodactylus we describe in this work contribute to these two aspects: we characterize the parasites of a representative fish family originating and widely distributed in the Neotropics; and support the notion that the parasites were co-distributed with their fish hosts as they migrated through Central America following the emergence of the Isthmus of Panama.
Both G. chulini n. sp. and G. juili n. sp. are morphologically similar to gyrodactylids that infect siluriform catfishes in South America, when contrasting the taxonomically-informative marginal hooks of the new parasite species found on Rhamdia spp. in Mexico: G. chulini n. sp. resembles G. lilianae and G. superbus; while G. juili n. sp. is akin to G. anisopharynx, G. bueni and G. corydori. Similarly, the molecular analysis of ITS rDNA and COII genes shows that the Gyrodactylus species collected in the present study from Rhamdia in Mexico are a sister group to G. lilianae from South America. A phylogenetic hypothesis based on both ITS rDNA and COII sequences suggests that G. chulini n. sp. and G. juili n. sp. are sister species and unequivocally recognized as two new species; and the ITS rDNA and COII divergence among the two new taxa is consistent with species-level divergence for other species pairs within Gyrodactylus (Razzolini et al., Reference Razzolini, Levay-Maurari, Baldisserotto and Boeger2019). We also show that all the gyrodactylids infecting Heptapteridae are related to other Gyrodactylus spp. found on Siluriformes from South America (fig. 5). This suggests that the two new species of Gyrodactylus infecting Rhamdia in Mexico have South American origins; and that, as suggested by Choudhury et al. (Reference Choudhury, García-Varela and Pérez-Ponce de León2017), they seem to have co-migrated through Central America with their catfish hosts.
Northward co-migration of Neotropical parasites with Rhamdia hosts could have also occurred with other monogeneans, as members of dactylogyridean genera such as Amelloblastella Kritsky, Mendoza-Franco & Scholz, 2000, Aphanoblastella Kritsky, Mendoza-Franco & Scholz, 2000, Pavanelliella Kritsky & Boeger, 1988, and the gyrodactylid Scleroductus Java & Cone, 1989, have been recorded infecting these catfishes throughout southern Mexico, Central and South America, and Trinidad in the Caribbean (Kohn et al., Reference Kohn, Cohen and Salgado-Maldonado2006; Salgado-Maldonado, Reference Salgado-Maldonado2006, Reference Salgado-Maldonado2008; Mendoza-Garfias et al., Reference Mendoza-Garfias, Garcia-Prieto and Pérez-Ponce de León2017). To corroborate this hypothesis, extensive surveys are needed to close the parasitological knowledge gap still existing in Central America (e.g. Santacruz et al., Reference Santacruz, Barluenga and Pérez-Ponce de León2022); this would also contribute to address the overall knowledge shortfalls identified for biodiversity data (Hortal et al., Reference Hortal, de Bello, Diniz-Filho, Lewinsohn, Lobo and Ladle2015).
Acknowledgements
We thank Dr Walter A. Boeger, Universidade Federal do Paraná, who kindly provided microphotographs of Gyrodactylus species described from siluriform hosts in Brazil, which we examined and used for comparisons. We also thank Dr Diego Santiago for the loan of microscope to take microphotographs and Luis García Prieto from the Colección Nacional de Helmintos, Universidad Nacional Autónoma de México for the loan of Gyrodactylus type material. Field sampling was performed according to Mexican codes of practice and received approval of the Mexican Ministry of the Environment and Natural Resources under scientific collection permit SGPA/DGVS/02967/14.
Financial support
This research was supported by funds awarded to MRG by Consejo Nacional de Ciencia y Tecnología (CONACyT, grant CB 168306) and INECOL institutional funds. AGV thanks CONACyT (Becario 20340, 2013-2015) and INECOL (2017-2022) for her postdoctoral fellowship. MCR acknowledges the support of the Programa de Doctorado en Ciencias, INECOL and CONACyT for a doctoral scholarship.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of wild animals.
Author contributions
MRG conceived and designed the study; AGV, CDPP, IGV, MCR and MRG carried out the field and laboratory work; AGV took the microphotographs and did the drawings; AGV, CDPP, IG, and MRG analysed the data; AGV, CDPP and MRG wrote the manuscript. All authors checked and agreed with the final manuscript.










