Summary
The use of energy has defined our civilization and governs our daily lives. Throughout the day and night modern humans consume enormous quantities of energy resources for the preparation of their food; transportation; lighting, heat, ventilation, and air-conditioning of buildings; entertainment; and a myriad other applications that define modern life. A gigantic global energy industry transports and inconspicuously transforms the energy resources to convenient forms (gasoline, diesel, electricity) that are vital to the functioning of the modern human society. This introductory chapter surveys the types of global primary energy sources, how they are transformed to useful energy forms and how they are used by the consumers. The chapter introduces succinctly the two laws of thermodynamics that govern the conversion of energy from one form to another; it explains the methodology of thermodynamics, which is essential for the understanding of energy conversion processes; and delineates the physical limitations on energy conversion. The thermodynamic cycles for the generation of power and refrigeration are extensively reviewed and the thermodynamic efficiencies of the cycles and energy conversion equipment (turbines, compressors, solar cells, etc.) are defined.
1.1 Energy – Whither Does It Come? Whence Does It Go?
The human civilization has been defined by the utilization of energy forms for space heating, lighting, and food preparation. When the prehistoric humans mastered the use of fire for domestic comfort and cooking in their caves, human civilization began. In the later stages of human civilization, the utilization of animal power (primarily power of horses and oxen) contributed to the development of the agricultural society; helped nourish an increasing human population; and continued with the development of the ancient and medieval social structures. Also, wind-propelled sailboats facilitated trade and brought communities in contact. In the last three centuries the invention of powerful and energy-voracious engines ushered the industrial revolution and the mechanized society. Today’s knowledge-based society, where citizens live in relatively great comfort; have more food and natural resources than any other society in the past; use fast modes of transportation to experience far-away lands and social events; and are continuously in contact with electronic devices, necessitates the use of enormous quantities of energy and the production of previously unmatched quantities of power.
Throughout the centuries, the human society has evolved by using the various forms of energy in increasingly larger quantities. Today, the utilization of vast amounts of energy – in thermal, chemical, mechanical, and electrical form – is absolutely necessary for the functioning of the modern society, the prosperity of the nations, and the survival of our civilization.
While the word energy is used as a colloquial term for several related concepts – including work, power, heat, and fuel – the human society primarily has the need for mechanical work, and mechanical or electrical power, which are produced by engines that utilize our energy resources. Airplanes and automobiles use liquid hydrocarbon fuels to produce motive power. Tractors and combines are fitted with engines that use hydrocarbons for the production of agricultural goods and food. Households use electricity for lighting, domestic comfort, and entertainment. Computers and wireless networks use electricity to function. The needed power is generated in power plants that convert primary resources – coal, natural gas, nuclear, petroleum, solar, wind, geothermal, and hydroelectric – to electricity.
Because energy is such a vital element of our lives, elaborate networks for the transportation and supply of energy forms to consumers have been developed in the last two centuries. Electricity is fed into cities and households by the transmission lines of the electric grid at very high voltages. Natural gas is transported by complex systems of pipelines, which often transcend national boundaries. Tanker ships crisscross the oceans daily to transport crude oil to refineries, which then supply the consumers with gasoline and diesel fuel via an elaborate system of pipelines, train cars, and trucks. Trainloads of coal are transported daily from coal mines to the electric power plants. In today’s world energy enters all aspects of human life, economics, and politics. Actually, the need for increasingly larger quantities of energy resources has defined the lives and geopolitics of humans in the last two centuries.
The global population consumed 392.9 HJ (1 HJ = 1018 J) of energy resources in 2015 in several forms [1], which are shown in Table 1.1. It is observed that petroleum products and electricity account for approximately 60% of the energy forms used globally. Petroleum products are primarily used for transportation. Electricity is used in several human activities including lighting, air-conditioning, and entertainment. Most of the other energy forms are utilized by the various industries.
| Amount (HJ) | Percent (%) | |
|---|---|---|
| Coal | 43.6 | 11.1 |
| Petroleum products | 161.1 | 41.0 |
| Natural gas | 58.5 | 14.9 |
| Biofuels and waste | 44.0 | 11.2 |
| Electricity | 72.7 | 18.5 |
| Other forms | 13.0 | 3.3 |
| Total | 392.9 | 100 |
The energy forms we use daily (and are depicted in Table 1.1) are not naturally occurring. They are products of the energy resources, often called primary energy sources. Some of these resources are naturally occurring minerals that are mined in a few locations (e.g. crude oil, natural gas, uranium ore, and coal), while other resources are more widely distributed and free to be used (e.g. solar energy, wind power, and hydraulic power from rivers). The mineral resources are traded daily in the international markets; they are exported and imported in all the countries of the world; and represent significant entries in the energy and financial balance sheets of nations. Table 1.2 depicts the primary energy sources that have been used globally in the year 2015 to satisfy the demand for energy [1].
| Amount (HJ) | Percent (%) | |
|---|---|---|
| Coal and coal products | 160.6 | 28.1 |
| Crude oil | 181.1 | 31.7 |
| Natural gas | 123.4 | 21.6 |
| Nuclear | 28.0 | 4.9 |
| Hydraulic | 14.3 | 2.5 |
| Wood, biomass and wastes | 55.4 | 9.7 |
| Other Renewables | 8.6 | 1.5 |
| Total | 571.4 | 100 |
It is observed in Tables 1.1 and 1.2 that 571.4 HJ of energy resources have been used to supply the human society with the needed 392.9 HJ. The difference of 178.5 HJ or 31.1% of the total resources represents the dissipation when one form of energy is converted to another. For example, approximately 60% of the chemical energy in coal is dissipated when this mineral is converted to electricity. Additional energy dissipation occurs during the consumption stage when energy forms are utilized in our engines [Reference Michaelides2]. For example, the internal combustion (IC) engines in automobiles dissipate (as heat and sound) approximately 75% of the energy in the gasoline and diesel fuels (both are petroleum products) when they convert the chemical energy of the fuel to motive power.
The subject of this book and the concept of exergy (the exergy method) comprise the energy dissipation when primary energy forms are converted to secondary and tertiary forms to fulfill the desired objectives of the human society. Chapter 1 of the book includes a summary of the fundamental thermodynamic concepts and the two principal laws of thermodynamics that govern all energy transformations. Chapter 2 introduces the exergy concept and derives quantitative measures for the exergy of the commonly used primary energy sources. Chapter 3 elucidates the application of exergy in several types of energy systems that produce electric and motive power and offers quantitative tools and methods for the minimization of energy resource consumption. Chapter 4 offers the quantitative tools to minimize the energy resource consumption in what is colloquially called energy conservation. Chapter 5 explains the exergy dissipation processes in biological systems – including humans – that produce as well as consume energy. Chapter 6 focuses on the applications of the exergy methodology in the minimization of waste, a cleaner environment, and a sustainable energy future. Finally, Chapter 7 deals with optimization methods for the minimization of exergy destruction, the economics of energy resource conservation and the uncertainty of the calculations.
1.2 Fundamental Concepts of Thermodynamics
Central to the theory of thermodynamics is the concept of the thermodynamic system, or simply the system, which is the part of the universe where our attention is focused. The system may or may not contain any material objects: A tank full of hydrogen gas is a system; a turbine where steam flows is a system; and a vacuum chamber is also a system. The system is enclosed by a boundary, which is permeable or impermeable to mass. Outside the boundary are the surroundings, which represent the part of the universe that is affected by changes in the defined thermodynamic system.
Thermodynamic systems are closed or open systems. Closed systems contain a fixed amount of molecules and mass. Open systems have one or more inlets and outlets, through which mass is allowed to flow. The quantity of mass inside open systems may be constant, but individual molecules enter and exit the systems. Work and heat cross the boundaries of both closed and open systems and are exchanged with the surroundings. In the case of closed systems, we are usually interested in the total work and heat, W and Q, or the specific work and heat, w and q, namely the work and heat per unit mass of the system. For open systems we typically perform calculations on the instantaneous rates of work and heat, ![]() (the power) and
(the power) and ![]() , as well as on the mass flow rates that enter and leave the systems. The vast majority of energy conversion machinery are open systems: pumps, boilers, turbines, compressors, nozzles, and jet engines are all open systems. At typical operating conditions these devices operate as open systems at steady state, with equal rates of masses flowing in and out.
, as well as on the mass flow rates that enter and leave the systems. The vast majority of energy conversion machinery are open systems: pumps, boilers, turbines, compressors, nozzles, and jet engines are all open systems. At typical operating conditions these devices operate as open systems at steady state, with equal rates of masses flowing in and out.
The properties of the thermodynamic system are measurable variables associated with the system. Temperature, pressure, volume, enthalpy, entropy, electrical conductivity, and viscosity are examples of thermodynamic properties. When the properties of a thermodynamic system do not change with time, the system is in thermodynamic equilibrium or simply at equilibrium. At equilibrium the properties may be measured, calculated via equations of state, or determined from thermodynamic tables. For homogeneous substances (substances that have stable and uniform composition), knowledge of two independent properties is sufficient to determine all the other properties. The equations of state are algebraic equations that give one property in terms of other (measurable) properties, as for example the ideal gas equation of state, Pv = RT. When a simple equation is not adequate for the accurate determination of properties, the properties are calculated by numerical computations and become available in thermodynamic tables or via computer algorithms, for example, the Steam Tables for the properties of water/steam, Refrigerant-134a Tables, and the REFPRO software that calculate the properties of tens of common materials.
The state of the system is another fundamental concept of thermodynamics, and is simply defined as the set of all the properties of the system.Footnote 1 When the properties change, the state of the system changes too, and the system undergoes a process. The thermodynamic process is central to energy conversion and takes place within a finite amount of time. When the timescale of the change of the system’s properties is much less than the characteristic time of the process, τsystem ≪ τprocess, the system responds fast to the external changes and is considered to be in internal thermodynamic equilibrium during the process. In this case the process is called reversible. In all other situations, the system is not in internal thermodynamic equilibrium during the process and the process is considered to be irreversible.
Mechanical work is a primitive concept in the discipline of mechanics and is associated with the concept of force: A force performs work when its point of application moves. The amount of work performed when the force moves its point of application by a path defined by the end states 1 and 2 is:
 (1.1)
(1.1)Work is a scalar quantity and depends on the path followed by the force between the end states 1 and 2.
Let us consider a system composed of a compressible substance enclosed in a cylinder fitted with a weightless piston. The force acting on the system is equal to the external force acting on the piston. When the system is in equilibrium the external force is equal to the product of the internal pressure and the area of the piston (Fext = PA). Under these conditions, any process that moves the piston from position 1 to position 2 is reversible and Eq. (1.1) yields an expression for the work performed in terms of the two properties of the enclosed compressible substance, the pressure, P, and the volume, V:
 (1.2)
(1.2)Equations such as Eq. (1.2) prove that the work, which is performed to the system or by the system, depends on the details of the process 1-2 (the path between 1-2) and does not correspond to a potential function (a function whose difference would be equal to the work regardless of the path). The subscript 12 in the symbol for work is added to denote this path dependence. The amount of work performed by compressible substances, such as gases and vapors, during several common processes may be calculated from the details of the process and is given by one of the following expressions:
 (1.3)
(1.3)where m is the mass of the compressible substance in the cylinder-piston system that performs the work; R is the gas constant, R = ![]() /M (where
/M (where ![]() is the universal gas constant 8.314 kJ/kg K); and n is the polytropic index, an exponent that defines the general polytropic process (when n = 1, the process is isothermal and when n is equal to the ratio of the specific heats, n = cp/cv, the process is isentropic).
is the universal gas constant 8.314 kJ/kg K); and n is the polytropic index, an exponent that defines the general polytropic process (when n = 1, the process is isothermal and when n is equal to the ratio of the specific heats, n = cp/cv, the process is isentropic).
The rate of work is often called the power or the mechanical power:
 (1.4)
(1.4)where 0 − t is the time of duration of the process 1-2.
Heat is transferred spontaneously from a system at higher temperature to another system at a lower temperature (a temperature difference is a prerequisite for the transfer of heat). The transfer of heat takes place via one of the following three modes:
1. Heat Conduction occurs when a temperature gradient exists and heat flows down this gradient. The rate of heat conducted through an area A is given by the expression:
 (1.5)
(1.5)where k is the thermal conductivity, a property of the materials and dT/dx is the spatial gradient of temperature. The negative sign in the r.h.s (right hand side) of Eq. (1.5) signifies that heat is transferred from the high-temperature to the lower temperature, that is, opposite to the sign of the gradient dT/dx.
2. Heat Convection is caused by the motion of fluids. The heat convection may be forced convection, (when a fluid is pumped or blown by mechanical means, as in the case of car radiators, where colder air is blown by the fan over a heat exchanger to cool the engine coolant) or natural convection (when the fluid moves without any mechanical forcing by density differences, as in the case of the plume generated by a fire). The rate of heat transferred by forced or natural convection from an object with an area A, which is at temperature TH, to a fluid at temperature TL is:
where the coefficient h is the convective coefficient. This variable is a function of the flow conditions as well as of the properties of the fluid (in the boundary layer of the surface) that facilitates the heat transfer. The negative sign, again, signifies that heat is transferred from the system at the higher temperature TH to the system at the lower temperature TL.
3. Heat Radiation does not require any intermediate materials to be transferred. Radiation may pass through vacuum as well as transparent fluids and solids. All material objects radiate heat. The sun heats the earth (and the rest of the universe) by radiation to provide solar energy. Similarly, the earth transfers heat to the rest of the universe by radiation. The rate of heat absorbed by radiation by an object at temperature TL from another at temperature TH is:
 (1.7)
(1.7)where σ is the Stefan-Boltzmann constant, 5.67 ∗ 10−8 W/m2 K4; A is the surface area of the object at absolute temperature TL; FHL is a geometric factor related to the area the two objects “see” through straight radiation rays; and α is the absorptivity of the receiving object surface, an empirical factor that characterizes all types of surfaces. The absorptivity is, for most surfaces, equal to the emissivity of the surface ε.
The work and heat exchanged between a system and its surroundings during processes are not potential functions and depend on the details, such as the “path” of the processes. The mechanical engineering community has adopted the following sign convention for the quantities of work and heat exchanged by a thermodynamic system for all processes:
A quantity of work, W, is positive when it leaves the thermodynamic system and negative when it enters the system.
A quantity of heat, Q, is positive when it enters the system and negative when it leaves the system.
The same convention applies to the rates of work (power) and heat. Figure 1.1 illustrates schematically the sign convention for the heat and work exchanged by a thermodynamic system as it will be used in this book. The algebraic sign of the quantities of heat and work that enter or leave the system are clearly shown in Figure 1.1.

Figure 1.1 The sign convention for heat and work exchanged by a thermodynamic system.
1.3 First Law of Thermodynamics
The first law of thermodynamics defines what is commonly referred to as the energy conservation principle. Several formulations of the first law – some of which claim to be more mathematically rigorous than others – abound in the literature [Reference Kestin3, Reference Bejan4]. All the formulations may be summarized by the general statement of energy conservation: Energy is neither created nor destroyed. Energy may only be transformed from one form to another. Expressions of the first law for closed systems, open systems, and systems undergoing thermodynamic cycles (all at steady state) will be given in the following subsections.
1.3.1 Closed Systems
The energy balance for a closed system is best given in terms of a thermodynamic process that takes a system from state 1 to state 2: The heat entering a closed system minus the work produced by the system during a process 1-2 is equal to the difference of the total energy of the system between these two states. This statement is written in symbolic form as follows:
where the total energy of the system Uo is a potential function defined as the sum of the internal (thermal) energy of the system, U, the potential energy, mgz, the kinetic energy, 1/2 mV2, and any other form of energy the system may possess, and which may be described by potential functions as for example, electric energy, magnetic energy, surface tension energy, elastic energy, etc.Footnote 2 Thus:
 (1.9)
(1.9)In the vast majority of thermal systems operating within the terrestrial environment the internal energy difference is by far greater in magnitude than the differences of kinetic, potential, and all other forms of energy. For example, when 10 kg of water boil to produce steam, the internal energy of this mass increases by 20,880,000 J. The same mass of water would gain approximately 10,000 J if it were raised to a height of 1,000 m; and 50,000 J if it were accelerated from rest to 100 m/s (360 km/hr or 225 mph). Because for most thermal processes the changes in kinetic and potential energies are very small in comparison to the internal energy change, one may approximate Eq. (1.9) as follows:
The specific internal energy, u, is a property of the system and the difference u2 − u1 may be obtained from thermodynamic tables; using a closure equation with the specific heat capacity at constant volume: u2 − u1 = cv(T2 − T1); from computer software; or from databases. Figure 1.2 depicts schematically the first law of thermodynamics for closed systems.

Figure 1.2 The first law of thermodynamics for closed systems.
It must be emphasized that, unlike the internal energy, work and heat are not potential functions and depend on the process 1-2. Equation (1.10) simply states that, for a closed system at steady state undergoing a process, the energy entering the system in the form of heat, minus the energy exiting the system in the form of work, is equal to the difference of the internal energy of the system between the two end states (2 and 1) of the process.
1.3.2 Open Systems
Masses cross the boundaries of open systems through several inlets and outlets. At the same time a net rate of heat enters the open system and a net rate of work (power) is delivered by the system. Because the operations of open systems are continuous, the pertinent conservation equations are expressed in terms of rates, which are denoted by a dot (.) above the symbol of the corresponding variable.
The laws of mass and energy conservation apply to open systems. For the open system at steady state, which is depicted in Figure 1.3, the mass conservation equation is:
 (1.11)
(1.11)where i denotes the inlets and e the exits of the system. For simple, steady-state, open systems with only one entrance and one exit, such as pumps, compressors, most turbines, nozzles, etc., the mass conservation equation simplifies as follows:
The first law of thermodynamics (energy conservation law) for open systems at steady state becomes:
 (1.13)
(1.13)where the property of specific total enthalpy, ho, is defined as: ho = uo + Pv. The total enthalpy per unit mass incorporates the specific enthalpy, h = u + Pv, as well as all the other potential forms of energy that make up the total energy of the system:
 (1.14)
(1.14)The power, ![]() , that appears in Eqs. (1.13) and (1.14) pertains to the useful power the system produces or consumes. The useful power produced is used for the fulfillment of engineering tasks and processes, such as the production of electric power with steam and gas turbines, the propulsion power for jet engines, and the motive power for automobiles. As in the case of internal energy and Eq. (1.10), the enthalpy difference in most open systems is by far more significant than the difference of the other forms of energy. For typical open systems – pumps, turbines, compressors, heat exchangers, etc. – the first law of thermodynamics at steady state may be simply written as:
, that appears in Eqs. (1.13) and (1.14) pertains to the useful power the system produces or consumes. The useful power produced is used for the fulfillment of engineering tasks and processes, such as the production of electric power with steam and gas turbines, the propulsion power for jet engines, and the motive power for automobiles. As in the case of internal energy and Eq. (1.10), the enthalpy difference in most open systems is by far more significant than the difference of the other forms of energy. For typical open systems – pumps, turbines, compressors, heat exchangers, etc. – the first law of thermodynamics at steady state may be simply written as:
 (1.15)
(1.15)For open thermodynamic systems at steady state with a single inlet and a single outlet, such as pumps, most turbines, and most compressors, Eq. (1.15) simplifies to the following form:
The first law of thermodynamics is also applicable to open systems with chemical reactions, such as boilers, burners, and combustors as well as for the planet Earth. A useful property of the chemical reactions is the heat of the reaction (or heat of combustion for combustion reactions), which is expressed as the enthalpy difference between reactants and products per kg of the fuel, Δh, or as the enthalpy difference between reactants and products per kmol of the fuel, ![]() . In both cases, the heat of reaction is calculated and tabulated when the reactants and the products are at 25°C (298 K). This quantity is sometimes called the heating value of the fuel and is expressed as kJ/kg of the fuel (for Δh) or as kJ/kmol of the fuel (for
. In both cases, the heat of reaction is calculated and tabulated when the reactants and the products are at 25°C (298 K). This quantity is sometimes called the heating value of the fuel and is expressed as kJ/kg of the fuel (for Δh) or as kJ/kmol of the fuel (for ![]() ).
).

Figure 1.3 The first law of thermodynamics for open systems.
In most industrial chemical reactions, including all combustion reactions, the useful power produced or consumed in the combustion chamber vanishes, that is ![]() = 0. When the reactants and the products of the reaction are at 25°C the first law of thermodynamics may be used in a simplified form to calculate the rate of heat released by the open system – combustor, boiler, burner, etc. – where the combustion takes place:
= 0. When the reactants and the products of the reaction are at 25°C the first law of thermodynamics may be used in a simplified form to calculate the rate of heat released by the open system – combustor, boiler, burner, etc. – where the combustion takes place:
where ![]() denotes the flow rate of the mols of the reactant. When
denotes the flow rate of the mols of the reactant. When ![]() is measured in kmol/s and
is measured in kmol/s and ![]() in J/kmol, the rate of heat is measured in W.
in J/kmol, the rate of heat is measured in W.
1.3.3 Systems Undergoing Cycles
A thermodynamic cycle is a series of processes with the same end states. Thermodynamic systems undergoing cycles are commonly used for the production of electric power in thermal power plants, in most of the engines used in the transportation industry, and in HVAC systems. Let us consider a system undergoing a series of n processes that constitute a cycle: 1-2, 2-3, 3-4, ... , n-1. The last process, n-1 in the cycle, ends at the initial state of the system, as shown in Figure 1.4. In this case one may write the first law of thermodynamics for each one of the n processes and add the resulting n equations to obtain the following expression:
The left hand side of Eq. (1.18) represents the difference of the net heat entering the cyclic system and the net work produced by the system when the latter performs one cycle 1-2-3- … -n-1. The right-hand side of Eq. (1.18) is equal to zero. Therefore, Eq. (1.18) may be written succinctly as:
During a complete cycle, the net amount of heat that enters a cyclic engine is equal to the net amount of work performed by the engine.

Figure 1.4 A system undergoing a cycle with n states. The (n + 1)th state is the same as the 1st state.
1.4 Second Law of Thermodynamics
We deduce from common experience that all the natural processes proceed only in one way and exhibit a definite directionality: if left unsupported, an apple always falls down; a moving billiards ball finally stops; all fluids flow from a higher pressure to a lower pressure; a drop of perfume evaporates in a room with the perfume molecules not spontaneously condensing to form the drop again; and heat always flows from hotter to colder bodies. When the natural processes are completed, it is not possible to reverse them without spending mechanical work for their reversal. For example, if we bring together two bodies, one at high temperature, TH, and the other at lower temperature, TL, and allow them to come to thermal equilibrium (without any heat losses or gains from other systems), they will finally come to a common temperature TW, which is between the two original temperatures, TL < TW < TH, as it is depicted in Figure 1.5. During this process, when the two bodies progress from state 1 to state 2, the total internal energy, which was originally contained in the two bodies, is conserved: the sum of the internal energies of the two bodies at state 1 is equal to the same sum at state 2. If we wish to reverse this process and restore the two bodies to their original temperatures, TH and TL, we will soon find out that this cannot be done without the use of a refrigeration devise, which consumes work. Despite the fact that the total energy of state 2 is equal to the total energy at state 1, the process 2 to 1 is impossible without the addition of work. We will draw the same conclusion when we try to reverse other naturally occurring processes. In order to restore a fallen apple from the ground (state 2) to the tree level (state 1) we must perform work by lifting it. To transport a fluid from low pressure (state 2) to a higher pressure (state 1) we must spend pumping or compression work. To reconstruct the evaporated drop of perfume, the perfume molecules in the air must be condensed (liquefied) by refrigerating the air/perfume mixture to a lower temperature, a process that only becomes possible with the consumption of work.

Figure 1.5 Two bodies at different temperatures, TH and TL, brought together adiabatically will finally attain an equilibrium common temperature, TW, which is between the two original temperatures, TL < TW < TH. This process cannot be reversed without work consumption.
The second law of thermodynamics explains the directionality of natural processes, by defining the property entropy. Entropy increases in all the possible natural processes of adiabatic systems (systems with zero heat transfer to and from their surroundings). The second law may be expressed by the following two simple statements [Reference Kestin3]:
1. There is a property of every system, entropy, which is defined as:
 (1.20)
(1.20)2. For every natural process 1-2 that takes place in an adiabatic system:
S2 − S1 > 0.(1.21)
The superscript “0” in the differential of heat denotes that this integral must be calculated during a reversible process. Although such processes are idealized processes, following standard procedures of thermodynamics [Reference Kestin3–Reference Moran and Shapiro5], one may express the differential dQ0 in terms of the system’s properties and then carry the integration using only these properties, which are potential functions and independent of the process. For example, for a system containing a compressible substance, dQ0 = dU + PdV = dH − VdP, and Eq. (1.20) may be written as:
 (1.22)
(1.22)The last four integrals only include material properties and may be easily calculated.
The inequality sign in Eq. (1.21) – the second part of the second law – indicates the directionality of the natural processes. Any thermodynamic system is part of a greater adiabatic system that includes the system proper and its surroundings. Processes in these greater and adiabatic thermodynamic systems always proceed in the direction where their entropy increases.Footnote 3 Actually, without any loss of generality, one may write the inequality in Eq. (1.21) as follows:
It must be noted that there is not an a priory established limit on how high or low the entropy change of a system during a process may be or should be. Processes where the entropy change approaches zero (S2 − S1 → 0) are usually idealized as isentropic processes with S2 = S1. In several actual processes, such as expansion in turbines or compression in pumps and compressors, the entropy differences are small and the processes are idealized as isentropic processes. In all the other processes (e.g. in isothermal and isobaric processes) the changes of the entropy property are significant and are not neglected.
1.4.1 Implications of the Second Law on Energy Conversion
The most important implication of the second law on energy conversion processes is that work may not be produced spontaneously by a cyclic engine, when this engine solely receives heat.Footnote 4 Most power plants – including nuclear, gas turbines, jet engines, and car engines – are cyclic engines. As a consequence of the second law these cyclic engines must reject heat to a heat sink – typically their surroundings – a fraction of the heat received. Figure 1.6 depicts the schematic diagram of the operation of all cyclic engines. During a complete cycle, the engine is in contact with two heat reservoirs, receives heat QH, rejects heat QL, and produces net work, Wnet, equal to the difference: Wnet = QH − QL. Typically, the heat is rejected in the atmosphere (in gas turbines, jet engines, and car engines via the exhaust gases) or the hydrosphere (in larger steam power plants through their condensers and cooling systems). The rejected heat is sometimes called waste heat. As a consequence of the second law and the necessity for heat rejection, the thermal efficiency of all cyclic engines, ηt, cannot exceed the Carnot efficiency, ηC:
 (1.24)
(1.24)
Figure 1.6 Net work produced and heat exchanged with two heat reservoirs during the operation of a cyclic engine.
Typical thermal efficiencies of fossil fuel thermal power cycles are close to 40% and typical efficiencies of nuclear power cycles are close to 33%. This implies that a coal power plant, which produces 400 MW of electric power, receives approximately 1,000 MW of heat and rejects 600 MW of heat, usually to a river or a lake. For a typical nuclear power plant, which generates 1,000 MW of electric power, the rate of heat produced in the reactor is approximately 3,000 MW and the rate of heat rejected to the environment is close to 2,000 MW.
It is apparent that the most important consequence of the second law of thermodynamics for the energy conversion systems is that, even though heat may readily be converted to work, it is only a fraction of the heat that is actually converted into work in all thermal engines. The rest must be rejected to the environment as low temperature heat and is referred to as the waste heat.
Ratios, such as Wnet/QH, which appears in the first part of Eq. (1.24), compare the useful work output to the heat (or energy) input of a thermal cyclic engine and are often called first law efficiencies. The concept of exergy generates other types of efficiencies that are usually called exergetic efficiencies.
1.4.2 Efficiencies of Engine Components
In addition to the overall efficiencies of cyclic engines (the power plants), engineers have defined the component efficiencies, to characterize the operation of the separate components that are parts of the cyclic engines. Such components are turbines, compressors, heat exchangers, fans, and pumps. The component efficiencies are defined as ratios of two variables: the actual work, Wact, produced or consumed by the component and the corresponding ideal work, Wid, which is produced or consumed in idealized, isentropic processes. All efficiencies are defined using the absolute values of work and heat (not the thermodynamic convention of Figure 1.1) in a way that their numerical values are between 0 and 1 (0–100%). The definitions of component efficiencies for commonly used equipment are:
1. For turbines:
 (1.25)
(1.25)2. For pumps:
 (1.26)
(1.26)3. For compressors and fans:
 (1.27)
(1.27)4. For Solar Cells:
 (1.28)
(1.28)
In the last expression I is the incident solar irradiance, usually expressed in W/m2, and A is the area of the solar cell.
Typical values of efficiencies of industrial steam turbines are in the range 78–87% and those of gas turbines are within 80–90%. The efficiencies of large industrial compressors are in the range 75–85%, while the efficiencies of compressors used in domestic appliances (e.g. small refrigerators) may be as low as 30%. Pump efficiencies are in the range 70–82%. For commonly used, commercial solar cells, typical efficiencies are in the range 14–25%, with some prototype, experimental cells having efficiencies as high as 40%. For larger industrial units, component efficiency charts are supplied by the manufacturers for the entire range of the operation of the components. For the calculations, an engineer first calculates the ideal isentropic work, Wid, using the first and second laws of thermodynamics and then calculates the actual work, Wact, using the pertinent expression from Eqs. (1.25)–(1.28). It must be noted that the concept of exergy, which is central for this book, gives rise to several other efficiencies and figures of merit.
1.5 Practical Cycles for Power Production and Refrigeration
The vast majority of thermal electric power plants utilize one of two generic types of cycles: vapor cycles and gas cycles. This section provides a succinct description of the essential components and processes of the two types of cycles that will assist with the applications of the exergy method. More detailed descriptions, improvements of the basic cycles, and details of practical cycles may be found in textbooks of Engineering Thermodynamics, such as [Reference Moran and Shapiro5].
1.5.1 Vapor Power Cycles – The Rankine Cycle
The Rankine cycle is the most commonly used vapor power cycle. In most practical power systems, water is the working fluid of the cycle and the produced vapor is steam. A few systems in operation utilize an organic fluid, typically a hydrocarbon and produce the vapor of the organic fluid. These cycles are referred to as Organic Rankine Cycles (ORCs). Water-steam Rankine cycles and their variations are the principally used cycles of all coal and nuclear power plants, which currently produce more than one-third of the global electricity [1].
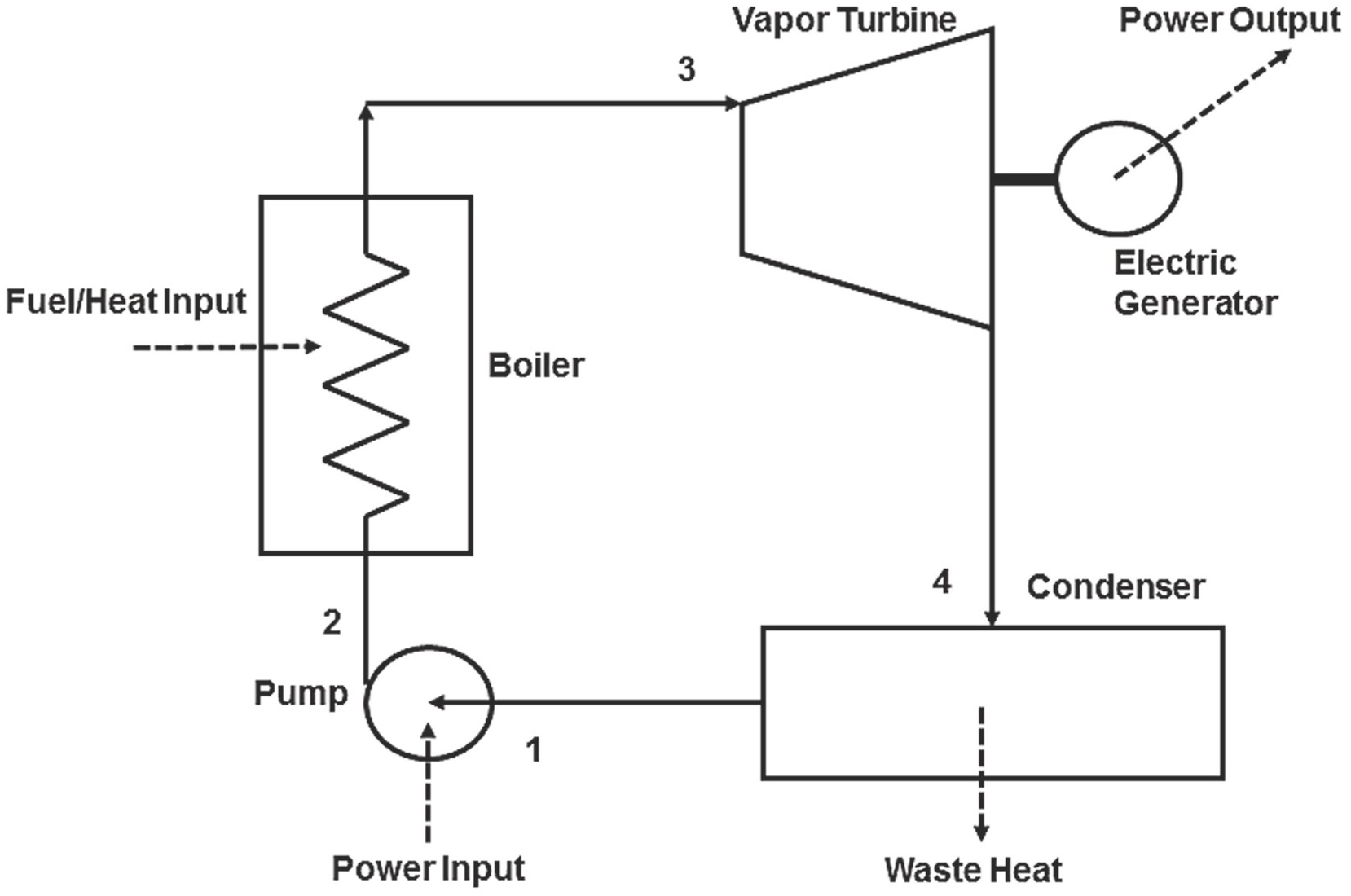
The schematic diagram of this thermodynamic cycle with its four basic components is shown in Figure 1.7. Liquid water at state 1 is pressurized in the pump from where it exits at state 2, which is at much higher pressure. The pressurized water enters the boiler, where it absorbs heat, and exits as superheated steam at high temperature, state 3. The high-temperature and high-pressure steam enters the vapor turbine, where it expands to a much lower pressure (usually subatmospheric) and its temperature drops to almost the ambient temperature. From the turbine the steam exhausts at state 4 into the condenser where the spent steam exits as liquid water and is fed back to the pump at state 1 to repeat the cycle. The condenser is cooled by the cooling system of the power plant, typically a water-cooling system.

Figure 1.7 Schematic diagram of the basic Rankine cycle with its four essential processes.
The P,v and T,s diagrams that represent the four processes of the basic Rankine cycle and the four states of the working fluid are shown in Figure 1.8, which also includes the saturation curve (liquid-vapor dome) for water. The basic processes in this cycle are:
1. Process 1-2 is the pressurization of the liquid water effluent from the condenser in the pump and is almost isentropic. In large steam units, typical inlet conditions at state 1 are 6–10 kPa (6–10% of atmospheric pressure) and the outlet pressures at state 2 vary from a few MPa to 30 MPa for supercritical cycles. The pump is driven by a motor that consumes a small fraction (on the order of 1%) of the power produced by the steam turbine.
2. The boiler (sometimes called steam generator, burner, or combustion chamber) is the component where fossil fuel combustion (coal, petroleum, natural gas) or nuclear reactions produce a large amount of heat (QH in Figure 1.6), which is transferred to the pressurized water to produce steam. The process, 2-3 in the diagrams, produces steam at high temperature and pressure. The boiler is essentially the high temperature reservoir for the cycle. The heating process 2-3 occurs with very low pressure loss and is considered to be isobaric. Therefore, P2 ≈ P3.
3. After the boiler, the steam enters a single turbine in smaller units, or several steam turbines in larger power plants. The expansion of the steam in the turbine(s) provides the motive power that drives an electricity generator via the prime shaft. The pressure and temperature of the steam are significantly reduced in the turbine and the steam exhausts in the condenser at very low pressure, in the range 6–10 kPa, and low temperature, in the range 32–50°C. The steam expansion process, 3-4, is almost isentropic. The net work produced by the ideal cycle, Wnet, is equal to the difference of the work produced by the turbine and the work consumed by the pump. In actual cycles a small amount of work is also consumed for the circulation of the cooling water in the condenser.
4. The condenser is a heat exchanger that receives steam from the turbine and typically cooling water from the cooling system of the power plant. The steam transfers its latent heat to the cooling water and condenses to become liquid. Heat is rejected in this process from the cycle to the cooling water and, finally, to the environment. This is the waste heat of the power plant, QL, in Figure 1.6. The condensation process, 4-1, occurs at constant pressure, which implies that P4 ≈ P1. If cooling water is not available, the condenser is cooled by air.
If the mass flow rate of the circulating water in the cycle is denoted by
 , the power consumed by the pump is:
, the power consumed by the pump is:  ;
;the rate of heat transferred to the water/steam in the boiler is:
 ;
;the power produced by the turbine is:
 ;
;and the rate of heat rejected by the condenser via the cooling water to the environment is:
 .
.

Figure 1.8 Thermodynamic states of the basic Rankine cycle in the P,v and T,s coordinates.
The net power produced by the basic Rankine cycle is equal to the difference of the power produced by the turbine and the power consumed by the pump: ![]() . Hence, the thermal efficiency (first-law efficiency) of the cycle is:
. Hence, the thermal efficiency (first-law efficiency) of the cycle is:
 (1.29)
(1.29)The enthalpy and the other properties of the water and steam at the states 1, 2, 3, and 4 may be obtained from steam tables, which are standard appendices in books of Thermodynamics [Reference Kestin3, Reference Moran and Shapiro5].
1.5.2 Gas Cycles – The Brayton Cycle
All the gas turbine cycles are variations of the basic Brayton cycle and typically use air as the working fluid. A few advanced gas cycles for nuclear reactors have used carbon dioxide, argon, and helium. The arrangement of the basic components of the Brayton cycle is shown in Figure 1.9, while the pressure-volume and temperature-entropy diagrams of this cycle are depicted in Figure 1.10. The four processes of the Brayton cycle are:
1. Air at ambient temperature and pressure at state 1 enters the compressor where its pressure and temperature increase to state 2. Typical pressures at the exit of the compression process are 8–30 atm. Oftentimes intercoolers are used to reduce the work input for the compression. The compressor consumes a significant fraction (between 30 and 45%) of the power produced by the turbine. In order to avoid additional losses, the compressor is coupled mechanically to the turbine by the prime shaft and is driven directly by the turbine.
2. The compressed air enters the combustor (burner) where a fossil fuel (pulverized coal, natural gas, or liquid hydrocarbons) is injected and burns by combining with the oxygen in the air. The combustion product gases are at very high temperatures in the range 1,000–1,800°C.
3. The high-temperature combustion products are fed to the gas turbine where they expand to atmospheric pressure and are discharged to the environment at state 4. The temperature at this state is significantly higher than the ambient temperature T1.
The electric generator – coupled to the turbine and the compressor via the prime shaft – produces electric power equal to the difference of the power produced by the turbine and the power consumed by the compressor.

Figure 1.9 The basic components of a Brayton cycle.

Figure 1.10 Thermodynamic states of the basic Brayton cycle in the P,v and T,s coordinates.
The gas cycle is not a cycle per se because the turbine exhaust is at a different state and has different composition (it contains the combustion products) than the ambient air at the compressor input. This series of processes is treated as a cycle, because the input to the compressor is always air at ambient temperature. In this case, the atmosphere plays the role of a heat and mass reservoir, which receives the hotter output of the turbine, cools it, purifies it to the ambient temperature and composition and, finally, allows it to be fed back to the compressor at the prevailing, ambient pressure, temperature, and composition. The fictitious cooling process in the atmosphere that completes the cycle is denoted by the broken lines 4-1 in the diagrams of Figure 1.10. The thermal efficiency of the Brayton cycle is defined in the same way as in the vapor cycles and may be expressed as follows in terms of the states in Figure 1.10:
 (1.30)
(1.30)As with the vapor cycles, the enthalpy and other properties of the air at the states 1-2-3-4 may be obtained from air tables that are standard appendices in Engineering Thermodynamics books [Reference Kestin3, Reference Moran and Shapiro5]. Oftentimes the air and the combustion products are modeled as ideal gases and the ideal gas relations are used for approximate calculations.
1.5.3 Refrigeration, Heat Pump, and Air-Conditioning Cycles
If the operation of the power generation cycle in Figure 1.6 is reversed, the cycle absorbs heat, QL, from a colder heat reservoir and transfers a higher amount of heat, QH, to a hotter reservoir, while simultaneously consuming work, Wnet. Such reversed cycles are used in refrigerators, heat pumps, and air-conditioning systems. The working fluid in the refrigeration cycles is one of the common refrigerants, which are chemical compounds of carbon, hydrogen, chlorine, and fluorine. The four basic components of the refrigeration cycle are shown in Figure 1.11 and the thermodynamic diagram – in the T,s coordinates – is shown in Figure 1.12. A comparison of Figures 1.12 and 1.8 proves that the refrigeration cycle is a modified reversed Rankine cycle that comprises the following processes:
1. The compressor admits the vapor refrigerant at state 1 and raises its pressure and temperature to those of state 2. The compressor is driven by an electric motor, which consumes power,
 . The refrigerant exits the compressor as superheated vapor at higher temperature.
. The refrigerant exits the compressor as superheated vapor at higher temperature.2. The superheated refrigerant is directed to the condenser, where it undergoes the condensation process 2-3. During this process the refrigerant dissipates heat to a heat sink at the higher temperature TH. In a heat pump, the heat sink is the interior of the building. In a refrigerator, the heat sink is the room (or kitchen) where the refrigerator is located.Footnote 5 In an air-conditioner, the heat sink is the outside air or the ground.
3. Process 3-4 is caused by the expansion valve. During this process, the pressure of the refrigerant is reduced abruptly to the original pressure, P1, and a mixture of liquid and vapor at significantly lower temperature, T4 = T1 is produced. The expansion valve is small and well insulated for this process to be considered isenthalpic, h3 = h4. Because this process entails significant irreversibilities, it is usually denoted with a broken line.
4. The refrigerant passes through the evaporator and undergoes the evaporation process 4-1. Because the temperature of the refrigerant at this stage is very low, it evaporates by absorbing heat from the heat source at the lower temperature, TL, and leaves the evaporator at the original state 1, typically as saturated vapor or slightly superheated vapor. In an air-conditioning cycle, the lower temperature heat source is the air in the interior of the building. In a refrigeration cycle it is the interior of the refrigerator, which is kept at lower temperature. In a heat pump cycle the lower temperature heat source is the outside ambient air or the ground.
The main difference between a reversed Rankine cycle and the refrigeration cycle is that the process 3-4 of the refrigeration cycle is isenthalpic and does not produce work. The work that could be produced from the expansion of the liquid refrigerant at state 3 is too low to justify the additional cost of an expander/turbine. The simple expansion valve adopted for the process 3-4 is by far cheaper and fulfills the function to significantly lower the temperature of the refrigerant.

Figure 1.11 Schematic diagram of the components and states in a refrigeration cycle.

Figure 1.12 Thermodynamic (T,s) diagram of the refrigeration cycle.
The figure of merit most often used in refrigeration cycles is commonly referred to as the coefficient of performance (COP) and is defined as the ratio of the rate of heat removal (the benefit of the cycle) and the power input (the cost) to the cycle:
 (1.31)
(1.31)As with the heat engines, the second law of thermodynamics also imposes a limit on the COP of refrigerator cycles:
 (1.32)
(1.32)1.6 A Note on the Heat Reservoirs
The notion of heat reservoirs is central to the theory of thermodynamics and essential in the application of the second law. A heat reservoir is a natural or artificial thermodynamic system that may supply or absorb any quantity of heat without appreciable change of its temperature. Natural heat reservoirs (e.g. the atmosphere and the hydrosphere) are massive systems that receive and supply heat to the thermal engines. As a result of the heat exchange, the extensive properties of the heat reservoir change according to whether heat is received or supplied, but their temperature remains approximately the same. For example, when during a process 1-2 heat Q12 is supplied to a heat reservoir by a thermodynamic system, the properties of the reservoir change according to the two laws of thermodynamics:
 (1.33)
(1.33)If the heat exchange is accomplished without phase change and chemical reactions (e.g. only sensible heat is exchanged between the system and the reservoir), the first law of thermodynamics yields the following relationship between the temperature changes of the system and of the reservoir:
where the subscripts S and R denote the system and the reservoir, respectively. For most thermodynamic systems and reservoirs, the two specific heats, cPS and cPR, are of the same order of magnitude. If the temperature of the heat reservoir is to remain approximately constant, (T2 − T1)R ≈ 0, Eq. (1.34) leads to the following result:
 (1.35)
(1.35)which implies that the mass of the heat reservoir is much greater than the mass of the system.
Natural heat reservoirs – the atmosphere, large lakes, rivers, and oceans – are massive and satisfy this condition. The sun and other stars, which are also massive, may be considered as heat reservoirs at high temperatures but, at present, we do not utilize these extraterrestrial, high-temperature heat reservoirs for the production of work and mechanical power. Instead, we have developed artificial systems, within the biosphere, that are often modeled as heat reservoirs: boilers, burners, superheaters, and combustion chambers, which are components of power plants, jet engines, and automobiles. They supply the engines with heat at high temperature and are often modeled as heat reservoirs. Energy is continuously supplied to these “reservoirs” by the combustion of fuels that maintain their “reservoir’s” temperature at high levels. Effectively, it is the continuous supply of the fuels (the natural energy resources) that creates and maintains the terrestrial high-temperature heat “reservoirs.”
Problems
1. A waterfall has 50 m height. Assuming that there is no other change and zero evaporation during the fall, what is the increase of the water temperature at the bottom?
2. A nuclear power plant generates 1,200 MW of electric power and has 32.5% overall thermal efficiency. What are the rate of heat input from the nuclear fuel and the rate of waste heat from this power plant? How much heat, in J, does the power plant reject every day?
3. A gas turbine produces 80 MW of power and has 52% thermal efficiency. What is the thermal power input to the turbine? The fuel of the gas turbine is methane (CH4) with heating value 802 MJ/kmol. Determine the rate of fuel supply in kmol/s and kg/s.
4. The interior of a refrigerator is kept at 4°C, while the ambient temperature is 32°C. What is the maximum COP for the refrigerator?
5. A pump is used to elevate 15 kg/s of water by 52 m. Frictional and other losses are 18% of the ideal power required to lift the water. If the combined efficiency of the pump and its motor is 75%, what is the rate of electric power to be supplied?
6. Convert to J the following: 3.4 MeV; 15 Btu; 35 kWh; 98 Quads; 450 TJ; 115 bbl of crude oil.