Introduction
The study of drill holes in benthic marine communities is a valuable input for a better understanding of biotic interactions both from an ecological and evolutionary point of view. This is particularly due to the fact that they are commonly preserved in the palaeontological record, allowing comparisons at different scales (see Vermeij, Reference Vermeij1980; Kabat, Reference Kabat1990; Kelley, Reference Kelley1991; Tull and Bohning, Reference Tull and Bohning-Gaese1993; Alexander and Dietl, Reference Alexander and Dietl2001; Kowalewski, Reference Kowalewski2002; Kelley and Hansen, Reference Kelley, Hansen, Kelley, Kowalewski and Hansen2003; Harper et al., Reference Harper, Peck and Hendry2009; among many others).
In addition, several authors such as Paine (Reference Paine1966) have recognized the strong control of predation on local communities. In this context, molluscan assemblages (composed of live and dead specimens) are not conditioned by annual events, but rather the average of what occurs over an interval of time; offering valuable ecological and environmental information about the communities to which they belong (Kidwell, Reference Kidwell2001, Reference Kidwell2002, Reference Kidwell2013; Tomašových and Kidwell, Reference Tomašových and Kidwell2009). One of the most interesting predation marks on mollusc shells are the drill holes, which have a long and rich palaeontological record from the Precambrian to the Holocene (Kowalewski et al., Reference Kowalewski, Dulai and Fursich1998). Researchers find these holes interesting because they provide a variety of additional information on predator–prey interactions, including the size and selectivity of a predator for prey, and the success of the predation attempt (Kelley and Hansen, Reference Kelley, Hansen, Kelley, Kowalewski and Hansen2003 and references therein).
In Argentinean Patagonia, most of the available information on drilling predation associated with benthic communities comes from the shallow coastal environment, both on hard and soft bottoms (Martinelli et al., Reference Martinelli, Gordillo and Archuby2013; Gordillo and Archuby, Reference Gordillo and Archuby2014; Archuby and Gordillo, Reference Archuby and Gordillo2018; Gordillo et al., Reference Gordillo, Malvé, Morán and Boretto2020). Other works in coastal environments were carried out further south, in Tierra del Fuego, mainly in the Beagle Channel (Gordillo, Reference Gordillo, Johnston and Haggart1998, Reference Gordillo2001; Gordillo and Amuchástegui, Reference Gordillo and Amuchástegui1998; Gordillo and Archuby, Reference Gordillo and Archuby2012). These previous works include sampling, field observations and laboratory experiments, which provided the first data on marks produced by different drilling predators, main prey, site and size selectivity and predation intensity at local level.
In this study, we explore drilling predation on molluscan assemblages in Argentinean Patagonia, covering ~1800 km along the Patagonian Continental Shelf (PCS) (Figure 1; Supplementary material S1). Because there are no previous studies on this topic in this area at these depths, and, even hemispherically, most data come from the Northern Hemisphere (Mondal et al., Reference Mondal, Chakraborty, Saha, Dey and Sarkar2021), this work aims to improve the knowledge of this type of biotic interaction in southern South America where the little information available is restricted to the intertidal to subtidal coastal environments as mentioned before.

Figure 1. Map of the study area showing the sampling sites (stations 1–19). A general characterization of this area is in Supplementary material S1. For details about location numbers, see Supplementary Table S2.
An aspect of interest regarding the intensity of predation at the regional scale is to evaluate whether there are spatial trends as a latitudinal predation pattern. Previous studies on this topic reveal that predation pressure may decrease or increase with increasing latitude, or even show no trend, questioning the generality of any large-scale latitudinal or biogeographic pattern (e.g. Vermeij et al., Reference Vermeij, Dudley and Zipser1989; Hansen and Kelley, Reference Hansen and Kelley1995; Alexander and Dietl, Reference Alexander and Dietl2001; Hoffmeister and Kowalewski, Reference Hoffmeister and Kowalewski2001; Visaggi and Kelley, Reference Visaggi and Kelley2015; Mondal et al., Reference Mondal, Chakraborty, Saha, Dey and Sarkar2021). In southern South America, this issue has been previously treated by Martinelli et al. (Reference Martinelli, Gordillo and Archuby2013) for a sector (between 42° and 48° S) along the Patagonian coast, concluding that drilling predation does not indicate a latitudinal/thermodependent trend, but rather seems to be linked to local factors, which includes anthropogenic reasons, among others. With this background and interests, our objective is to understand if the variation in predation intensity along the PCS follows a latitudinal pattern or spatial trend, or if it rather depends on local factors, in accordance with previous data from the shallow Patagonian coastline.
Potential predators
In the modern seas, drill holes are primarily – but not exclusively (e.g. see Kelley and Hansen, Reference Kelley, Hansen, Kelley, Kowalewski and Hansen2003) made by two families of gastropods, the Muricidae and the Naticidae. Drilling process in these two families involves alternating phases of mechanical rasping with a radula and chemical dissolution with acid produced by the accessory boring organ (the ABO) (Carriker, Reference Carriker1981). Although Muricidae and Naticidae converge in this chemo-mechanical process, they differ in the location of the ABO: in muricids, it is in the mid-ventral part of the foot, while in naticids at the tip of the proboscis (Carriker and Gruber, Reference Carriker and Gruber1999).
There is an ecological difference between the two main families of drilling gastropods: the muricids are more associated with epifaunal prey, while the naticids are associated with infaunal prey. However, it is important to highlight that muricids search for prey epifaunally, but also unearth shallow infaunal prey (Kelley and Hansen, Reference Kelley, Hansen, Kelley, Kowalewski and Hansen2003), which has also been pointed out in previous studies in Patagonia and Tierra del Fuego (Gordillo, Reference Gordillo, Johnston and Haggart1998; Gordillo and Archuby, Reference Gordillo and Archuby2014).
Complete drill holes by muricids are usually cylindrical with external and internal diameters about the same size, while drill holes by naticids have a wide conical shape (Kelley and Hansen, Reference Kelley, Hansen, Kelley, Kowalewski and Hansen2003). However, there are cases in which muricid holes resemble those made by naticids and vice versa. For example, drill holes produced by the muricid Trophon geversianus vary from conical to cylindrical depending on the prey species drilled (Gordillo, Reference Gordillo, Johnston and Haggart1998). For these cases, a more precise feature to differentiate the two potential families is the incomplete holes: in muricids the bottom of the hole is flat or concave, while in the naticids, it usually (but not always) has a central hump (Vermeij et al., Reference Vermeij, Dudley and Zipser1989; Kabat, Reference Kabat1990).
Other groups, such as octopuses, can also make holes (Carriker, Reference Carriker1981; Bromley, Reference Bromley1993). However, octopuses use a salivary papilla provided with horny thorns, resulting in relatively smaller holes (considering the size of the prey) than those produced by the drilling snails (Bromley, Reference Bromley1993).
Materials and methods
Sampling was conducted in March/April 2012 during a campaign aboard the R/V ARA Puerto Deseado. Molluscan assemblages were collected from 19 stations (Figure 1) at depths ranging from 27 to 135 meters using a demersal bottom trawl pilot net. The net had a total length of 6 m, with 25 mm mesh on the wings and 10 mm mesh at the cod end. It had a vertical opening of 0.6 m and a horizontal aperture of 1.8 m.
In addition, environmental data collected during the fieldwork include depth, temperature, salinity and substrate type (Supplementary Table S2). Water temperature and salinity data were measured using a Seabird SBE 21 thermosalinograph (Sea-Bird Scientific, Bellevue, WA, USA), whereas depth was measured using a SIMRAD EA 600 (Kogsberg Maritime, Horten, Norway) echo-sounder. Sediment samples were taken by a Shipek or Snapper dredge (Borel and Franco Arias, Reference Borel and Franco Arias2012).
While working on board, molluscan assemblages, composed of live/dead specimens and empty shells and disarticulated valves, were stored in sealed plastic bags containing 4% buffered formaldehyde solution until further analysis. Then, in the laboratory, the material was cleaned, separated, sorted and labelled.
Taxonomic composition of molluscan shells and their relative abundance were determined for each station. To count bivalves from disarticulated valves, half of the total number of single (left or right) valves was taken for each species, except when they corresponded to different specimens, which were added to the rest as independent right and left valves. Only specimens with complete valves were counted due to the impossibility of determining whether a broken valve had drilling or not (although few specimens were broken and not included in the count).
Molluscs were identified at the species level or the lowest possible taxonomic level. The validity of species names was reviewed from the World Register of Marine Species (WoRMS) database (revision 05.2023).
Molluscs were classified according to their relationship with the bottom substrate: those that live on the bottom or other surface (epifaunal) or those that live within the substrate (infaunal). Also based on their mobility, molluscs were grouped into three categories: attached or resting bivalves, active burrowing bivalves and mobile gastropods. To classify the species according to their feeding mode, we used the family to which they belong as a reference. Bivalves were classified into three subtypes by feeding behaviour: suspension-feeders (SF) that consume material suspended in the water column, deposit-feeders (DF) that consume sediments and facultative suspension- and deposit-feeders (D/SF) as occur in certain cases (e.g. Nuculidae). Gastropods were further subdivided into grazers (G), carnivores (C) and suspension feeders and grazers (SF/G; e.g. Calyptaeidae).
Shells were examined for marks of predation. Holes with circular to oval outlines were considered as drill holes, and therefore indicators of biotic interactions. Previous works in Patagonia and southern South America (Gordillo, Reference Gordillo, Johnston and Haggart1998, Reference Gordillo2013; Gordillo and Amuchástegui, Reference Gordillo and Amuchástegui1998; Gordillo et al., Reference Gordillo, Bayer and Martinelli2010, Reference Gordillo, Malvé, Morán and Boretto2020, among others) were used as a reference for identifying these predatory marks.
To evaluate drilling predation, specimens were examined for traces of complete/incomplete drill holes (Vermeij et al., Reference Vermeij, Dudley and Zipser1989). Complete drill holes are identified as those that penetrate completely through the prey shell, here interpreted as successful attempts. Incomplete drill holes were identified as those that do not penetrate completely through the prey shell, representing unsuccessful attempts. However, incomplete drill holes may overestimate the frequency of failed attacks because in some instances, incompletely drilled prey can be killed (Kowalewski, Reference Kowalewski2004; Chattopadhyay and Baumiller, Reference Chattopadhyay and Baumiller2007).
In addition, we looked for signs of repair of drill holes following failed predation. The reason is because successful attempts (complete drillings) do not always result in a successful attack. In other words, an unsuccessful attack would be when there is evidence that the predator did not eat the prey, as manifested by shell repair (e.g. O'Neill et al., Reference O'Neill, Mala, Cafiso, Bignardi and Taylor2018), indicating that the potential prey survived the attack.
We used the drilling frequency (DF) to estimate how often shelled organisms are attacked (here it includes complete and incomplete holes) by drilling predators and the successful attacks frequency (SAF) to estimate how often these attempts result in a successful attack. DF was calculated by dividing the total number of shells with complete and/or incomplete drill holes by the total number of specimens (and multiplying it by 100), while SAF was obtained by dividing the total number of shells with complete and not repaired drill holes by the total number of specimens (and multiplying it by 100). Chi-squared tests (χ2) were conducted to test the statistical significance of differences in DF and SAF between stations. The standard error in the graph bars was calculated by dividing the standard deviation by the square root of the number of values in each dataset.
Then, the calculated DF was arbitrarily classified as low (up to 5), moderate (>5–15) and high (>15), taking as reference average values from previous works (Kelley and Hansen, Reference Kelley, Hansen, Bromley, Buatois, Mángano, Genise and Melchor2007; Martinelli et al., Reference Martinelli, Gordillo and Archuby2013; Visaggi and Kelley, Reference Visaggi and Kelley2015; Gordillo et al., Reference Gordillo, Malvé, Morán and Boretto2020).
To interpret how much of the DF can be explained by water temperature and depth, we conducted linear regressions which are widely used in many different contexts in aquatic ecology. The criterion used was that all predictor variables were required to be significant at α = 0.05.
We also compared two subregions in terms of their faunal composition: San Jorge Gulf (GSJ) and Tierra del Fuego (TDF), given that they concentrate the largest number of localities and are separated by ca. 600 km.
To assess size selectivity, we compared the size-frequency distributions of drilled specimens of three common prey (Tawera elliptica, Proteopitar patagonicus and Retrotapes exalbidus) in different stations. For this, the specimens were arbitrarily grouped into size intervals based on length (in mm): in T. elliptica (10–30 mm) four classes of 0.5 mm; in P. patagonicus (10–60 mm) five classes of 10 mm; and in R. exalbidus (10–40 mm) three classes of 10 mm. Size selectivity was evaluated using the χ2 goodness-of-fit test with α = 0.05.
In order to assess site selectivity, we analysed the positions of the drill holes on the shell surface of the most abundant prey, the bivalve T. elliptica. To do so, we arbitrarily divided the shell surface into nine sectors (umbo, centre and marginal region and each one of them divided into anterior, middle and posterior area, thus defining nine sectors) (Kelley, Reference Kelley1988). Although these sectors are of uneven size (uneven sector approach; Kowalewski, Reference Kowalewski2002), for practical purposes and to avoid somewhat complicated calculations, they were statistically treated as having the same surface area. With this consideration, site selectivity was evaluated using the χ2 goodness-of-fit test with α = 0.05. Statistical analyses were conducted in R Software (R Core Team, 2015).
One final comment is that in the Results section, we also include unpublished data on drilling predation on brachiopods, which were collected in the same campaign trip (Gordillo et al., Reference Gordillo, Muñoz, Bayer and Malvé2018). Brachiopods are not included in the analyses, except when specified and only for comparative purposes given that they provide complementary information about the sampled stations.
Results
Molluscan assemblages: taxonomic composition arranged by family
A total of 2179 specimens, including bivalves and gastropods, were recovered. Most of them (n = 2172) were identified to species level. The bivalves (n = 1574) are distributed in 22 families, while gastropods (n = 598) are in 15 families. The most abundant families in terms of numbers of specimens and species were Mytilidae (n = 112, 3 species), Pectinidae (n = 399, 3 species), Mactridae (n = 133, 5 species), Veneridae (n = 419, 7 species), Calliostomatidae (n = 62, 6 species), Calyptraeidae (n = 319, 6 species) and Muricidae (n = 90, 8 species). Naticidae (n = 28, 3 species) were present in smaller numbers than muricids.
When comparing molluscan assemblages from two sub-regions within the study area (GSJ, San Jorge Gulf to the north vs TDF, Tierra del Fuego to the south; Figure 1), some differences in the more common families were observed. To the north, there is a high abundance of Neilonellidae, with Pectinidae, Veneridae and Pandoridae also abundant. To the south, the commonest family is Pectinidae, with Mactridae, Veneridae and Calyptraeidae also abundant. The predominant sediments in the GSJ area are muds (with gravels and shells) to sandy muds while in the TDF subregion they are mostly fine to coarse-grained sands (Supplementary Table S2).
When considering depth, the best represented bivalve families throughout the range were Pectinidae and Veneridae and, among the gastropods, Calliostomatidae and Calyptraeidae. Other families such as Mactridae and Semelidae were not present in the deeper stations, while Glycymeridae and Pandoridae appeared below 50 m, and Neilonellidae were only recorded below 100 m depth (Supplementary Table S3).
Intensity of drilling predation
Of the total number of specimens analysed, 9.9% had a drill hole. When including repaired shells and failed attempts, the frequency of successful attacks gave a slightly lower average value of 9.5%. Stations with high values (greater than 15%) were S3, S6, S10 and S15, stations with moderate values (between 5 and 15%) were S1, S2, S4, S7, S12, S13, S17, S18 and S19 and stations with low values (less than 5%) were S9, S11, S14 and S16. Two stations, S5 and S8, did not present cases of drilling predation. Table 1 and Figure 2 summarize the data for each station and Supplementary data are in Table S4. Moreover, significant differences were found among stations for pooled DF and SAF (χ2DF = 95.882; dfDF = 18; P DF < 0.0001; χ2SAF = 102.53; dfSAF = 18; P SAF < 0.0001).
Table 1. Summary of drill hole data and main inferred predators for each station
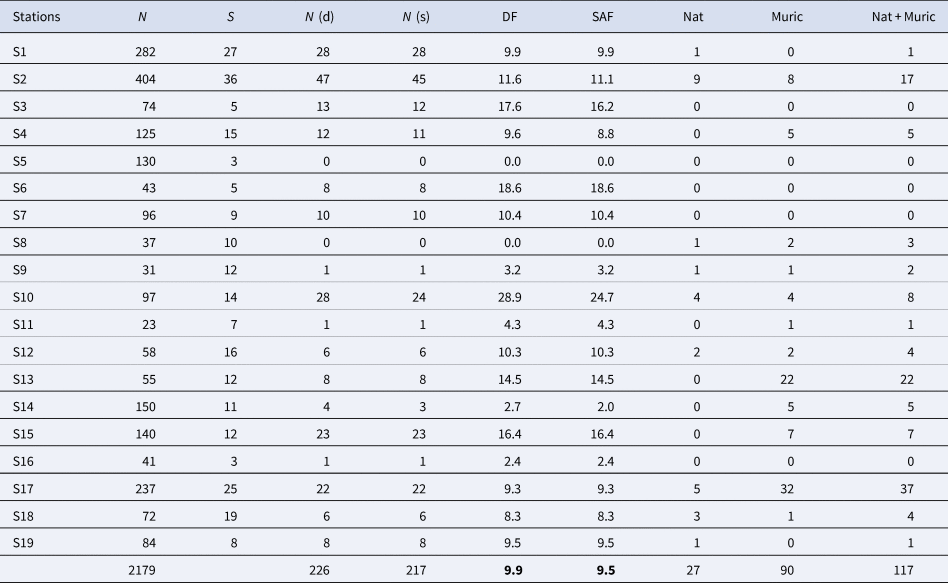
N, number of specimens; S, number of species; N (d), number of specimens with drill holes; N (s), number of specimens with successful attacks; DF, drilling frequency; SAF, successful attacks frequency; Nat, number of naticid specimens; Muric, number of muricid specimens; Nat + Muric, sum of naticid and muricid specimens.

Figure 2. Graph showing the distribution and relative abundance of drilled (black bar) and undrilled (white bar) mollusc specimens per station. Data pooled from all specimens. Error bars are 95% confidence intervals.
Drilling frequency, latitude and depth
We examined the relationships of DF to latitude and depth (Figure 3). The graph shows the great variability in predation intensity between stations with values of DF ranging between 0–2 and 29%, not evidencing any latitudinal trend or pattern (R = −0.051; F = 0.2236; P = 0.6431), nor with depth (R = −0.037; F = 0.4248; P = 0.5244), at least from the pooled data.

Figure 3. Bar graphs showing drilling frequency (%) per station from north (left) to south (right), indicating depth (dashed line) and water temperature (continuous line) taken at each site at the time of sampling.
Identity of prey
Drill holes were found in at least 33 species (21 families), of which 23 are bivalves. Figure 4 illustrates some specimens of different species recovered with traces of drilling predation.

Figure 4. Shells with drill holes. (A–R) Bivalves. (A) Ennucula grayi (d'Orbigny, 1846), station 4, 92 m. (B) Malletia cumingii (Hanley, 1860), station 4, 92 m. (C) Glycymeris ovata (Broderip, 1832), station 7, 108 m. (D) Plicatula gibbosa Lamarck, 1801, station 2, 56 m. (E) Zygochlamys patagonica (P. P. King, 1832), station 4, 92 m. (F) Aequipecten tehuelchus (d'Orbigny, 1842), S2, 56 m. (G) Limea pygmaea (R. A. Philippi, 1845), station 18, 115 m. (H) Cyclocardia compressa (Reeve, 1843), station 18, 115 m. (I) Diplodonta patagonica (d'Orbigny, 1842), station 18, 115 m. (J–K) Mactra guidoi Signorelli & F. Scarabino, 2010, station 1, 41 m. (L) Mactra fuegiensis E. A. Smith, 1905, station 15, 43 m. (M) Retrotapes exalbidus (Dillwyn, 1817), station 9, 102 m. (N–O) Tawera elliptica (Lamarck, 1818), station 11, 36 m. (P) Proteopitar patagonicus (d'Orbigny, 1844), station 3, 74 m. (Q) Semele proficua (Pulteney, 1799), station 2, 56 m. (R) Psammotreta brevifrons (Say, 1834), station 2, 56 m. (S–W) Gastropods. (S) Trochita pileus (Lamarck, 1822), station 17, 81 m. (T) Photinula coerulescens (P. P. King, 1832), station 13, 29 m, (U) Naticidae, station 11, 36 m. (V) Xymenopsis buccineus (Lamarck, 1816), station 17, 81 m. (W) Pareuthria atrata (E. A. Smith, 1881), station 17, 81 m. (X–Y) Brachiopods. (X) Liothyrella uva (Broderip, 1833), station 11, 36 m. (Y) Terebratella dorsata (Gmelin, 1791), station 11, 36 m. Scales: 10 mm.
The highest values of predation occurred within the Mactridae, Veneridae, Semelidae and Calliostomatidae with DF values ranging between 15 and 35 (Table 2). The most drilled bivalves were T. elliptica, R. exalbidus, Zygochlamys patagonica, Mactra fuegiensis, P. patagonicus and Semele proficua. They are common species in the region, mainly infaunal in soft substrates, except for Z. patagonica, which can live as epifauna on sandy, muddy or gravel and shell covered bottoms. Among gastropods, the trochoid Photinula coerulescens, which is the gastropod that scored the highest value of DF, is also epifaunal living on rocky bottoms or hard surfaces.
Table 2. Most preyed taxa
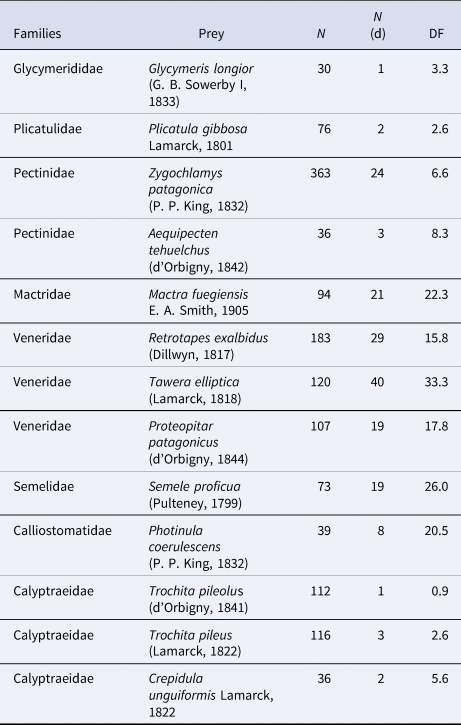
N, number of specimens; N (d), number of specimens with drill holes; DF, drilling frequency.
Pooled data. See also all species discriminated by station in Table S4.
Besides bivalves and gastropods, there are brachiopod species present in the same stations (i.e. Liothyrella uva, Terebratella dorsata and Magellania venosa), which also present specimens with drill holes (Figure 4X, Y, Supplementary Tables S4 and S5).
The only two bivalve families that show drilling throughout the depth range considered are the Pectinidae and the Veneridae (Figure 5A). Among pectinids, the most preyed species present throughout the depth interval sampled is Z. patagonica, more abundant at deeper waters. Among venerids, the most preyed are T. elliptica, more abundant at intermediate depths, and R. exalbidus, at depths up to 50 m. In relation to gastropods with drill holes, P. coerulescens (Calliostomatidae) and Trochita pileus (Calyptraeidae) also were found sparsely throughout the range, with P. coerulescens occurring more in the shallower depths (up to 50 m) (Figure 5B).

Figure 5. Bathymetric distribution of drilled specimens of bivalves and gastropods, expressed as drilling frequency (proportions) according to three depth categories: up to 50 m (black), between 50 and 100 m depth (grey) and more than 100 m (white). In the first graph (A), the molluscs were grouped by family. In the second graph (B) only the main species are shown.
Life habits and feeding mode of prey
Discriminating the taxa of the molluscan assemblages by life habits and feeding strategies, we found that 47.4% live as infauna and 52.6% as epifauna, 43.8% are active burrowing bivalves, 28.4% are attached or resting bivalves and 27.8% mobile gastropods; and according to feeding mode, 64.4% are suspension feeders, 14.7% SF/grazers and the remaining 20.9% make up the other trophic types, including deposit feeders and carnivores (first column, Table 3).
Table 3. Taxonomic and ecological summary of drilling predation pooled across stations
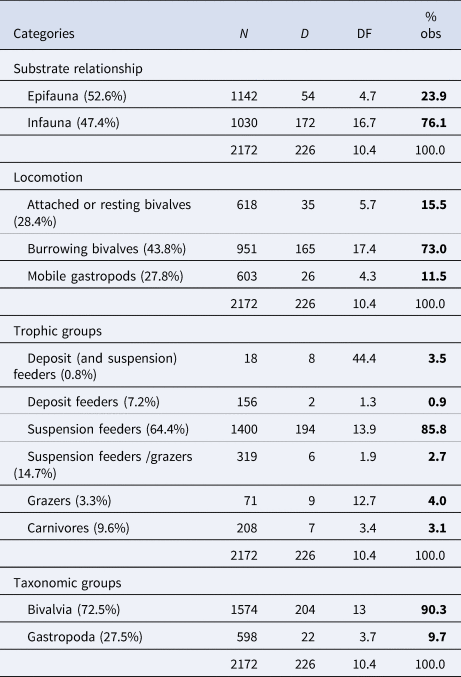
N, total number of specimens; D, number of specimens with drill holes; DF, drilling frequency; % obs., % observed.
When considering specimens with drill holes, more than 85% are suspension feeders, while the rest are categorized among the other five trophic types (Table 3 and Figure 6). The value of 85.8% of suspension feeders with drill holes slightly rises to 86.8% (not shown in the table) when brachiopods (N = 820; DF = 0.5), recovered from the same stations, are included.
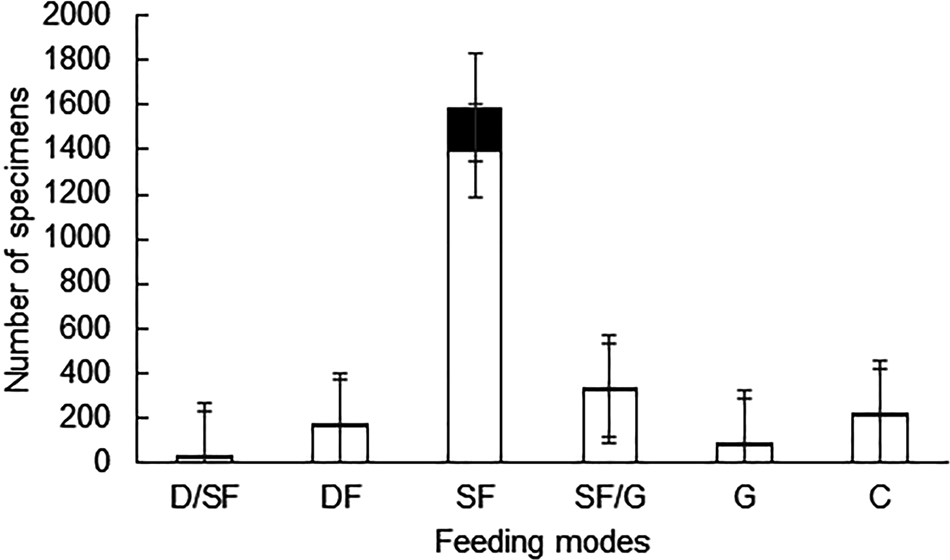
Figure 6. Comparison of the relative abundance of specimens with drill holes (black) in relation to the total number of specimens (white) for each trophic group. Error bars are 95% confidence intervals. D/SF, deposit-suspension feeder; DF, deposit feeder; SF, suspension feeder; SF/G, suspension feeder-grazer; G, grazer; C, carnivore.
DF was significantly different between suspension feeders and most of the other trophic groups. Suspension feeders showed significant differences with deposit feeders (χ2 = 194.92, P < 0.0001), also with suspension/grazers (χ2 = 62.372, P < 0.001), grazers (χ2 = 174.95, P < 0.0001) and with carnivores (χ2 = 180.47, P < 0.001). According to mode of life, DF was significantly different between infaunal and epifaunal organisms (χ2 = 14.924, P < 0.001) with infaunal organisms having a higher DF than epifaunal ones. Comparisons between different locomotion preferences showed that DF was significantly different between attached or resting bivalves compared with burrowing bivalves (χ2 = 44.954, P < 0.0001), but not statistically different compared with mobile gastropods (χ2 = 0.9072, P = 0.3408). There were significant differences in DF between burrowing bivalves and mobile gastropods (χ2 = 56.989, P < 0.0001).
Incomplete and multiple drill holes
Although in most cases these were specimens with a single complete drill hole, there were also specimens with multiple drill holes, which had incomplete holes and at least one complete drill hole (Supplementary Table S4). This was the case for five T. elliptica specimens recovered from station 17. Similarly, one specimen assigned to Xymenopsis muriciformis from station 13 had one incomplete and two complete drill holes. A second gastropod specimen from the same station (P. coerulescens) had one complete and one incomplete hole.
In addition, there were seven specimens with only incomplete holes. These include four specimens of T. elliptica (station 10), one of Z. patagonica (station 14) and one of S. proficua and Crepidula unguiformis (both from station 2). In all cases, the incomplete holes have flat bottoms.
Repair signals
Finally, there were two specimens from different species and sites, P. patagonicus (station 3) and Ennucula grayi (station 4), which in life apparently repaired the drill holes (i.e. they occluded the hole by depositing material on the inner side of the shell). By considering the latter cases (incomplete drill holes and repaired shells), it is possible to calculate the frequency of successful attacks (Table 1, Supplementary Table S4) that excludes those specimens interpreted as ‘not eaten’.
Drilling and size
To assess size selectivity, we compared drilled shells from three venerid species (T. elliptica, P. patagonicus and Retrotapes exabidus) from different localities. For the bivalve T. elliptica, middle sizes were the most preyed upon in three localities (S2, S10 and S17) (χ2 = 8.5882, P = 0.035) (Figure 7A). In the case of P. patagonicus, there was no overlap between sizes when comparing specimens with drill holes from two localities where this species was found (S1 and S3) (χ2 = 4, P = 0.406) (Figure 7B). Finally, R. exalbidus specimens with drill holes from stations 1 and 10 were compared in size. We observed that at station 1, specimens from only one size category were drilled (χ2 = 6.4853, P = 0.0108), while at station 10, drilled specimens were distributed over a wider size range, and specimens of middle size are more heavily eaten (χ2 = 7.505, P = 0.006) (Figure 7C).

Figure 7. Size distribution of specimens with drill holes in three common bivalve species discriminated by stations. Tawera elliptica (A), Proteopitar patagonicus (B) and Retrotapes exalbidus (C).
Site selectivity
When examining whether there was a preference for any particular sector of the T. elliptica shell surface in the three localities studied (as depicted in Figure 8), it was found that there was an average of 58.5% in sector v (mid-central) and 27.9% in sector ii (umbo-central). The null hypothesis of equal DF for each shell sector is rejected (χ2 = 24.829; df = 9; P = 0.00316). Then, there are a high number of drill holes located in the mid-central and umbo-central sectors of the shell, indicating that these two sectors were the most extensively drilled.

Figure 8. Distribution of drill holes on the shell surface of Tawera elliptica compared across three stations (S2, black; S10, grey; S17, white). The scheme indicates the subdivision of the shell surface into nine sectors.
Other holes
In addition to typical drill holes, some specimens showed other types of holes (Figure 9), which have been included as they are not isolated breaks, but denote a pattern and could be caused by predators (see discussion). These ‘other holes’ were registered in some specimens of Tegula patagonica and Photinula coelurescens from station S13 (Figure 9A–C) exhibiting elongated holes and in some mactrid specimens from station S15 (Figure 9D–F) that showed two breaks in each shell.

Figure 9. Shells with ‘other holes’. (A) Tegula patagonica (d'Orbigny, 1838). (B–C) Photinula coerulescens (P. P. King, 1832), station 13, 28 m. (D) Mactra fuegiensis E. A. Smith, 1905. (E) Mulinia edulis (P. P. King, 1832). (F) Mactridae, station 15, 43 m. Scales: 10 mm.
Discussion
Shelled assemblages and drilling predation
The shell assemblages collected in this study included over 2000 specimens from 37 families, with 22 being bivalves and 15 being gastropods. They are composed of live, dead and empty specimens. Then, these assemblages were formed from both contemporaneous and non-contemporaneous generations of organic remains. As a result, time-averaging and spatial mixing could have occurred. However, despite these factors, they provide useful ecological information and can be used as proxies for living communities at regional spatial scale. This has been demonstrated in previous studies with brachiopod specimens from the same sample collection (Gordillo et al., Reference Gordillo, Muñoz, Bayer and Malvé2018). Based on these studies, the assemblages collected during 2012 by ARA Puerto Deseado could be interpreted as local assemblages or in situ shelled assemblages.
Thus, despite inherent taphonomic biases of these time-averaged assemblages, they provide valuable information on the structure of the original communities through the analysis of the preservable fraction (Kidwell, Reference Kidwell2001, Reference Kidwell2002, Reference Kidwell2013; Tomašových and Kidwell, Reference Tomašových and Kidwell2009). From an ecological point of view, these assemblages consist of epifaunal and infaunal/semi-infaunal organisms, able to live on different types of bottoms from cohesive mud to coarse sand with gravels and shell fragments. When classifying shelled taxa by feeding mode, they are mainly suspension feeders, but also carnivores, grazers and deposit feeders.
The pooled DF value of 9.9% falls within the moderate range, but the range of variation is wide with very low (0–2.4%) to high (28.9%) values. Something similar happened with the values obtained by Martinelli et al. (Reference Martinelli, Gordillo and Archuby2013) for the coastal sector with pooled DF between 3 and 36%. Concerning the intensity of drilling predation, no latitudinal gradient was recognized, and regarding depth a trend was not detected within the analysed range. Further data collection at greater depths is required to obtain more comprehensive understanding of this topic.
Based on our findings (highly variable and lacked a spatial trend), it appears that predation control is primarily linked to local biological or environmental factors within each local community. Several factors such as the type of substrate, facies, water depth, water temperature, salinity and the local ecological composition of the predator and prey (i.e. tiering, size class, diversity) could have a local impact on drilling predation (Mondal et al., Reference Mondal, Chakraborty, Saha, Dey and Sarkar2021).
Concerning faunal composition, the main families with drill holes in terms of abundance were Veneridae, Mactridae, Pectinidae and Semelidae, representing 70.1% of the specimens with holes as predation marks. When considering ecological attributes of the molluscan assemblages, the DF was more intense in the infaunals than in the epifaunals (16.7 vs 4.7%), and in the burrowing bivalves (17.4%) than the resting or attached bivalves (5.7%) or mobile gastropods (4.3%). All these results argue that both infaunal and the active burrowing life habits do not deter drilling predators, and these predators eat a wide variety of available prey, including carnivores, among others, which gave lower values.
Regarding potential predator snails, muricids are more abundant and diverse than naticids. Among them, X. muriciformis was the most abundant in the pooled sample, followed by Xymenopsis buccineus, Trophon plicatus and T. geversianus, plus others in small proportion; and among the naticids, the involved predators would be Notocochlis isabelleana, Falsilunatia carcellesi and Tectonatica impervia. In addition to gastropods, octopuses may also be responsible for some holes; however, as detailed below, these predators are very versatile in their strategies, which makes identification and interpretation more difficult.
Taking into account that muricids feed on epifauna, but they can also dig up shallow infaunal prey, added to the fact that they are more diverse and abundant than naticids, it is likely that muricids are responsible for a large proportion of the drill holes. However, sometimes naticids and muricids can eat without leaving marks (e.g. Frey et al., Reference Frey, Howard and Hong1986; Kabat, Reference Kabat1990; Vermeij and Carlson, Reference Vermeij and Carlson2000), also masking the results of the frequency of predation.
The most preyed upon species was the venerid T. elliptica, which also indicated size and site selectivity. Preference for the same species was also reported for benthic communities of the Beagle Channel, in the extreme south of South America, where T. elliptica (as Tawera gayi, the old name) was the main shelled prey of the muricids T. geversianus and X. muriciformis (Gordillo, Reference Gordillo, Johnston and Haggart1998; Gordillo et al., Reference Gordillo, Martinelli, Cárdenas and Bayer2011). Tawera elliptica is a small infaunal venerid able to live semi-infaunally or partially buried which may increase predation risk by epifaunal gastropods (Gordillo et al., Reference Gordillo, Martinelli, Cárdenas and Bayer2011).
A similarity between drill holes on T. elliptica shells of the present study and those of the Beagle Channel is that in both cases they are preferentially located in the central area of the shell. This stereotyped behaviour is probably related to the horizontal position (lying on the sediment) that venerid clams adopt when they are exhumed outside the sediment during storms or by the same predators (Kondo, Reference Kondo1987; Gordillo and Archuby, Reference Gordillo and Archuby2014). This horizontal position would explain the preference of the predator (when handling the prey) for the central area, whereas drill holes on the umbonal zone would be more feasible when the clam remains semi-buried in a vertical position.
Regarding the presence of breaks and holes (called here ‘other holes’) on some recovered shells we cannot rule out that they are produced by predators such as octopuses. This interpretation (although speculative) is mainly based on the fact that octopuses are opportunistic predators with high versatility and precision, using different mechanisms to overcome their prey (Nixon and Maconnachie, Reference Nixon and Maconnachie1988; Bromley, Reference Bromley1993; Cooke et al., Reference Cooke, Whitelaw, Norman, Caruana, Strugnell, Gopalakrishnakone and Malhotra2017; Fee et al., Reference Fee, Mather, Landschoff and Griffiths2023). Some prey are killed with a toxin (Nixon and Boyle, Reference Nixon and Boyle1982) and shelled molluscs are usually drilled, although some are killed by pulling apart their valves (Fujita, Reference Fujita1916; Pilson and Taylor, Reference Pilson and Taylor1961; Arnold and Arnold, Reference Arnold and Arnold1969; Hartwick et al., Reference Hartwick, Thorarinsson and Tulloch1978). The holes produced by octopus are highly variable in shape: oval, rounded, elongated, even irregular (Arnold and Arnold, Reference Arnold and Arnold1969; Nixon, Reference Nixon1979; Kabat, Reference Kabat1990; Bromley, Reference Bromley1993). In addition, many cephalopods use a combination of their parrot-like beak and/or toothed radula to inject venomous saliva into its prey through a bite wound (Cooke et al., Reference Cooke, Whitelaw, Norman, Caruana, Strugnell, Gopalakrishnakone and Malhotra2017). Therefore, marks produced by the radula with chemical secretions or venom, and marks produced by the beak, or both, are feasible to occur on shelled molluscs.
More specifically, a first pattern observed in three trochid shells from station 13 consists of elongated holes, similar to each other. Although there are no comparable examples in the literature, a deformation (as a groove or gutter) of a typical hole could be produced when the octopus manipulates and rotates the prey (Bromley, Reference Bromley1993). In addition, another trochid shell recovered at the same station have two small drill holes, which is consistent with typical octopus behaviour of drilling two or more drill holes in the same prey (Bromley, Reference Bromley1993). A further comment of interest as it involves Patagonian species and could also explain differences in predation marks produced by octopus on this type of prey was observed under laboratory conditions by Iribarne et al. (Reference Iribarne, Fernandez, Diaz and Clemente1993). These authors mention that Octopus vulgaris makes holes on T. patagonica when these shells are occupied by hermit crabs, whereas if the Tegula is alive, they eat them but leave no marks on the shell.
A second pattern (two irregular holes) was observed in some mactrid shells from station 15 and could be caused when the octopus holds the prey with its beak (upper and lower beak) while releasing saliva that acts by softening the surrounding area. For comparison, a similar pattern was described for bivalve shells belonging to Antarctic communities (Gordillo et al., Reference Gordillo, Morán and Malvé2021), where, moreover, there are no crabs. However, at the moment we have no other information on the behaviour and feeding patterns of octopus's species in the studied region, except for the work by Iribarne et al. (Reference Iribarne, Fernandez, Diaz and Clemente1993) and some observations of marks on limpets (perhaps produced by Robsonella fontaniana and/or Octopus tehuelchus) from coastal environments in northern Patagonia (Archuby and Gordillo, Reference Archuby and Gordillo2018). For all this, our interpretation of octopus marks should be taken with caution and other alternatives should also be considered (for example, that they be taphonomically enlarged gastropod drill holes). This indicates that further work is needed to address these uncertainties.
Finally, the scarcity of incomplete or multiple drill holes and signs of shell repair (meaning a sublethal injury) would support evidence in favour of successful predation. Previous work under controlled laboratory experiments (Gordillo, Reference Gordillo, Johnston and Haggart1998) has shown a low percentage of multiple and incomplete drill holes; however, as this behaviour was achieved under artificial conditions, it is also difficult to project in natural environments. This indicates that this type of study requires different complementary methodologies in order to improve our interpretation.
Drilling predation in an heterogeneous and dynamic habitat
To contextualize the results of this work, which do not support latitudinal or bathymetric trends within the range studied, but on the contrary suggest that drilling predation would have a strong local imprint, it is important to mention certain characteristics of the region as a setting for the events. Studied shelled molluscs, as members of benthic communities, interact in a region characterized by its great dynamism, where two western boundary currents (the Brazil and Malvinas currents) and several oceanographic fronts converge (Martos and Piccolo, Reference Martos and Piccolo1988; Acha, et al., Reference Acha, Mianzan, Guerrero, Favero and Bava2004; Matano et al., Reference Matano, Palma and Piola2010; Dogliotti et al., Reference Dogliotti, Lutz and Segura2014). This makes it a large and rich biological area where both phytoplankton and zooplankton have very variable compositions (Sabatini and Álvarez Colombo, Reference Sabatini and Álvarez Colombo2001; Segura et al., Reference Segura, Lutz, Dogliotti, Silva, Negri, Akselman and Benavides2013; Dogliotti et al., Reference Dogliotti, Lutz and Segura2014; Sabatini et al., Reference Sabatini, Reta, Lutz, Segura and Daponte2016). There are natural physiological elements, such as water temperature and available dissolved oxygen, that would explain at least part of the differences in the species distribution, mainly for those species present only to the north or south of the area under consideration; although other physical factors such as geomorphological features, sediment types, freshwater discharges, among others, appear to play an important role in their distribution (Parker et al., Reference Parker, Paterlini, Violante and Boschi1997; Podestá, Reference Podestá and Boschi1997; Acha et al., Reference Acha, Mianzan, Guerrero, Favero and Bava2004; Violante et al., Reference Violante, Costa, Cavallotto, Paterlini, Marcolini and Bozzano2014; Sabatini et al., Reference Sabatini, Reta, Lutz, Segura and Daponte2016).
This dynamic scenario leads to a heterogeneous habitat with variations at short distances and at different depths giving rise to local benthic communities, which adopt a patchy distribution in association with different biological assemblages (Fainburg et al., Reference Fainburg, Trassens, Bastida, Farenga, Isla and Bastida2012). This spatial heterogeneity was also analysed in the light of environmental changes during the Quaternary and was referred to as a dynamic mosaic of benthic habitats (Gordillo et al., Reference Gordillo, Bernasconi, Cusminsky, Coronato and Rabassa2013; Bayer et al., Reference Bayer, Gordillo and Morsan2016). Therefore, spatial and time variability of environmental factors most probably played an important role in shaping molluscan assemblages along the PCS.
For now, for the study area, the analyses previously carried out on articulate brachiopods (collected from the same stations considered here; Gordillo et al., Reference Gordillo, Muñoz, Bayer and Malvé2018) showed that the main physical factors that would have affected the distribution of at least these suspensivorous organisms were the substrate (sediment grain size) and the currents (water flow velocities); and larger sizes appeared to be associated with higher productivity areas rich in phytoplankton. In the same way, different abiotic and biotic factors could also influence the predator–prey interaction at a local level. Also Mondal et al. (Reference Mondal, Chakraborty, Saha, Dey and Sarkar2021) suggested that the nature of substrate can determine the prey and predator composition, including life mode and taxonomic compositions at any location, influencing local drilling predation.
Thus, this complex mosaic of different habitats with the patchy distribution of suspension feeders, mainly bivalves and also brachiopods, may also have an impact on different sorts of predators, such as fishes and slow-moving gastropods (Harper, Reference Harper2016), among others. The latter also prevailed in the sampled shelled assemblages, and more than half correspond to drilling predators, which would be mainly responsible for the drill holes analysed here.
A final comment of a more general nature about these communities is linked to the problem that ocean acidification represents for marine species. The rapid acidification of the Patagonian shelf (Orselli et al., Reference Orselli, Kerr, Ito, Tavano, Mendes and Garcia2018) and the changes in water masses (Silvy et al., Reference Silvy, Guilyardi and Sallée2020; Franco et al., Reference Franco, Ruiz-Etcheverry, Marrari, Piola and Matano2022) have a significant effect on benthic communities and their biotic interactions including predation (Melatunan et al., Reference Melatunan, Calosi, Rundle, Widdicombe and Moody2013; Miller, Reference Miller2013; Kroeker et al., Reference Kroeker, Sanford, Jellison and Gaylord2014; Sanford et al., Reference Sanford, Gaylord, Hettinger, Lenz, Meyer and Hill2014; Fortunato, Reference Fortunato2015; Duquette et al., Reference Duquette, McClintock, Amsler, Pérez-Huerta, Milazzo and Hall-Spencer2017; Watson et al., Reference Watson, Fields and Munday2017; Lord et al., Reference Lord, Harper and Barry2019; Ashton et al., Reference Ashton, Freestone, Duffy, Torchin, Sewall, Tracy, Albano, Altieri, Altvater, Bastida-Zavala, Bortolus, Brante, Bravo, Brown, Buschmann, Buskey, Barrera, Cheng, Collin, Coutinho, De Gracia, Dias, DiBacco, Flores, Haddad, Hoffman, Erquiaga, Janiak, Campeán, Keith, Leclerc, Lecompte-Pérez, Longo, Matthews-Cascon, McKenzie, Miller, Munizaga, Naval-Xavier, Navarrete, Otálora, Palomino-Alvarez, Palomo, Patrick, Pegau, Pereda, Rocha, Rumbold, Sánchez, Sanjuan-Muñoz, Schlöder, Schwindt, Seemann, Shanks, Simoes, Skinner, Suárez-Mozo, Thiel, Valdivia, Velez-Zuazo, Vieira, Vildoso, Wehrtmann, Whalen, Wilbur and Ruiz2022). Therefore, it is essential to gather information about the structure and functioning of benthic communities to make better decisions regarding their conservation.
Conclusions
This work provides novel information on drilling predation for a large area of the PCS in southern Argentina. Molluscan assemblages are dominated by infaunal active burrowing bivalves and suspension feeders.
DF across all stations was moderate but varied from low to high values, and does not show a trend linked to latitude or depth. On the contrary, variations between sampled stations would respond to both biological and environmental local factors.
The main prey items were the venerids, with T. elliptica and R. exalbidus having the highest predation frequency. As main potential predators, both Muricidae and Naticidae were well represented in the molluscan assemblages, with the former being more abundant, diverse and widespread. These results are also consistent with previous works in coastal environments of Patagonia and southern South America showing that shallow infaunal venerids can be common prey of muricids. Other marks that follow other patterns (here called ‘other holes’) are tentatively attributed to octopuses, made by drilling or using the beak.
To our understanding, there is still a lack of basic information on the predator–prey interactions between molluscs living on the PCS. Therefore, more ecological studies are necessary in order to increase the knowledge on these benthic communities.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424000249
Acknowledgements
The authors thank Gustavo Lovrich (Scientific Head) for logistic support and everyone on board the R/V ARA Puerto Deseado during March/April 2012, for their cooperation and assistance during the sampling. To Marcos Tatián for the logistical support for the transfer of the samples to the city of Córdoba. We are also grateful to the editor Chris Hauton and the reviewers, Patricia Kelley and Elizabeth M. Harper, for their insightful comments and suggestions that greatly improved the manuscript. This study is a contribution to the knowledge of the benthic marine fauna on the Argentine Continental Shelf and the manuscript is a contribution to the Argentine ‘Pampa Azul’ Marine National Science Project.
Financial support
This study was carried out with financial support from Consejo Nacional de Investigaciones Científicas y Técnicas (PIP CONICET 114-200801-00260).














