1. Introduction
Williams syndrome (WS; also known as Williams–Beuren syndrome) is a rare genetic disorder that occurs in approximately 1:7,500 births (Strømme et al., Reference Strømme, Bjømstad and Ramstad2002). It is caused by a submicroscopic hemideletion of 25–27 genes on chromosome 7q11.23, and, as a consequence, a distinctive multisystem disorder appears, involving the cardiovascular, central nervous, gastrointestinal and endocrine systems, although it may affect other organ systems (for a review, see Kozel et al., Reference Kozel, Barak, Kim, Mervis, Osborne, Porter and Pober2021). Neurodevelopmental characteristics include a cognitive profile characterised by an almost universal developmental delay (i.e., most individuals diagnosed with WS present intellectual disability or borderline IQ, although some individuals may present severe intellectual disability or, to the contrary, average intellectual ability; Martens et al., Reference Martens, Wilson and Reutens2008; Mervis & Greiner de Magalhães, Reference Mervis, Greiner de Magalhães, Beauchamp, Peterson, Ris, Taylor and Yeates2022; Mervis & John, Reference Mervis and John2010; Morris et al., Reference Morris, Braddock, Chen, Trotter, Berry, Burke, Geleske, Hamid, Hopkin, Introne, Lyons, Scheuerle and Stoler2020), and a phenotypic pattern of relative peaks and valleys based on the overall intellectual ability. Among the weaknesses, visuospatial construction skills stand out (e.g., handwriting and block construction), whilst, among the strengths, linguistic (e.g., phonological processing, vocabulary breadth or verbal short-term memory) and nonverbal reasoning skills are noticeable (Mervis & Greiner de Magalhães, Reference Mervis, Greiner de Magalhães, Beauchamp, Peterson, Ris, Taylor and Yeates2022; Mervis & John, Reference Mervis and John2010; Miezah et al., Reference Miezah, Porter, Rossi, Kazzi, Batchelor and Reeve2021).
In this manuscript, we will focus precisely on analysing the evidence about performance in a general linguistic skill in WS: lexical-semantic processing. By lexical semantics, we refer to the processing of the meaning of a word (e.g., Race & Hillis, Reference Race, Hillis and Tonga2015), when presented either in isolation (e.g., in vocabulary tasks) or in context (e.g., in sentence comprehension or priming tasks). Importantly, studying lexical-semantic processing in WS may enrich the debate on the genetic/constructivist origin of certain linguistic processes, as discussed in modular and neuroconstructivist theoretical perspectives (Thomas et al., Reference Thomas, Purser and Richardson2013; Westermann et al., Reference Westermann, Thomas, Karmiloff-Smith and Goswami2011; see also below). Previous studies have shown, in a relatively consistent manner, a number of difficulties and/or strengths in WS with respect to phonological and grammatical processing. For instance, the literature on WS systematically highlights weaknesses in phonological processing, except for syllable awareness and rhyme performance (e.g., Garayzábal Heinze & Cuetos Vega, Reference Garayzábal Heinze and Cuetos Vega2008; Menghini et al., Reference Menghini, Verucci and Vicari2004). As for grammatical processing, the abilities of people with WS are similar to those of individuals with other types of intellectual disabilities matched in chronological or mental age (e.g., Gosch et al., Reference Gosch, Städing and Pankau1994; Udwin & Yule, Reference Udwin and Yule1990), with the exception of people diagnosed with Down Syndrome, who tend to show lower abilities (e.g., Bellugi et al., Reference Bellugi, Marks, Bihrle, Sabo, Bishop and Mogford1988, Reference Bellugi, Lichtenberger, Jones, Lai and St. George2000). However, the scientific literature on lexical-semantic processing in WS is much less clear.
For instance, regarding vocabulary skills, Bellugi et al. (Reference Bellugi, Marks, Bihrle, Sabo, Bishop and Mogford1988), in a seminal study, examined the cognitive and linguistic profile of three adolescents with WS, finding that, despite their intellectual disability and their difficulties with visuospatial tasks, they had an excellent vocabulary and used grammatically complex sentences (see also Bellugi et al., Reference Bellugi, Lichtenberger, Jones, Lai and St. George2000). Other studies have also reported relative strengths in WS in areas related to receptive and expressive vocabulary (Brock, Reference Brock2007; Levy & Hermon, Reference Levy and Hermon2003; Martens et al., Reference Martens, Wilson and Reutens2008). Nevertheless, some results indicate that there is a pattern of strengths and weaknesses in WS vocabulary skills: performance in concrete vocabulary tasks (names for objects, actions and descriptors) would be better than performance in relational vocabulary tasks (e.g., labels for spatial, temporal and quantitative concepts) (Garayzábal Heinze et al., Reference Garayzábal Heinze, Osório, Lens and Sampaio2014; Mervis & John, Reference Mervis and John2008, Reference Mervis and John2010). This pattern would suggest that there is not as such a strength in vocabulary skills in WS, but rather that they experience difficulties when processing certain concepts.
It is also unclear whether semantic processing/integration is spared in WS. As an example, studies using electroencephalographic recordings have found diverse patterns of semantic information processing in WS during online sentence comprehension: whilst some results suggest that there is an increased use of semantic-contextual cues in WS (vs. typical controls and individuals in the autistic spectrum) when comprehending sentences (Fishman et al., Reference Fishman, Yam, Bellugi, Lincoln and Mills2011), others point out that people diagnosed with WS may experience more difficulties when integrating semantic information than their typically developing (TD) peers (Pinheiro et al., Reference Pinheiro, Galdo-Álvarez, Sampaio, Niznikiewicz and Gonçalves2010). Results are also inconclusive in semantic priming studies: some studies report normal semantic priming effects in WS individuals (Tyler et al., Reference Tyler, Karmiloff-Smith, Voice, Stevens, Grant, Udwin, Davies and Howlin1997), whilst others show that semantic priming in WS individuals can only be compared to the abilities of TD individuals matched to the experimental group for their reading abilities, but not to the abilities of TD individuals with the same chronological age as the WS group (Lee & Binder, Reference Lee and Binder2014).
Another of the most controversial issues regarding lexical-semantic processing in WS is semantic fluency. In an influential article, Bellugi et al. (Reference Bellugi, Bihrle, Jernigan, Trauner and Doherty1990) asked individuals to provide as many examples as they could of a given category (i.e., animals), and observed that children with WS produced more responses, and more atypical (e.g., ‘chihuahua’ and ‘ibex’; i.e., less prototypical exemplars of the corresponding semantic categories), than children with Down Syndrome. These results were widely cited and taken as evidence that people diagnosed with WS showed atypical semantic processing. Nevertheless, most subsequent studies have found opposing results, indicating that there would be no overall differences between individuals with WS and other groups in terms of typicality or frequency of responses in semantic fluency tasks (Garayzábal Heinze & Cuetos Vega, Reference Garayzábal Heinze and Cuetos Vega2010; Jarrold et al., Reference Jarrold, Hartley, Phillips and Baddeley2000; Johnson & Carey, Reference Johnson and Carey1998; Levy & Bechar, Reference Levy and Bechar2003; Lukács et al., Reference Lukács, Pléh, Racsmány, Bartke and Siegmüller2004; Rossen et al., Reference Rossen, Klima, Bellugi, Bihrle, Jones, Beitchman, Cohen, Konstantareas and Tannock1996; Scott et al., Reference Scott, Mervis, Bertrand, Klein, Armstrong and Ford1995; Volterra et al., Reference Volterra, Capirci, Pezzini, Sabbadini and Vicari1996).
These controversies regarding language processing in WS have led to several hypotheses about how language skills develop in this population. The results of some of the early studies exploring language processing in WS, which suggested that the language skills of these individuals were much better than other cognitive skills (e.g., Bellugi et al., Reference Bellugi, Marks, Bihrle, Sabo, Bishop and Mogford1988), were quickly picked up by proponents of the modularity theory, the classic view which maintains that the mind contains domain-specific modules (Fodor, Reference Fodor1983). Following this theoretical approach, some authors suggested that WS was a prototypical example of the existence of a language module, since whilst other cognitive abilities were affected in this syndrome, linguistic abilities (or at least some linguistic processes) were spared (e.g., Clahsen & Almazan, Reference Clahsen and Almazan1998; Clahsen & Temple, Reference Clahsen, Temple, Levy and Schaeffer2003; Pinker, Reference Pinker1999). This strong interpretation of the modularity hypothesis, however, gradually ceased to receive support. On the other hand, weaker interpretations of modularity suggest that the ‘modules’ (or functional specialisation) are built up over the course of development by more basic innate modules. Thus, when a selective deficit is observed in cognitive functioning, this could arise from the failure of one or more modules that contribute to its development (e.g., Baron-Cohen, Reference Baron-Cohen1998). The modules or low-level factors that would contribute to impairment in the development of high-level skills (such as lexical-semantic processing) would be more general than the specific domain that is affected.
These latter ideas gave rise to neuroconstructivism, with an even greater emphasis on development (e.g., Karmiloff-Smith, Reference Karmiloff-Smith1997; Mareschal et al., Reference Mareschal, Johnson, Sirois, Spratling, Thomas and Westermann2007), following the publication of several studies showing that knowledge of grammar and morphosyntax was compromised in WS. In these studies, the authors also indicated that people with WS may learn language by using cognitive mechanisms that are different (e.g., altered) from those used by TD individuals (Elman et al., Reference Elman, Bates, Johnson, Karmiloff-Smith, Parisi and Plunkett2001; Karmiloff & Karmiloff-Smith, Reference Karmiloff and Karmiloff-Smith2001; Karmiloff-Smith, Reference Karmiloff-Smith1997, Reference Karmiloff-Smith1998; Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2005; Westermann et al., Reference Westermann, Mareschal, Johnson, Sirois, Spratling and Thomas2007). Under the neuroconstructivist perspective, if development has been impaired, it is unlikely that only one module or a reduced set of modules is impaired, and the rest have developed normally. Thus, even if in some cases behaviours are observed that fall within the normal range for some cognitive function, this could be masking subtle differences in the nature of the underlying cognitive processes. More specifically, in terms of lexical-semantic processing, the proponents of the neuroconstructivist theory suggested that, although the performance of people with WS could be similar to that of different control groups in some tasks, their semantic representations would be shallower, containing less abstract information (Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2003). Or, in other words, people with WS would show a ‘frozen’ vocabulary: the use of more complex terms than expected for their mental age could be due to the hypersociability of this population (e.g., Jones et al., Reference Jones, Bellugi, Lai, Chiles, Reilly and Lincoln2000), retrieving them from memory in an invariant way, as a mechanism to attract the attention of the interlocutor (Thomas, Reference Thomas2010). However, there would not be a deep implicit knowledge about the meaning of this vocabulary, nor a complex online processing of lexical-semantic information (Annaz et al., Reference Annaz, Van Herwegen, Thomas, Fishman, Karmiloff‐Smith and Rundblad2009).
Finally, Thomas and Karmiloff-Smith (Reference Thomas and Karmiloff-Smith2003) also proposed the ‘conservative hypothesis’, a kind of null hypothesis according to which the processes of language acquisition would be delayed, but not altered, in WS. That is, the combination of delayed development and low IQ would explain the linguistic development of people with WS. This hypothesis received considerable support over the years (e.g., Brock, Reference Brock2007; Tager-Flusberg et al., Reference Tager-Flusberg, Plesa-Skwerer, Faja and Joseph2003; Thomas, Reference Thomas2010).
Therefore, the aim of the present study is to perform a systematic search and meta-analysis on lexical-semantic processing in WS. This aims to fulfil a double purpose: on the one hand, to add some clarity to the literature on lexical-semantic processing in WS, which, as we have seen above, often offers contradictory results. A meta-analysis could also be useful to overcome some of the typical limitations in WS studies, such as the use of small samples and the wide heterogeneity (in terms of age, cognitive and linguistic abilities, etc.) between participants. On the other hand, this meta-analysis will allow us to test the three main theories that have been offered to explain linguistic development in WS: modularity, neuroconstructivism and the conservative hypothesis. If the postulates of the modularity approach (strong interpretation) were true, suggesting that linguistic skills may be spared in WS (e.g., Clahsen & Almazan, Reference Clahsen and Almazan1998; Clahsen & Temple, Reference Clahsen, Temple, Levy and Schaeffer2003), we would expect to find similar performance in lexical-semantic tasks in individuals with WS when compared to their TD peers, or at least, to TD individuals of the same mental age. However, if the neuroconstructivist hypothesis were correct, which stated that even if the performance in some lexical-semantic tasks can be optimal in WS, the internal representations are shallower (Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2003), we would expect to observe that individuals with WS would have deficits in certain cognitive mechanisms that come into play when processing lexical-semantic information (e.g., Karmiloff-Smith, Reference Karmiloff-Smith1998). Finally, if the conservative hypothesis (i.e., performance would mostly depend on the level of development of the individual, irrespective of their diagnosis) received the most support, we would expect to find similar patterns in individuals with WS, TD individuals matched for mental age and individuals with other intellectual disabilities (e.g., Thomas, Reference Thomas2010).
2. Method
2.1. Study selection and inclusion criteria
We conducted a systematic literature search in English and Spanish, covering a time-window from January 1990 to January 2023 (we selected this time-window because it is from the 1990s onwards that studies comparing experimentally the cognitive phenotype of WS with other populations began to emerge), and using the following online databases: PubMed, EMBASE, Scopus, Web of Science (including its main collection, Current Content Connect, Derwent Innovation Index, Korean Journal Database, MEDLINE, Russian Science Citation Index and SciELO Citation Index), EBSCO, ProQuest, ERIC, PsychINFO and Dialnet. Search criteria were set as: English – (Williams syndrome AND semantic*); Spanish – (síndrome Williams AND semantic*).
In the literature search, we identified a total of 581 items. After eliminating duplicate entries, 284 items remained and were assessed for eligibility based on the following inclusion criteria: (a) studies published in peer-reviewed journals; (b) between-subjects study designs; (c) direct comparison/s between WS and a control group [i.e., participants matched by chronological age; participants matched by mental age (either by overall mental age or by language/reading/vocabulary level) or participants with other disabilities]; (d) tasks measuring lexical-semantic processing and (e) effect sizes were reported or could be calculated. Finally, after examination, we found 33 studies matching our criteria. Then, we reviewed the references included in these studies, and we added nine more studies matching our criteria, obtaining a total of 42 studies that entered the meta-analysis. If a study included more than one comparison between WS and a control group, we separately coded each comparison. In total, 180 effects were obtained.
The studies included were conducted between 1991 and 2021. The sample size of the WS group varied from 1 to 69; as for the control groups, the sample size varied from 5 to 166. As previous studies on lexical-semantic processing in WS have mainly focused on three aspects: (1) vocabulary knowledge; (2) integration of lexical-semantic information in context (e.g., Bellugi et al., Reference Bellugi, Lichtenberger, Jones, Lai and St. George2000; Martens et al., Reference Martens, Wilson and Reutens2008) and (3) organisation of semantic categories (e.g., Rossen et al., Reference Rossen, Klima, Bellugi, Bihrle, Jones, Beitchman, Cohen, Konstantareas and Tannock1996), we classified the tasks used in the studies into the following categories: (a) vocabulary tasks; (b) semantic processing/integration tasks; (c) semantic memory organisation tasks (e.g., semantic fluency tasks) and (d) added verbal working memory tasks in which lexical-semantic factors were manipulated (i.e., tasks in which the lexical frequency of words, their concreteness or the level of processing with which they were studied were manipulated; see Table A.1 in the Appendix for more information on the studies and effects included in the meta-analysis).
2.2. Effect size calculation and data analysis
We used the RStudio (RStudio Team, 2015) package compute.es (Del Re, Reference Del Re2013) to calculate Hedges’ g (a standardised mean difference that accounts for sampling variance differences between conditions; Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009) for each comparison. When a study reported more than one comparison between the same groups (e.g., WS vs. participants matched by chronological age) and using the same type of task (e.g., a vocabulary task), the effect sizes were aggregated using the RStudio package MAd (Del Re & Hoyt, Reference Del Re and Hoyt2014), through the function agg. Thus, in the end, 78 effect sizes entered the meta-analysis (see Table A.1 in the Appendix for more information).
In addition to the overall meta-analysis, we performed two meta-regressions in order to assess whether the observed effect was moderated by the control group (i.e., participants matched by chronological age, participants matched by mental age or participants with other disabilities) used for comparison with the WS group, and/or by the type of task (i.e., vocabulary tasks, semantic processing/integration tasks, semantic memory organisation tasks or verbal working memory tasks) used in the studies.
Also, we used Cochran’s Q and Higgins’ I 2 to examine heterogeneity (i.e., to assess the consistency of effects across studies). Although heterogeneity may be expected in a meta-analysis, because the systematic review brings together studies that are diverse (e.g., in terms of inclusion criteria for participants, the groups that are compared, or the tasks that are used), it is important to measure to what extent this heterogeneity may affect the conclusions of the meta-analysis (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). Whilst a significant p-value in the Q test may imply that the effects are inconsistent across the studies, Higgins’ I 2 additionally allows us to measure the magnitude of the heterogeneity (i.e., the percentage of total variation across studies that is due to heterogeneity rather than chance). For instance, high levels of inconsistency in the results of the studies included in a meta-analysis may reduce the confidence in the meta-analysis outcomes (I 2 values of 25%, 50% and 75% can be categorised as low, moderate and high, respectively; Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003).
Finally, publication bias was evaluated using a funnel plot and Egger’s test (Egger et al., Reference Egger, Smith, Schneider and Minder1997), which examines the correlation between the effect sizes included in the meta-analysis and their sampling variance, and which would indicate publication bias if significant (Lin & Chu, Reference Lin and Chu2018).
All analyses were performed in JASP Version 0.14.1 (JASP Team, 2020).
3. Results
The overall random effects model (k = 78) showed a small-to-medium negative effect size, g = −.34, 95% CI [−.52, −.16], z = −3.77, p < 0.001, indicating that, in general, people diagnosed with WS performed worse than the control groups in lexical-semantic processing tasks. Total heterogeneity was significant, QT = 383.55, p < 0.001, I 2 = 82.52% (that Higgins’ I 2 value may be considered high; Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003), suggesting that the studies reported mixed effect size magnitudes. Egger’s test was not significant, z = −.61, p = 0.54, indicating no publication bias (Fig. 1).

Figure 1. Funnel plot on studies assessing lexical-semantic processing in WS. The X-axis represents the magnitude of the effect measured in the studies (Hedges’ g), while the Y-axis represents a measure of precision (i.e., standard error). Funnel plots should be symmetrical, and if this is not the case, a publication bias should be suspected (e.g., if they are not symmetrical, they could indicate that mainly studies with positive results are published, in which the measure of precision is low, due to a small sample size).
The meta-regression performed using the control group as a moderator revealed a significant effect, g = .73, 95% CI [.53, .93), z = 7.09, p < 0.001; heterogeneity remained significant, but lower, QT = 244.08, p < 0.001, I 2 = 70.15% (that Higgins’ I 2 value may be considered moderate-to-high). Subgroup analyses showed a large negative effect when comparing WS versus chronological age controls (N = 21), g = −1.07, 95% CI [−1.33, −.81], z = −8.18, p < 0.001; a small negative effect when comparing WS versus mental age controls (N = 40), g = −.24, 95% CI [−.42, −.05], z = −2.54, p = 0.01 and a small-to-medium positive effect when comparing WS versus other disabilities (N = 17), g = .39, 95% CI [.02, .76], z = 2.05, p = 0.04 (Fig. 2).

Figure 2. Random effect analyses on studies comparing WS vs. chronological age controls (CA; left panel); vs. mental age controls (MA; central panel); and vs. people diagnosed with other disabilities (right panel; DS = Down syndrome; ASD = Autism Spectrum Disorder; MLD = Moderate Learning Difficulties; MR = Mental Retardation; DLD = Developmental Language Disorder; NDD = Nonspecific developmental difficulties). Hedges’ g and confidence intervals are reported for each study. Below, the cumulative results are showed.
On the other hand, the meta-regression performed using the type of task as a moderator showed a marginally non-significant effect, g = −.17, 95% CI [−.35, .02), z = −1.79, p = 0.07; heterogeneity remained significant, QT = 369.63, p < 0.001, I 2 = 81.96% (that Higgins’ I 2 value may be considered high). Subsequent analyses showed no significant difference between WS and the control groups in vocabulary tasks (N = 22), g = −.03, 95% CI [−.32, .26], z = −.20, p = 0.84; but significant differences (negative effects) when comparing WS and the control groups in semantic processing/integration tasks (N = 18), g = −.54, 95% CI [−.97, −.11], z = −2.48, p = 0.01; semantic memory organisation tasks (N = 33), g = −.41, 95% CI [−.68, −.15], z = −3.03, p = 0.002 and verbal working memory tasks (N = 5), g = −.56, 95% CI [−1.02, −.10], z = −2.38, p = 0.02 (Fig. 3).

Figure 3. Random effect analyses on studies comparing WS vs. different control groups in vocabulary tasks (leftmost panel), semantic processing/integration tasks (center-left panel), semantic memory organization tasks (center-right panel), and verbal working memory tasks (rightmost panel). Hedges’ g and confidence intervals are reported for each study. Below, the cumulative results are showed.
4. Discussion
In this study, we had a twofold purpose: on the one hand, to bring some stability to the field of lexical-semantic processing in WS, which so far has obtained very contradictory findings; and, on the other hand, to test different theories about linguistic development in WS. In order to achieve these goals, we carried out a meta-analysis on lexical-semantic skills in WS, including 42 studies and 180 effects (later aggregated when, within a study, the same groups were compared using the same type of task, providing the final number of 78 effect sizes that were included in the meta-analysis). In a nutshell, our results show that:
-
• Individuals with WS have worse lexical-semantic skills than TD individuals, whether matched by chronological or mental age.
-
• On the contrary, individuals with WS have better lexical-semantic skills than people diagnosed with other disabilities (i.e., Down Syndrome, autism spectrum disorder, moderate learning difficulties, mental retardation or developmental language disorder).
-
• Whilst there are no significant differences in performance in vocabulary tasks between individuals with WS and the different control groups, there are significant differences in semantic processing/integration tasks, semantic memory organisation tasks and verbal working memory tasks.
The first observation is contrary to strong interpretations of the modularity approach. Under this theoretical framework, the mind would contain domain-specific modules (e.g., a module specialised in processing language, or in certain language features; Fodor, Reference Fodor1983). As we indicated in the introduction, some followers of this perspective suggested that WS might be a prototypical example of the modularity of the mind (e.g., Clahsen & Almazan, Reference Clahsen and Almazan1998; Clahsen & Temple, Reference Clahsen, Temple, Levy and Schaeffer2003), since some of the early studies on cognitive and linguistic development in WS seemed to indicate that language skills were spared in people diagnosed with this syndrome, whereas other cognitive abilities, such as visuospatial skills, were negatively affected (e.g., Bellugi et al., Reference Bellugi, Marks, Bihrle, Sabo, Bishop and Mogford1988). However, the observation that, in general, individuals with WS display worse lexical-semantic skills than TD individuals, even when matched by mental age, is contrary to this postulate. If there really were a mental module specialised in linguistic processing (or in lexical-semantic processing), and this was spared in WS, we would not expect to find differences with respect to TD individuals. On the other hand, our results could be compatible with weaker versions of the modular hypothesis. For example, just as it has been argued that deficits in grammar in specific language impairment might be due to an initial deficit in processing information about speech sounds (e.g., Joanisse & Seidenberg, Reference Joanisse and Seidenberg1998), or to a limitation of processing capacity (e.g., Bishop, Reference Bishop1994), difficulties at the lexical-semantic processing level in WS might be due to deficits in innate basic modules, or general processes, that would affect the development of lexical-semantic skills.
More surprising are the observations that, in general, individuals with WS have better lexical-semantic skills than people with other cognitive disabilities, and that they do not seem to be particularly impaired in their ability to perform vocabulary tasks. These results seem to go against the conservative hypothesis about cognitive and linguistic development in WS, which has perhaps been the theoretical perspective that has received the most support in this field in recent years. More specifically, this hypothesis suggests that language development in WS is usually delayed, as a function of the IQ of each individual, so that if a person diagnosed with WS has a low IQ, they will have a more delayed language development than another person with a higher IQ (e.g., Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2003). Although the most optimal way to test the conservative hypothesis within WS would be to obtain the IQ of each participant and contrast it with their level of lexical-semantic development, unfortunately, the studies reviewed in this meta-analysis do not provide us with the information to perform this analysis. Nevertheless, we can test the conservative hypothesis through different disabilities. In most of the studies we included comparing WS versus other cognitive disabilities, both groups were matched by mental age (see Table A.1 in the Appendix), so the observation that individuals with WS reached a better performance than people with other disabilities in lexical-semantic tasks could be taken as evidence against the conservative hypothesis. Also, although it is true that most of these studies used people with Down Syndrome as a control group, and these people usually present relative weaknesses in some linguistic processes (Silverman, Reference Silverman2007), differences between WS and autism spectrum disorder, for example, are remarkable in some of the studies included in the meta-analysis, and even more so if we consider that the group with autism spectrum disorder had a higher IQ than the WS group (Fishman et al., Reference Fishman, Yam, Bellugi, Lincoln and Mills2011). But, of course, future studies in this field should try to broaden the range of disabilities used as a control group to compare with WS.
In addition, finding that individuals with WS present similar abilities in vocabulary tasks when compared to the control groups, whilst they present worse semantic processing/integration, semantic memory organisation and verbal working memory abilities, fits better with the neuroconstructivist perspective than with the conservative hypothesis. More concretely, the former theory claims that, even though performance in some lexical-semantic tasks might be similar between individuals with WS and some control groups, their semantic representations might be shallower, and the cognitive mechanisms responsible for these tasks might be different, or work differently during the tasks (Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2003). This would fit perfectly with our results, since, although people with WS may have proficient vocabulary skills (i.e., they may access words, or word knowledge information), other skills could be impaired, such as semantic processing, or the retrieval of semantic relationships between different items, as suggested by the results obtained for semantic processing/integration tasks and semantic memory organisation tasks. Thus, despite their striking drive to engage with other people (e.g., Jones et al., Reference Jones, Bellugi, Lai, Chiles, Reilly and Lincoln2000; Klein-Tasman & Mervis, 2003), which could lead them to acquire advanced vocabulary skills for their mental age, the cognitive mechanisms responsible for language processing would be altered in individuals with WS, or different mechanisms would come into play compared to language processing in TD individuals (Thomas & Karmiloff-Smith, Reference Thomas and Karmiloff-Smith2003). This, in turn, could affect pragmatic skills in WS, as shown in previous studies (e.g., Stojanovik, Reference Stojanovik2006).
Within the neuroconstructivist framework, WS and other developmental disorders could be explained through altered constraints that generate atypical developmental trajectories. For example, genetic effects during brain development in WS could generate different neurocomputational properties in certain cortical structures compared with typical developmental patterns (Thomas et al., Reference Thomas, Purser and Richardson2013). Also, differences in input encoding may have cascading effects on the acquisition of other cognitive abilities. For instance, alterations in the level of abstraction achieved when acquiring internal representations, or in the encoding of those representations, may affect the way other cognitive functions employ that information to drive different processes (Westermann et al., 2010). Thus, an extensive practice (e.g., in WS there would be a substantial vocabulary practice due to the socioemotional rewards of interacting with other people) may enable a suboptimal system to achieve normal performance in a task (e.g., vocabulary task) that is relatively insensitive to how information is processed to achieve that level of performance. For that matter, just as adults with WS have been shown to perform in the normal range in face recognition tasks, even though the underlying neural activity is different compared with TD controls (Grice et al., Reference Grice, Spratling, Karmiloff-Smith, Halit, Csibra, de Haan and Johnson2001), future research in this field should deepen the study on the underlying neural patterns of vocabulary and lexical-semantic processes in WS.
As for what previous literature indicated about the performance of individuals with WS on different lexical-semantic tasks, our study also provides some clarity. Regarding vocabulary skills, our results confirm that this is a relative strength in WS (e.g., Brock, Reference Brock2007; Levy & Hermon, Reference Levy and Hermon2003; Martens et al., Reference Martens, Wilson and Reutens2008). However, the effects included in the meta-analysis do not allow us to confirm whether there are differences between concrete versus relational vocabulary processing, as indicated by some studies (e.g., Garayzábal Heinze et al., Reference Garayzábal Heinze, Osório, Lens and Sampaio2014; Mervis & John, Reference Mervis and John2008, Reference Mervis and John2010). Concerning semantic processing/integration skills, our results suggest that individuals with WS present difficulties in this type of task, contradicting previous studies that proposed that these skills are spared in WS (e.g., Tyler et al., Reference Tyler, Karmiloff-Smith, Voice, Stevens, Grant, Udwin, Davies and Howlin1997), or even that individuals with WS use semantic information to a greater extent than people with typical development to derive meaning during sentence comprehension (Fishman et al., Reference Fishman, Yam, Bellugi, Lincoln and Mills2011). With respect to semantic memory organisation, our results differ from previous observations suggesting that individuals with WS are able to handle this type of information with ease, being capable, for instance, to access numerous exemplars of semantic categories (e.g., Bellugi et al., Reference Bellugi, Bihrle, Jernigan, Trauner and Doherty1990). However, it is true that the results of this meta-analysis do not allow us to explore in depth whether semantic memory organisation itself might be different in this population (i.e., whether the semantic relationships established in the mental lexicon are similar/dissimilar between WS and other neurodevelopmental profiles); future studies in this field should continue to explore this issue. Finally, our results suggest that people with WS do not make use of the lexical-semantic information present in the studied materials to improve their performance in verbal working memory tasks, contradicting certain claims made in previous studies in this field (e.g., Greer et al., Reference Greer, Hamiliton, Riby and Riby2014; Laing et al., Reference Laing, Grant, Thomas, Parmigiani, Ewing and Karmiloff-Smith2005).
Before concluding, we would like to discuss some potential limitations of our study. First, we observed a high heterogeneity between the different studies included in the meta-analysis, which suggests that the observed effects were inconsistent across studies. Although heterogeneity is to some extent unavoidable in a meta-analysis, as the included studies differ in various aspects, the observed values were generally high. This heterogeneity could be explained, at least partially, by the fact that people with WS present very diverse cognitive and linguistic profiles (e.g., Mervis et al., Reference Mervis, Morris, Bertrand, Robinson and Tager-Flusberg1999, Reference Mervis, Robinson, Bertrand, Morris, Klein-Tasman and Armstrong2000; Porter & Coltheart, Reference Porter and Coltheart2005), and only part of this cognitive variability is explained by variation in genetics (Porter et al., Reference Porter, Dobson-Stone, Kwok, Schofield, Beckett and Tassabehji2012; Serrano-Juárez et al., Reference Serrano-Juárez, Venegas-Vega, Yáñez-Téllez, Rodríguez-Camacho, Silva-Pereyra, Salgado-Ceballos and Prieto-Corona2018). Therefore, the results of our meta-analysis should be taken with caution, since they refer to WS as a whole, but certain people diagnosed with this syndrome could present a diverse pattern of lexical-semantic abilities. Importantly, though, heterogeneity levels were reduced from high to moderate-to-high when we introduced the control group as a moderator in the meta-regression, pointing out that a relevant part of the overall variability between studies was due to the fact that in some cases individuals with WS were being compared with TD individuals (matched either by chronological or mental age), whilst in other cases, they were being compared with people with other disabilities. Additionally, another potential source of heterogeneity between the different studies included in the meta-analysis could be the language spoken by the participants. Although it is difficult to estimate to what extent this factor could have increased the heterogeneity between studies, since on occasions the number of articles included in the meta-analysis in which the participants spoke a certain language (e.g., Spanish) was quite small, we must acknowledge that this is another potential source of variability between studies.
Second, the results of vocabulary tasks should also be taken with some caution. To begin with, the comparisons by task type follow from the meta-regression carried out with the moderator type of task, in which the p-value had a marginally non-significant value (p = 0.07). Despite this, we kept on exploring the performance of individuals with WS on the different tasks, as we found this to be an interesting cue. And, to continue, none of the vocabulary tasks used in the studies applied online or implicit measures that might index whether, although individuals with WS may be able to access word knowledge, the underlying processing might be impaired. Future studies in this area should use online measures (e.g., electroencephalographic recordings) to continue exploring whether lexical and semantic access processes are altered in WS.
To conclude, in this study, we show that people with WS have worse lexical-semantic skills than TD individuals, even when both groups are matched by mental age. However, individuals with WS appear to have better lexical-semantic skills than individuals diagnosed with other cognitive disabilities. Furthermore, they seem to have no difficulties in completing vocabulary tasks, whereas they do show deficits in semantic processing/integration tasks, semantic memory organisation tasks and verbal working memory tasks in which lexical or semantic factors are manipulated. These results support the neuroconstructivist hypothesis, according to which the cognitive mechanisms involved in lexical-semantic processing might be impaired, even when performance in some tasks might be optimal (e.g., Karmiloff-Smith, Reference Karmiloff-Smith1997, Reference Karmiloff-Smith1998). We hope that these results will bring some clarity to a field of study in which the available results were very contradictory at times and that they will encourage further research on the development of the cognitive mechanisms involved in language acquisition in WS.
Acknowledgements
We would like to thank Miguel Vadillo for his help throughout the preparation of the manuscript.
Data availability statement
The data used to perform the analyses are uploaded to the Open Science Framework repository at the following link: https://osf.io/2ab9g/?view_only=None.
Funding statement
This study was funded by the Ministry of Science and Innovation of the Government of Spain (Grant No. PID2019-108092GA-I00/AEI/10.13039/501100011033; PI: CRR).
Author contribution
Conceptualisation: C.R.-R.; Data curation: all authors; Formal analysis: C.R.-R.; Funding acquisition: C.R.-R., S.R.-C., E.G.H.; Investigation: all authors; Methodology: C.R.-R., S.R.-C.; Project administration: C.R.-R.; Resources: C.R.-R.; Supervision: C.R.-R.; Validation: C.R.-R.; Visualisation: C.R.-R.; Writing – original draft: C.R.-R.; Writing – review and editing: all authors.
Competing interest
The authors declare none.
Appendix
Table A.1. Effects, studies, participants and tasks included in the meta-analysis
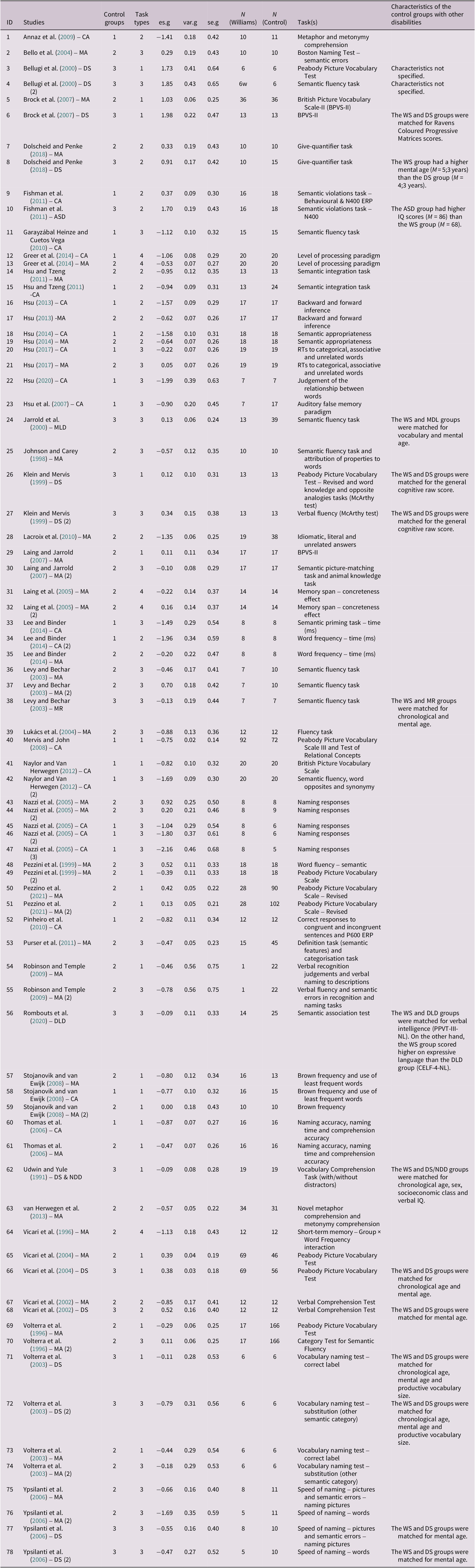
Note: Control group (1 = chronological age; 2 = mental age; 3 = other disabilities). Task type (1 = vocabulary task; 2 = semantic integration/processing task; 3 = semantic memory organisation task; 4 = verbal working memory task). es.g = Hedges’ g. var.g = Hedges’ g variance. se.g = Hedges’ g standard error.







