Introduction
The average prevalence of psychiatric disorders was about 22.1% with the severe disorders (schizophrenia, bipolar disorder, severe depression, severe anxiety and severe post-traumatic stress disorder) estimated to be 5.1% (Charlson et al., Reference Charlson, van Ommeren, Flaxman, Cornett, Whiteford and Saxena2019). In children, the prevalence has been reported to be about 6.7% but varies between different countries (Erskine et al., Reference Erskine, Baxter, Patton, Moffitt, Patel, Whiteford and Scott2017). The use of antipsychotics has increased over the years and has become one of the mainstays for the treatment of psychiatric disorders in children, despite lingering concerns over side effects (Harrison et al., Reference Harrison, Cluxton-Keller and Gross2012; Lao et al., Reference Lao, Tam, Wong, Besag, Man, Chui and Chan2017; Lee et al., Reference Lee, Song, Han and Kang2018). Specifically, in such young populations, antipsychotic medications may induce cardiovascular and metabolic abnormalities (such as obesity, hyperglycaemia, dyslipidaemia and diabetes mellitus) that could affect their physical, mental and social development (Hsu et al., Reference Hsu, Chien, Tan, Lin, Cheng, Chou and Chou2013). Moreover, some life-threatening cardiovascular side effects of antipsychotics such as stroke, ischaemic heart disease (IHD) and acute myocardial infarction (AMI) have also been reported (De Hert et al., Reference De Hert, Detraux, van Winkel, Yu and Correll2011). Meanwhile, increasing prescribing of antipsychotics is observed among children and young adults (Olfson et al., Reference Olfson, King and Schoenbaum2015), which leads to great concern regarding the safety of antipsychotics (Pillay et al., Reference Pillay, Boylan, Newton, Hartling, Vandermeer, Nuspl, MacGregor, Featherstone and Carrey2018). Currently available studies mainly focus on the outcomes of weight change or shifts in metabolic parameters over a short period of time (Sjo et al., Reference Sjo, Stenstrøm, Bojesen, Frølich and Bilenberg2017; Vandenberghe et al., Reference Vandenberghe, Najar-Giroud, Holzer, Conus, Eap and Ambresin2018). Not much is known about the specific risk of cardiovascular and metabolic events in children and young adults treated with antipsychotics, as previous studies were mainly of small sample size and thus may not have developed sufficient statistical power in their analyses (McIntyre and Jerrell, Reference McIntyre and Jerrell2008; Burcu et al., Reference Burcu, Zito, Safer, Magder, dosReis, Shaya and Rosenthal2018).
The risk of antipsychotic-associated cardiovascular and metabolic events may differ among countries (Man et al., Reference Man, Shao, Chaiyakunapruk, Dilokthornsakul, Kubota, Li, Ooba, Pratt, Pottegård, Rasmussen, Roughead, Shin, Su, Wong, Kao Yang and Lai2020). This could potentially be due to variations in healthcare systems and preferences of patients and clinicians in the choice of antipsychotics (Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley, Beck, Natesan, Efthimiou, Cipriani and Howes2020). From the genetic perspective, the variant HTR2C gene, which encodes the 5HT2c receptor, may increase metabolic risk, especially by the rs 1414334 C allele (Ma et al., Reference Ma, Maimaitirexiati, Zhang, Gui, Zhang, Xu and Hu2014). A survey indicated that this allele is present in a higher proportion of Americans (10%) and Europeans (15%), but, by contrast, is of very low frequency in Asians (1%). This suggests that variations between different ethnicities could affect the risk of cardiovascular and metabolic events (Mulder et al., Reference Mulder, Cohen, Scheffer, Gispen-de Wied, Arends, Wilmink, Franke and Egberts2009). To date, only limited evidence has been available comparing the corresponding risks among children and young adults receiving antipsychotics between different populations in real-world situations. We, therefore, conducted the current study with four large population-based datasets from Taiwan, Korea, Hong Kong and the UK, which have coverage of approximately 100 million individuals in total, to evaluate and benchmark the risk of specific cardiovascular events (stroke, IHD and AMI) and metabolic events (hypertension, T2DM and dyslipidaemia), associated with antipsychotics in children and young adults.
Method
Database sources
We included databases from Taiwan (Taiwan's National Health Insurance Database; NHID), Korea (Korea's NHID), Hong Kong (Clinical Data Analysis and Reporting System; CDARS) and the UK (The Health Improvement Network; THIN) in this study (Hsieh et al., Reference Hsieh, Su, Shao, Sung, Lin, Kao Yang and Lai2019; Ilomäki et al., Reference Ilomäki, Bell, Chan, Tolppanen, Luo, Wei, Lai, Shin, De Paoli, Pajouheshnia, Ho, Reynolds, Lau, Crystal, Lau, Man, Brauer, Chan, Shen, Kim, Lum, Hartikainen, Koponen, Rooke, Bazelier, Klungel, Setoguchi, Pell, Cook and Wong2020). Additional details about the included databases are presented in Supplementary Table 1. We applied a distributed network approach with a common data model (CDM) (Lai et al., Reference Lai, Man, Chaiyakunapruk, Cheng, Chien, Chui, Dilokthornsakul, Hardy, Hsieh, Hsu, Kubota, Lin, Liu, Park, Pratt, Roughead, Shin, Watcharathanakij, Wen, Wong, Yang, Zhang and Setoguchi2015). Briefly, the coordination centre distributed the common SAS program for analysis, generating aggregated results based on the CDM that standardised the structures and contents of participating databases, and collected the final summary of results from each participating site (Lai et al., Reference Lai, Man, Chaiyakunapruk, Cheng, Chien, Chui, Dilokthornsakul, Hardy, Hsieh, Hsu, Kubota, Lin, Liu, Park, Pratt, Roughead, Shin, Watcharathanakij, Wen, Wong, Yang, Zhang and Setoguchi2015). This approach preserved the confidentiality of the data because the raw data stayed in the local site while the analyses were executed respectively by the sites (Lai et al., Reference Lai, Ryan, Zhang, Schuemie, Hardy, Kamijima, Kimura, Kubota, Man, Cho, Park, Stang, Su, Wong, Kao and Setoguchi2018a). Moreover, we could maintain the consistency of analysis among the sites through the use of the common analysis program (Lai et al., Reference Lai, Shin, Kubota, Man, Park, Pratt, Roughead, Wong, Kao Yang and Setoguchi2018b). The details of the mapping codes for diagnosis and the CDM are presented in Supplementary Table 2 and Supplementary Table 3. The study has been approved by the Human Research Ethics Committee at National Cheng Kung University (No. NCKU HREC-E-105-259-2); the University of Hong Kong/Hospital Authority Hong Kong West Cluster (No. UW15-619); The Health Improvement Network Scientific Review Committee (19THIN087) and the Institutional Review Board of Sungkyunkwan University (SKKU 2018-04-106).
Study design
We applied a self-controlled case series (SCCS) design to investigate the association between antipsychotics and risk of metabolic/cardiovascular event, whereby the relative risk is estimated based on within-person comparisons rather than between-person comparisons (Hallas and Pottegård, Reference Hallas and Pottegård2014; Petersen et al., Reference Petersen, Douglas and Whitaker2016). As a result, both measured and unmeasured time-independent confounding factors, such as sex, ethnicity, environmental and cultural factors are eliminated. The design is especially important for multinational studies, benchmarking results from different countries with very heterogeneous healthcare conditions (Lai et al., Reference Lai, Ryan, Zhang, Schuemie, Hardy, Kamijima, Kimura, Kubota, Man, Cho, Park, Stang, Su, Wong, Kao and Setoguchi2018a).
Source population and exposure
We included patients aged 6–30 years diagnosed with a mental disorder (ICD-9-CM: 290–319) who were newly receiving oral antipsychotic drugs between 2004 and 2012 in Taiwan, 2010 and 2016 in Korea, 2001 and 2014 in Hong Kong and 1997 and 2016 in the UK. Incident use of antipsychotics was captured based on a 1-year washout period before the first record of antipsychotic prescription in the database. We excluded patients who had a record of congenital disorders, including congenital heart disease, familial hypercholesterolaemia and type 1 diabetes. We also excluded patients who had a cancer diagnosis record. Details of diagnostic codes are presented in Supplementary Table 3.
Case identification and ascertainments
We included patients who had a record of the outcome of interest (cardiovascular events included AMI, IHD and stroke, and metabolic events included hypertension, dyslipidaemia and T2DM) for the analyses. To improve the validity of diagnoses of metabolic events, we confirmed cases by the records for corresponding drug prescriptions, including oral hypoglycaemic agents (A10B) for T2DM, lipid-modifying agents (C10) for dyslipidaemia and antihypertensive drugs including diuretics (C03A and C03B), beta-blockers (C07, except propranolol; C07AA05), calcium channel blockers (C08CA) and angiotensin-converting enzyme inhibitors (ACEI) / angiotensin receptor blockers (ARB) (C09).
Definition of risk periods
We defined the observation period based on the availability of data sources as mentioned in the previous section. Observational periods began on the first available date in the corresponding database, or the sixth birthday of the patient (whichever was later) and ended on the last available date in the corresponding database, the 31st birthday of the patient, or registered date of death (whichever was earlier). For each included participant, we defined the risk periods with respect to the duration of exposure to antipsychotics and categorised them into five mutually exclusive windows: (1) 90 days before (pre-exposure), (2) 1–30 days, (3) 31–90 days, (4) more than 90 days of antipsychotics use and (5) 30-day after the end of antipsychotics use (post-exposure) (Fig. 1). Details of ATC codes for antipsychotics are presented in Supplementary Table 4. A pre-exposure period was added to take account of the possibility that the outcome of interest may affect the likelihood of antipsychotics treatment, which in turn may introduce bias into the risk estimate during treatment whereas the post-exposure period acts as a washout period. To manage possible confounding effects due to age, we performed 1-year age banding for all patients in the analysis (Petersen et al., Reference Petersen, Douglas and Whitaker2016).

Fig. 1. Schematic presentation of self-control case series.
Statistical analysis
We report characteristics of patients included in the analyses by countries and describe categorical variables (e.g., sex) by number with proportion and continuous variables (e.g., age) by mean with standard deviation (SD). We considered the entire study period and categorised the risk periods in a time-varying manner, i.e. the exposure status was updated according to the treatment time. We calculate incidence rate ratio (IRR) and 95% confidence interval (95% CI) by conditional Poisson regression, adjusted for age in 1-year age bands to evaluate the risk of specific cardiovascular and metabolic events associated with antipsychotics in the different risk windows (Petersen et al., Reference Petersen, Douglas and Whitaker2016). IRRs for each risk window in each site were pooled by the random-effect model (DerSimonian and Laird, Reference DerSimonian and Laird1986). The Taiwanese and Korean databases provided a sufficient number of cases to conduct a secondary analysis for the risk comparisons between individual antipsychotics.
Results
Antipsychotic users from each country
We identified a total of 107 425 patients from Taiwan, 284 843 from Korea, 19 034 patients from Hong Kong and 7770 patients from the UK. The mean age (±SD) upon receiving the first prescription was 21.1 ± 6.7 years in Taiwan, 23.3 ± 5.3 years in Hong Kong and 24.9 ± 3.8 years in the UK. We found more males than females in Taiwan (61%), Korea (60%) and the UK (64%) and about the same males and females in Hong Kong (Table 1). The patterns and rates of antipsychotics use are presented in Fig. 2. The proportion of antipsychotics use varied among countries. The most commonly prescribed drugs at initiation were sulpiride in Taiwan, risperidone in Korea, haloperidol in Hong Kong and olanzapine in the UK.
Table 1. Baseline characteristics of all antipsychotic users included in the analysis

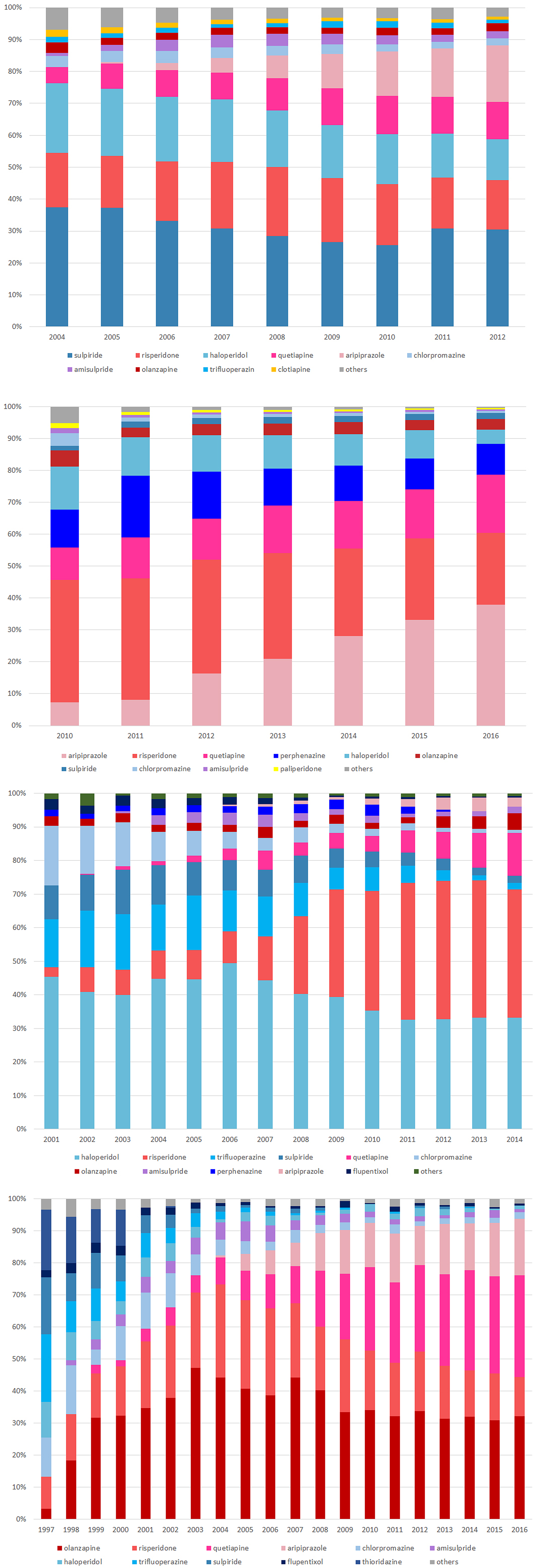
Fig. 2. Utilisation pattern of antipsychotics among countries: (a) Taiwan (b) Korea (c) Hong Kong (d) UK.
Pooled analysis
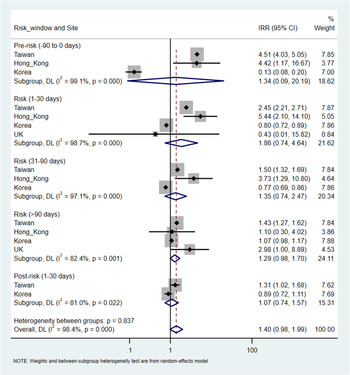
There were a total of 9730 (overall incidence rate, 11.5 per 100 person-years), 38 432 (12.4 per 100 person-years), 233 (7.6 per 100 person-years) and 120 (11.9 per 100 person-years) patients with at least one record of cardiovascular or metabolic events in Taiwan, Korea, Hong Kong and the UK, respectively. An increased risk of metabolic events was observed with more than 90-days of antipsychotics exposure with the pooled IRR of 1.29 (95% CI 1.20–1.38), but not in other risk windows. The corresponding IRR was 0.98 (95% CI 0.90–1.06) for 1–30 days and 0.88 (0.76–1.02) for 31–90 days of antipsychotics exposure. The I2 for heterogeneity ranged from 0% to 39.5% in the pooled estimates from metabolic events. For cardiovascular events, no significant association was identified in any risk windows in the pooled analysis. The pooled IRR was 1.86 (0.74–4.64) for 1–30 days, 1.35 (0.74–2.47) for 31–90 days and 1.29 (0.98–1.70) for more than 90-days of antipsychotics exposure, however, with high heterogeneity (I 2 ranged from 82.4% to 98.7%). No significant associations were found in the pre- and post-exposure windows for both outcomes (Figs 3 and 4).

Fig. 3. Pooled estimates of risk in metabolic events.

Fig. 4. Pooled estimates of risk in cardiovascular events.
Analysis by sites and specific events
The results of SCCS from individual sites varied. We found the risks of metabolic events were higher in the risk period of 90 + days in Taiwan and Korea. The IRR were 1.27 (1.12–1.45) and 1.48 (1.38–1.59) for hypertension, 1.35 (1.12–1.64) and 1.38 (1.28–1.49) for T2DM and 1.36 (1.20–1.55) and 1.36 (1.28–1.49) for dyslipidaemia in Taiwan and Korea, respectively. However, the risk of metabolic events in the period of 90 + days was higher but not reached statistical significance in Hong Kong and the UK (Table 2).
Table 2. Risk of metabolic events among countries

NHID, National Health Insurance Database; CDARS, Clinical Data Analysis and Reporting System, THIN, The Health Improvement Network, DM, diabetes mellitus, N/A, not available.
Compared to the non-exposure period, we found the risk of stroke to be higher in the pre-exposure period (5.49; 4.72–6.38), 1–30 days (3.51; 3.07–4.01), 31–90 days (2.06; 1.75–2.42) and 90 + days (2.07; 1.77–2.43) and the risk of IHD (1.59; 1.35–1.86) and AMI was higher in the pre-exposure period (6.42; 2.86–14.45) and the risk period of 1–30 days (2.29; 0.91–5.78) in Taiwan (Table 3). We did not find an association between antipsychotics and cardiovascular events in Korea, except for a higher risk of stroke in the period of 90 + days (1.38; 1.34–1.41) comparing with the non-exposure period. In Hong Kong, we found the risk of cardiovascular events, specifically for stroke (5.80; 1.91–17.61) was higher in the risk period of 1–30 days compared to the non-exposure period. We did not find any association between the use of antipsychotics and the risk of cardiovascular events in the UK.
Table 3. Risk of cardiovascular events among countries
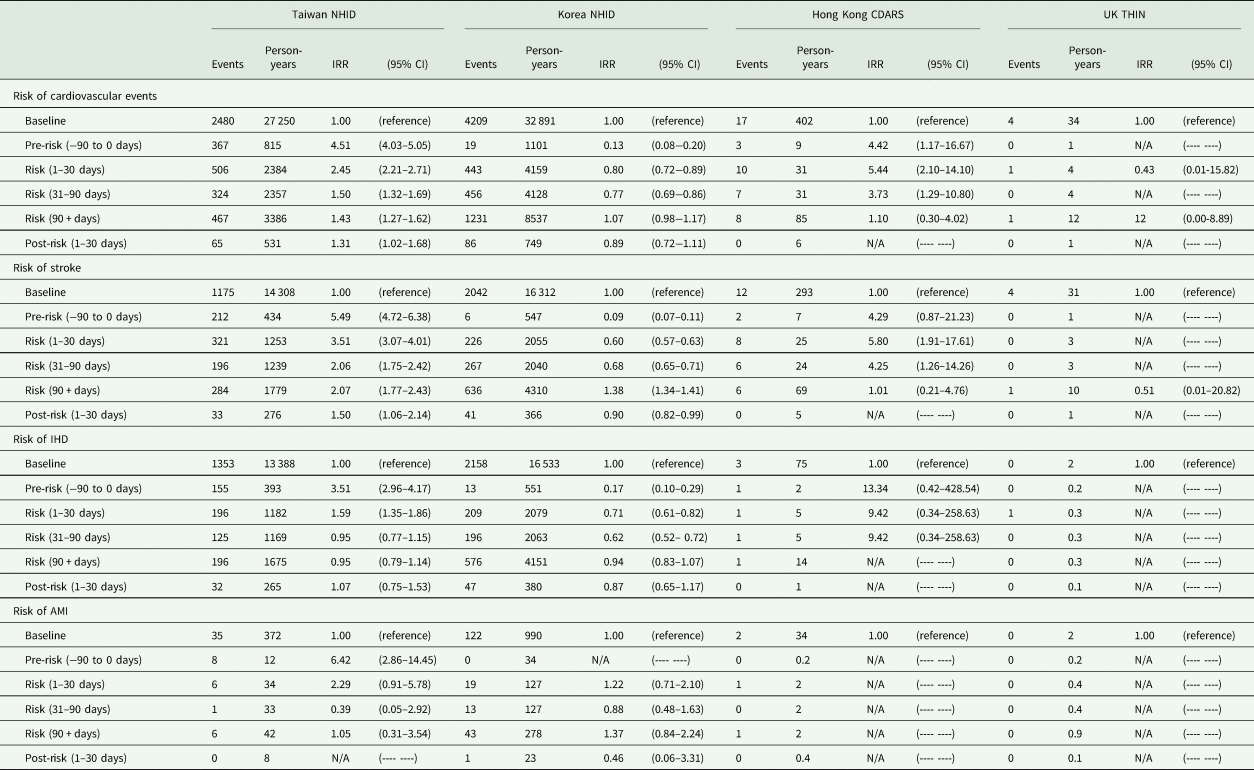
NHID, National Health Insurance Database; CDARS, Clinical Data Analysis and Reporting System; THIN, The Health Improvement Network; IHD, ischaemic heart disease, AMI, acute myocardial infarction; N/A, not available.
Discussion
Previous studies evaluating the safety of antipsychotics in young people were generally with a limited sample size to acquire a precise estimation (McIntyre and Jerrell, Reference McIntyre and Jerrell2008; Burcu et al., Reference Burcu, Zito, Safer, Magder, dosReis, Shaya and Rosenthal2018). The current study used four large databases from Taiwan, Korea, Hong Kong and the UK to evaluate the risk of cardiovascular and metabolic events associated with antipsychotics in children, adolescents and young adults. We identified an increased risk of metabolic events with exposure to antipsychotics for more than 90 days from the pooled results. However, no significant association was found between the use of antipsychotics and the risk of cardiovascular events. This suggested that the increased risk in the metabolic events may not be severe enough to trigger cardiovascular events in children, adolescents and young adults. Despite the varied risk pattern among countries, the conclusion is consistent with the overall results.
The results of our study were largely consistent with previous studies regarding the association between the risk of metabolic events with antipsychotics use (Man et al., Reference Man, Shao, Chaiyakunapruk, Dilokthornsakul, Kubota, Li, Ooba, Pratt, Pottegård, Rasmussen, Roughead, Shin, Su, Wong, Kao Yang and Lai2020). However, we found an increased risk of metabolic events only with the antipsychotics exposure for more than 90 days, implicating it may require a period of accumulative exposure of drugs to develop metabolic events. Our pooled analysis did not support the use of antipsychotics was associated with cardiovascular events. However, the finding was based on the results from countries with high heterogeneity. We found an increased risk of cardiovascular events in Taiwan and a specifically higher risk of stroke in Hong Kong in the first 30 days of antipsychotics treatment. In particular, we found patients receiving haloperidol had an increased risk of stroke in the initial stage of treatment in both Taiwan and Hong Kong. Haloperidol has a high affinity for binding to α1 and α2 receptors (Hensiek and Trimble, Reference Hensiek and Trimble2002), and the blockade of α receptors could cause fluctuations in blood pressure along with some symptoms such as hypotension, hypertension and QT interval prolongation, leading to a high risk of cardiovascular events (Cooper et al., Reference Cooper, Reynolds, Barnes, England, Haddad, Heald, Holt, Lingford-Hughes, Osborn, McGowan, Patel, Paton, Reid, Shiers and Smith2016; Hiremath et al., Reference Hiremath, Ruzicka, Petrcich, McCallum, Hundemer, Tanuseputro, Manuel, Burns, Edwards, Bugeja, Magner, McCormick, Garg, Rhodes and Sood2019). However, a more parsimonious interpretation of this pattern of temporal association is that the observed increased risk of cardiovascular events is not due to antipsychotics but precedes it because an increased risk was also observed in 90 days pre-exposure period from both Taiwan and Hong Kong. The changes in behavioural and mental health symptoms or associated impairment that lead to a medical consultation or comorbidities, which in turn may contribute to the decision to prescribe antipsychotics.
Besides, it is noteworthy that different risk profiles among countries could be attributed to different patterns of antipsychotic or prescribing preferences among the countries. From the study cohorts, we found that SGA uses increased over the years in Korea and the UK, and until 2016, SGA accounted for more than 80% of total antipsychotics use in children, adolescents and young adults. The frequent use of SGA may explain the higher risk of metabolic events within the observation window of more than 90 days after drug initiation, compared to the non-exposure period. We suggest interpreting the results cautiously considering countries’ specific situations. For instance, the healthcare accessibility or copayment of medical treatment may be different among countries, leading to various thresholds for seeking medical treatment (Lai et al., Reference Lai, Shin, Kubota, Man, Park, Pratt, Roughead, Wong, Kao Yang and Setoguchi2018b). The possibilities of the capture of events were different among countries. Moreover, the differences in the respective healthcare systems, cultures, behaviours of prescribing, preferences of clinicians among countries may also contribute to the heterogeneity of the results. Therefore we could not make inferences on the ethnic differences regarding the adverse effects in our study.
Limitations
We were unable to assess the actual medication adherence of patients, which may cause a bias towards null because patients may or may not be taking the medication. The self-controlled design eliminated time-constant unmeasured confounders, but the results may be influenced by time-variant factors that were not associated with age (Hallas and Pottegård, Reference Hallas and Pottegård2014; Petersen et al., Reference Petersen, Douglas and Whitaker2016). Protopathic bias should be noted with patients who had undetected cardiovascular events that caused psychosis and the use of antipsychotics. Because clinicians may avoid antipsychotics which are well known to increase metabolic side effects, such as olanzapine for patients with higher baseline risk, the sample size and power of the study may be decreased. On the other hand, because metabolic monitoring is not being implemented routinely in all study countries, we may omit some patients with mild metabolic syndrome.
Conclusion
Exposure to antipsychotics for more than 90 days was associated with an increased risk of a metabolic event, but did not trigger cardiovascular events in children and young adults. Although we found varied risk profiles of cardiovascular and metabolic events between countries, the conclusion remained consistent with the overall results. Nevertheless, clinicians should be mindful of the possible cardiovascular and metabolic risk as with the use of all antipsychotics in children and young adults in clinical practice while long-term use of antipsychotics is required.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796021000494.
Data
All data are not publicly available due to the issue of privacy. Requests for data require the review by the custodian from each country.
Acknowledgement
None.
Author contributions
Study concept and design: Man, KKC, YC Chang and ECC Lai.
Data analysis: Su CC and YC Chang.
Interpretation of results: All authors.
First draft of manuscript: ECC Lai.
Review and revise manuscript: All authors.
Financial support
This study was supported by a grant from the Ministry of Science and Technology of Taiwan (ID: 106–2320-B-006-025-MY2). The funding source had no role in the design, analysis, interpretation or reporting of results or in the decision to submit the manuscript for publication.
Conflict of interest
None.
Ethical standards
The study has been approved by the Human Research Ethics Committee at National Cheng Kung University (No. NCKU HREC-E-105-259-2); the University of Hong Kong/Hospital Authority Hong Kong West Cluster (No. UW15-619); The Health Improvement Network Scientific Review Committee (19THIN087) and the Institutional Review Board of Sungkyunkwan University (SKKU 2018-04-106).