Introduction
The first scientific expedition to explore the organisms of the deep sea, the voyage on the British HMS ‘Challenger’ from 1872–1876, was also the first cruise upon which bryozoans were described from around the central North Atlantic archipelago of the Azores (Busk, Reference Busk1881a, Reference Busk1884; Waters, Reference Waters1888). While a mere 21 species were recorded then, this pioneering expedition was quickly followed up by a series of French cruises to the archipelago in the late 19th and early 20th century, upon which the majority of the ~200 known Azorean bryozoan species were described (Jullien & Calvet, Reference Jullien and Calvet1903; Calvet, Reference Calvet1906, Reference Calvet1907, Reference Calvet1931). Another set of scientific expeditions to the Azores took place during the second half of the 20th century (e.g. d'Hondt, Reference d'Hondt1975; Harmelin, Reference Harmelin1978; Harmelin & Arístegui, Reference Harmelin and Arístegui1988). Since then, only a few species have been added to the bryozoan diversity of the Azores (Harmelin, Reference Harmelin2006; Reverter-Gil & Fernández-Pulpeiro, Reference Reverter-Gil and Fernández-Pulpeiro2007; Berning & Kuklinski, Reference Berning and Kuklinski2008; Berning, Reference Berning2013; Berning et al., Reference Berning, Achilleos and Wisshak2019; Harmelin et al., Reference Harmelin, Bishop, Madurell, Souto, Spencer Jones and Zabala2019; Haugen et al., Reference Haugen, Novosel, Wisshak, Berning, Wyse Jackson and Zágoršek2020). This is despite a renewed interest in the archipelago's marine fauna and ongoing sampling activities in the region (George et al., Reference George, Arndt, Wehrmann, Baptista, Berning, Bruhn, Carvalho, Cordeiro, Creemers, Defise, Domingues, Hermanns, Hohlfeld, Iwan, Janßen, Jeskulke, Kagerer, Kaufmann, Kieneke, Loureiro, Madeira, Meyer, Narciso, Ostmann, Pieper, Pointner, Raeke, Silva, Springer and Wilsenack2018; Baptista et al., Reference Baptista, Berning, Curto, Waeschenbach, Meimberg, Santos and Ávila2022), which is often driven by the necessity to identify non-indigenous species (e.g. Cardigos et al., Reference Cardigos, Tempera, Ávila, Gonçalves, Colaço and Santos2006; Amat & Tempera, Reference Amat and Tempera2009; Micael et al., Reference Micael, Marina, Costa and Occhipinti-Ambrogi2014, Reference Micael, Tempera, Berning, López-Fé, Occhipinti-Ambrogi and Costa2019; Vieira et al., Reference Vieira, Spencer Jones and Taylor2014).
This work is another in a series of modern revisions of cheilostomatid Bryozoa from NE Atlantic islands and seamounts, a great number of which were introduced during the late 19th and early 20th century (e.g. Reverter-Gil & Fernández-Pulpeiro, Reference Reverter Gil and Fernández Pulpeiro1999, Reference Reverter-Gil and Fernández-Pulpeiro2007; Berning & Kuklinski, Reference Berning and Kuklinski2008; Souto et al., Reference Souto, Reverter-Gil and Fernández-Pulpeiro2011, Reference Souto, Berning and Ostrovsky2016; Berning, Reference Berning2012, Reference Berning2013; Vieira et al., Reference Vieira, Spencer Jones and Winston2013; Reverter-Gil & Souto, Reference Reverter-Gil and Souto2015; Reverter-Gil et al., Reference Reverter-Gil, Berning and Souto2015; Berning et al., Reference Berning, Harmelin and Bader2017, Reference Berning, Achilleos and Wisshak2019, Reference Berning, Spencer Jones and Vieira2021; Harmelin et al., Reference Harmelin, Bishop, Madurell, Souto, Spencer Jones and Zabala2019; Madurell et al., Reference Madurell, Spencer Jones and Zabala2019). The modern species-taxon concept in bryozoans makes it necessary to reanalyse and redescribe the historical species, which were often insufficiently figured and described in the original literaure, in order to correctly assess bryozoan diversity, ecology and geographic distribution. We here deal with 12 historical cheilostomatid species from the greater Azores region that were recovered from four ‘Challenger’ stations in 1873, ranging in depth from 90–3060 m.
Materials and methods
Type and comparative material of species from the ‘Challenger’ Expedition, which were described by Busk (Reference Busk1881a, Reference Busk1884) and Waters (Reference Waters1888), and which are housed at the Natural History Museum London (NHMUK) and the Manchester Museum (MM), were studied using scanning electron microscopy (SEM). Material collected at the following HMS ‘Challenger’ stations in the vicinity of the Azores is considered here:
Station 70, 38°25′N 35°50′W (on the Pico Fracture Zone, i.e. ~400 km west of the Azores and therefore outside the Exclusive Economic Zone), 26/6/1873, 1675 fathoms (~3060 m), Globigerina ooze.
Station 75, 38°38′N 28°28′W (N of Pico Island), 2/7/1873, 450 fathoms (~820 m), volcanic mud and sand.
Station lacking a number and precise position, in the channel between the islands of Faial and Pico (here termed ‘Station FP’), 2/7/1873, washings from dredge, 50–90 fathoms (~90–165 m).
Station 76, 38°11′N 27°9′W (between the islands of Pico and São Miguel), 3/7/1873, 900 fathoms (~1650 m), Globigerina and pteropod ooze.
Additional specimens from the Azores, also those collected during the late 19th century on French scientific cruises, were examined at the Manchester Museum (MM), the Muséum national d'Histoire naturelle at Paris (MNHN), the Musée océanographique at Monaco (MOM), and the Biology Centre of the Oberösterreichische Landes-Kultur GmbH (formerly Oberösterreichisches Landesmuseum, collection Evertebrata varia; OLL). A substantial amount of material from the Azores was subsequently collected during the French ‘Biaçores’ Expedition in the 1970s, which is kept at the MNHN. A thorough review of that material was, however, beyond the scope of the present paper, and only a few specimens are given in the material list of the respective species. The specimens from the MNHN analysed during this study are hyperlinked with the respective online Collection Database entries whenever available (https://science.mnhn.fr/all/search); if existing, additional SEM images can be viewed and downloaded there. While in the text only the holotypes, lectotypes and neotypes are given for the sake of brevity, a complete list of the para(lecto)types and all other studied specimens is provided in the Supplementary Material (S1).
Suitable bryozoan colonies were digitally photographed at the NHMUK using a LEO 1455VP SEM, and at the MNHN using a Tescan VEGA SEM. Both machines allowed imaging of uncoated specimens with back-scattered electrons in low vacuum mode. Morphometrics were made on these micrographs using the image software ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012), and are given in the descriptions as mean ± standard deviation, minimum–maximum values and number of measurements (whenever >2 measurements were taken). Values are in μm unless otherwise noted. Character abbreviations: H = height (i.e. the zooid dimension normal to the basal surface), L = length, W = width.
Neither Busk (Reference Busk1881a, Reference Busk1884) nor Waters (Reference Waters1888) explicitly designated type material, while several species were newly described based on material from two or more stations separated by hundreds or even thousands of kilometres. In this case, the selection of a lectotype and the type locality of the species is primarily based on the figured specimen if it could be ascertained, and secondarily on the first mentioned station in Busk's (Reference Busk1884) ‘Habitat’ list at the end of each species description. As Busk distributed the original material among his colleagues and to different institutions (e.g. Waters (Reference Waters1888: 1) mentioned that duplicate specimens of the ‘Challenger’ material exist in Edinburgh), it is possible that additional paralectotypes will be discovered in the future.
The longitudes of the sampling stations of the ‘Talisman’, ‘Travailleur’ and ‘l'Hirondelle’ cruises, as given by Jullien & Calvet (Reference Jullien and Calvet1903) and Calvet (Reference Calvet1906, Reference Calvet1907), were initially measured with reference to the Paris meridian but have here been corrected to the Greenwich meridian. We also noted that the geographic position of ‘Prince Albert’ Station 882 (38°03′40″N 28°34′45″W, some 50 km south of Pico Island), as given by Calvet (Reference Calvet1931: 134) for specimens of Hemicyclopora canalifera, Buskea fayalensis and Reteporella atlantica (see below), is not in accordance with the depth of 98 m at that station. The nature and number of species recorded at this station by Calvet (Reference Calvet1931: 135) suggest that it is the geographic position that is erroneous. We thus presume that a transcription error has occurred in the minute reading, and that the correct latitudinal position is 38°30′40″N, which is in the southern Pico-Faial Channel and corresponding with a depth of 98 m. The corrected position is thus given as 38°30.67′N 28°34.75′W.
Systematics
Superfamily calloporoidea Norman, Reference Norman1903
Family farciminariidae Busk, Reference Busk1852
Genus Columnella Levinsen, Reference Levinsen1914
Columnella gracilis (Busk, Reference Busk1884)
(Figure 1A, B)
Part Farciminaria gracilis Busk, Reference Busk1884: 51, pl. 5, fig. 3.
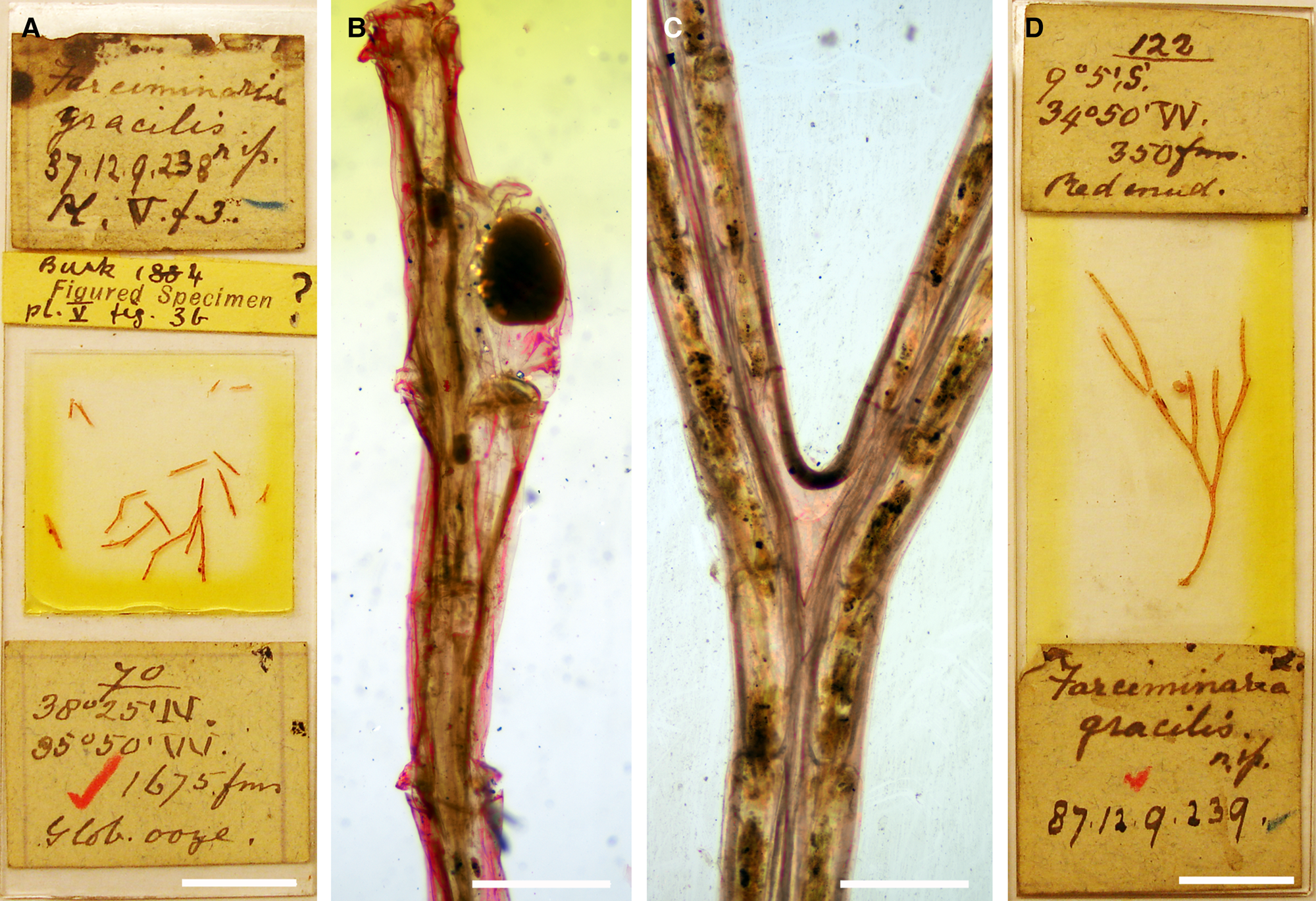
Figure 1. Columnella gracilis (Busk, Reference Busk1884): (A) the lectotype NHMUK 1887.12.9.238 from west of the Azores (Station 70); (B) optical image of an ovicellate branch (lectotype, NHMUK 1887.12.9.238). Columnella sp.: (C) optical image of a bifurcating branch segment of NHMUK 1887.12.9.239 from off Brazil (Station 122), note the distinctly greater branch width and absence of avicularia in this specimen; (D) same specimen, image of the slide. Scale bars: A, D, 1 cm; B, C, 500 μm.
Type material
Lectotype (here designated): NHMUK 1887.12.9.238, Busk coll., Station 70, several small fragments of a single colony, two with one ovicell each, ?figured specimen (Busk, Reference Busk1884: pl. 5, fig. 3b), in Canada balsam.
Description
Colony erect, unjointed but probably flexible, dichotomously branching at an angle of about 20–30°, over 1.5 cm in height; branches slender (~300 μm) and slightly curved, quadrangular in cross section; colony base not observed. Zooids lightly calcified, rectangular, very elongated and narrow (L: ~1100 μm; W: ~150 μm).
A small avicularium distal to every zooid.
Ovicell large and distinctly longer than wide (L: ~700 μm; W: ~400 μm), hyperstomial, ooecium proximally constricted and with an elevated rim.
Remarks
From the syntype series, only two specimens exist in the NHMUK collection. Whereas specimen NHMUK 1944.1.8.132 (Busk bequest, Station 70, on slide) is missing, one of the slides (NHMUK 1887.12.9.238) comprises several small fragments from Station 70 west of the Azores (Figure 1A). None of these specimens can be unequivocally attributed to Busk's drawings, although two fragments from Station 70, which is mentioned first in Busk's (Reference Busk1884) habitat list, comprise ovicells, one of which was presumably imaged in his fig. 3b. A note on the slide by Busk also supports this notion, and it is likely that the fragile colony broke apart between production of the image and preparation of the slide. This specimen is therefore designated here as lectotype. Both specimens are mounted in Canada balsam on slides and cannot be studied using SEM, which renders impossible a thorough redescription of the morphospecies. The short description and morphometrics given above are based on optical microscope images (Figure 1B), which do not show any detailed characteristics, as well as on Busk's (Reference Busk1884) original account. Both need to be taken cautiously. Nevertheless, Busk's original description and images (1884: 51, fig. 3a, b) match with the morphology of the lectotype.
Another specimen referred to Columnella gracilis by Busk (NHMUK 1887.12.9.239) is a large colony fragment from Station 122 off Brazil (Figure 1D). A comparison of the colonies from the Azores and Brazil, however, shows that the branches in the Azorean specimen are more slender than in the Brazilian one (300 μm vs 500 μm; Figure 1C), that the zooids are distinctly longer (~1100 μm vs ~300 μm), and that there are avicularia distal to every zooid, while these seem to be lacking in the southern Atlantic specimen. In concert with the geographic distance between the two stations, these morphometric differences suggest that the specimen from Brazil belongs to a different species.
Columnella gracilis was originally recorded from ~3000 m depth at the northern ridge of the Pico Fracture Zone, ~400 km WSW of the island of Flores, i.e. W of the Mid-Atlantic Ridge on the North American Plate. The conspecificity of subsequent records remains doubtful as most are, just like Busk's other specimen from Brazil, extremely far away from the type locality. For instance, d'Hondt (Reference d'Hondt1981: 13; Reference d'Hondt1983: 75) recorded the species from off Argentina. Other reports from the North Atlantic are also at a distance of several hundred to a few thousand kilometres to the original position (d'Hondt, Reference d'Hondt1983, Reference d'Hondt1985). In the absence of thorough descriptions and detailed illustrations from all of the above-mentioned publications, however, it is impossible to make any statements on its geographic and bathymetric range at present.
Other Columnella species are certainly present in the greater Azores region. Silén (Reference Silén and Petterson1951: fig. 1) reported a ~18 cm tall colony of C. magna (Busk, Reference Busk1884) from 4540–4600 m depth 370 km WNW of Flores (as Levinsenella magna). The type locality of that species is, however, in the southern hemisphere (the syntypes are from the Indian Ocean sector of Antarctica and from off southern Uruguay). Besides recording C. magna, d'Hondt (Reference d'Hondt1975) also reported two other farciminariid species from 1250–4700 m depth in and around the Azores.
Superfamily flustroidea Fleming, Reference Fleming1828
Family flustridae Fleming, Reference Fleming1828
Genus Carbasea Gray, 1848
Carbasea pedunculata Busk, Reference Busk1884
(Figure 2)
Carbasea pedunculata Busk, Reference Busk1884: 56, pl. 16, fig. 4.
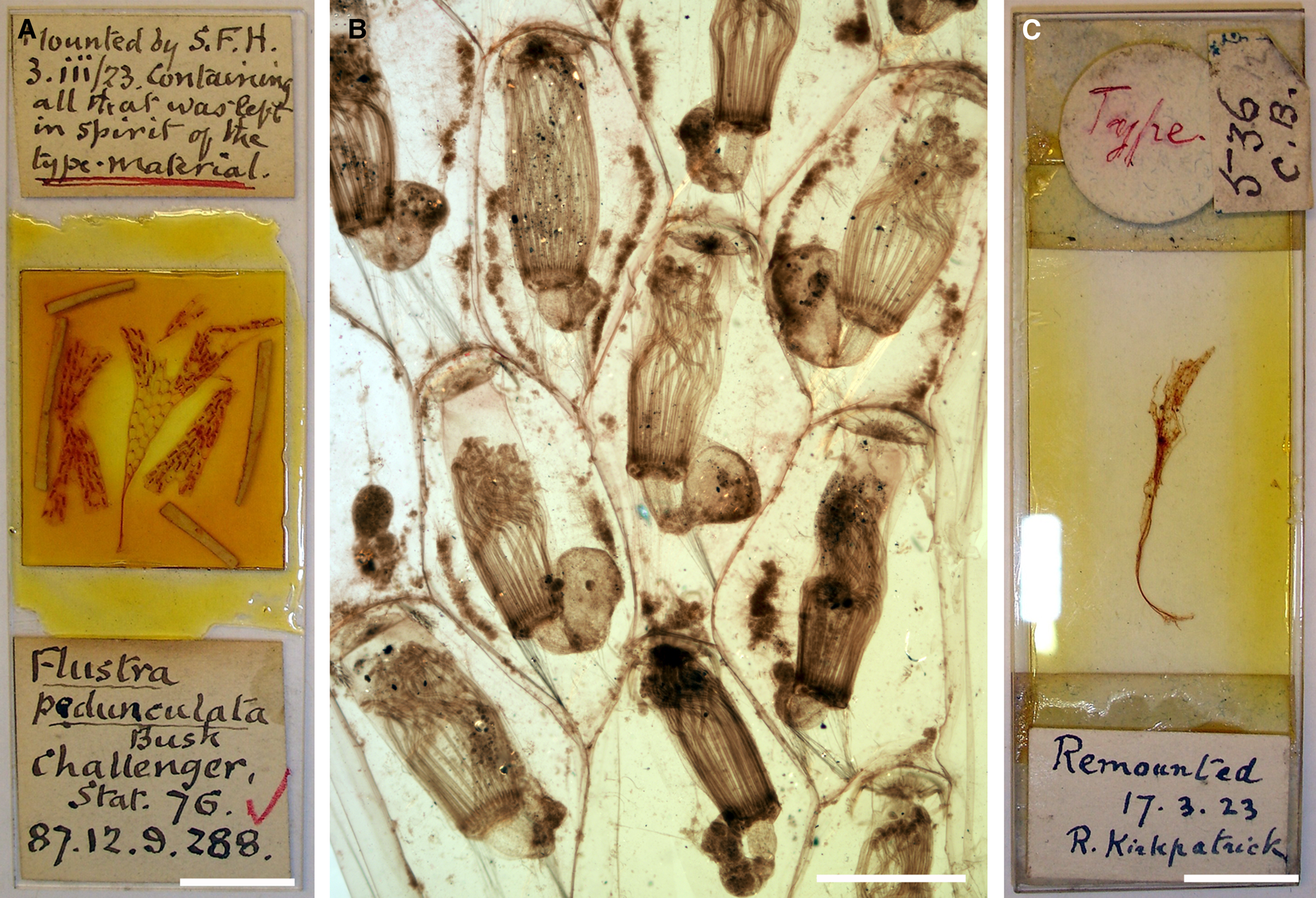
Figure 2. Carbasea pedunculata Busk, Reference Busk1884: (A) slide with the lectotype, NHMUK 1887.12.9.288; (B) optical image of autozooids (lectotype, NHMUK 1887.12.9.288); (C) paralectotype NHMUK 1887.12.9.286, note that the specimens were remounted by S.F. Harmer and R. Kirkpatrick, respectively. Scale bars: A, C, 1 cm; B, 500 μm.
Type material
Lectotype (here designated): NHMUK 1887.12.9.288, Station 76, several large colony fragments, figured specimen (Busk, Reference Busk1884: pl. 16, fig. 4).
Paralectotype: NHMUK 1887.12.9.286, Station 75, damaged colony base with rootlets.
Description
Colony erect, unilaminar, unjointed but flexible, dichotomously branching at an angle of ~20–30°, well over 2 cm in height (Figure 2A); branches ligulate, all in one plane, composed of three linear series of autozooids that are arranged in quincunx (Figure 2B), branches bordered on each side by a cuticular tube; colony fixed to the substratum by rootlets (Figure 3C). Zooecia very thinly calcified, rounded pentagonal to hexagonal, large (L: 1441 ± 91, 1300–1584, 8; W: 869 ± 101, 733–1044, 8). Polypide with some 24 tentacles. Operculum D-shaped, no oral or mural spines.

Figure 3. Notoplites bilobus (Busk, Reference Busk1884): (A) overview of lectotype (NHMUK 1887.12.9.64); (B) several ovicellate zoooids (lectotype, NHMUK 1887.12.9.64); (C) close-up of ovicell (lectotype, NHMUK 1887.12.9.64); (D) three autozooids, note the long distal spine (NHMUK 1945.9.14.1); (E) abfrontal, bifurcating branch with lateral rhizoidal tubes and distal avicularia on each zooid (paralectotype, NHMUK 1887.12.9.63); (F) close-up of abfrontal avicularium (paralectotype, NHMUK 1887.12.9.63); (G) intramural buds in zooids damaged by predation (paralectotype, NHMUK 1887.12.9.63); (H) abfrontal side of a branch, showing drill holes and damaged avicularia (NHMUK 1945.9.14.1). Scale bars: A, 500 μm; B, E, 200 μm; C, D, G, H, 100 μm; F, 50 μm.
Avicularia absent.
Remarks
Busk (Reference Busk1884: 56) remarked that ‘The single specimen included in the collection is unfortunately in a very imperfect condition – torn and ragged – so that the full dimensions of the growth cannot be determined from it.’ The first part of the quote is dubious as he reported the species from two ‘Challenger’ stations, and there are, accordingly, two specimens of the syntype series in the NHMUK collection. While the sample from Station 76 (NHMUK 1887.12.9.288) consists of several fragments of the erect colony (Figure 2A), another torn rhizoidal colony base is present from Station 75 (NHMUK 1887.12.9.286; Figure 2C), which is marked as ‘Type’. A note in the NHMUK archive also says that some material was destroyed during remounting of these specimens in the 1920s, which were originally kept in ethanol. It is probable that more material was initially present, which Busk either kept in his own collection (and which later arrived at the NHMUK with the Busk bequest, such as NHMUK 1944.1.8.148 from Station 76, but this specimen is also lost today), or which he distributed among colleagues.
While there may be a slight chance that more syntype material may be present in other collections, sample NHMUK 1887.12.9.288 from Station 76 is here chosen as lectotype as it is the only one in which autozooids are preserved. As all available material is mounted on slides (in Canada balsam), the redescription given above is based on observation by optical microscopy. A detailed analysis of zooecial characters using SEM will need to be carried out on newly collected topotypic material.
The species was hitherto assigned to the genus Flustra Linnaeus, 1761 (see Bock, Reference Bock2022). Based on its unilaminar colony growth as well as owing to the absence of spines and avicularia, however, Busk's (Reference Busk1884) original systematic placement in the genus Carbasea was correct.
Carbasea pedunculata has never been reported again since its discovery. It is thus endemic to the Azores, occurring in depths from 800–1700 m. The species has remarkably large zooids and polypides that are easily visible with the naked eye (Figure 2).
Superfamily buguloidea Gray, Reference Gray1848
Family candidae d'Orbigny, Reference d'Orbigny1851
Genus Notoplites Harmer, 1923
Notoplites bilobus (Busk, Reference Busk1884)
(Figure 3)
Cellularia biloba Busk, Reference Busk1884: 18, pl. 3, fig. 2.
Notoplites biloba (Busk): Harmer, Reference Harmer1923: 350.
Type material
Lectotype (here designated): NHMUK 1887.12.9.64, Busk coll., Station 76, a single specimen of >3 cm length with rootlet base, broken into several pieces, marked as ‘Type’ on the back, figured specimen (Busk, Reference Busk1884: pl. 3, fig. 2b).
Description
Colony erect, jointed, dichotomously branching, widely ramified, over 3 cm in height with slender branches (Figure 3A), attached by numerous basal rhizoids produced from a proximal pore. Branches formed by two series of alternating autozooids, branching angle between 30–40°, zooids opening on one side only, colony white in dried state. Branching points composed of a single proximomedian zooid and two distolateral ones, with the nodes developing immediately distal to the latter zooids by breakage of the narrow proximal parts of the two subsequent zooids (Figure 3E). Autozooids very elongate (Figure 3D, G), narrowest and tubular proximally, widening distally with the distal half of zooids turned outwards at an angle of ~40° (L: 736 ± 56, 640–812, 20; W: 212 ± 16, 183–242, 20), usually (much) less than half of total autozooid length occupied by the scutum/membranous area and orifice; skeletal surface smooth, convex, zooids separated by a distinct groove; ovicellate zooids slightly more bulky than autozooids. Rhizoids produced from a small oval pore near proximal end on the abfrontal side of some zooids, running along the lateral abfrontal side, basally closely approximated to form a single stalk; additional rhizoids are produced near branching points that run down on one interior side and up the other for some distance.
Opesia oval (L: 323 ± 21, 270–356, 20; W: 184 ± 14, 161–212, 20), proximal two-thirds on a distinctly raised rim (Figure 3D), outer cryptocystal rim thin and crenellate, the smooth inner cryptocyst steeply sloping towards a proximolateral shelf that is broadest proximally and gradually thinning distally to disappear in distal third (Figure 3G); outer distolateral opesial margin with 3 relatively thick spines that may be longer than an autozooid, proximal spine usually larger than the 2 distal ones, inner distolateral margin with 2 smaller and shorter spines plus scutum (Figure 3D); scutum on a short stalk at same level as proximal-most outer spine, arching over at some distance but not covering the entire opesia, much longer than wide (L: 237 ± 27, 196–314, 20; W: 110 ± 15, 82–150, 20), variably shaped but most often bilobed (Figure 3B–D), the slightly raised distal lobe generally shorter and narrower than proximal part, occasionally oval to reniform, surface with faint reticulate ridges; often slightly dimorphic in ovicellate zooids, with the distal lobe broadened.
A single adventitious avicularium placed near the bases of the oral spines at the distal abfrontal margin of every zooid (L: 214, 195–232, 2; W: 91, 88–93, 2), invisible in frontal view, directing towards the proximal end of zooid (Figure 3E, F, H), the proximal part of the avicularium (i.e. near the spine bases at the distal zooidal margin) forming a rim that is parallel with the abfrontal zooidal surface whereas the central and narrow distal part of rostrum is much raised, rostrum very elongate triangular (Figure 3F), distal part downcurved and with a hooked tip; mandible distally hooked, hinged on a pair of short lateral denticles, the semicircular area proximal to denticles with a narrow immersed proximal shelf and a semicircular opesia, palate (i.e. an immersed shelf in the rostrum) absent.
Ovicell hyperstomial, globular, large, distinctly longer than wide (L: 440 ± 24, 410–474, 7; W: 310 ± 20, 282–329, 7), covering the entire frontal shield of the distal zooid proximal of opesia (Figure 3B, C); ectooecium entirely calcified except for an elongated membranous fenestra of variable shape at the mid-proximal margin, surface smooth at first sight, faint reticulate ridges and growth marks visible at higher magnification, proximal ooecial margin concave and widely arched over opesia, covering the distal spine of the inner distolateral margin; ovicell closure acleithral.
Ancestrula not observed.
Remarks
Some 43 Notoplites species are recognized worldwide at present, which are distributed from the tropics to polar latitudes, although most species are confined to cooler waters (i.e. deeper water at lower latitudes). Besides Notoplites bilobus, two other Notoplites species are known to be present in the Azores: N. clausus (Busk, Reference Busk1884) (see below) as well as N. saojorgensis Berning, Reference Berning2013, which was newly described based on material erroneously reported as N. marsupiatus (Jullien, Reference Jullien1882) by Calvet (Reference Calvet1931). Both species have a fimbriated scutum that covers the entire opesia and are therefore distinctly different from N. bilobus. Other species recognized in the NE Atlantic are N. damicornis Hayward & Ryland, Reference Hayward and Ryland1978, N. evocatus (Jullien, Reference Jullien1882), N. evocatus trispinosus d'Hondt, Reference d'Hondt1987, N. harmeri Ryland, Reference Ryland1963, N. marsupiatus (Jullien, Reference Jullien1882), and N. smitti (Norman, Reference Norman1868). Notoplites bilobus differs from all of these species by, among other characters, the bilobed scutum and the presence of a single, distal, abfrontal avicularium.
Notoplites bilobus has never been reported after its original introduction, besides in a revision of the genus Notoplites by Harmer (Reference Harmer1923). It must therefore be regarded as endemic to the Azores, occurring at a depth of ~1600 m in the central group of islands. Many of the colonies' zooids are repaired and replaced by a second or occasionally even a third generation of intramural buds, which are distinctly raised above the level of the primary zooid (Figure 3G). The presence of drill holes in the abfrontal skeleton (Figure 3H), and also the structural damage to the abfrontal avicularia, shows that the formation of at least some of these intramural buds was induced by partial predators, most likely juvenile or micro-gastropods. This proves that predation pressure is also high in the bathyal, and that there is a necessity for the bryozoans to invest in defensive skeletal structures at these depths (see also Berning, Reference Berning2008; Berning et al., Reference Berning, Harmelin and Bader2017), while in this case the protective scutum on the frontal colony surface was sidestepped by attacking the abfrontal side.
Notoplites clausus (Busk, Reference Busk1884)
Menipea clausa Busk, Reference Busk1884: 20, pl. 4, fig. 5.
Scrupocellaria marsupiata Jullien: Waters, Reference Waters1888: 9.
part Notoplites marsupiatus (Jullien): Harmer, Reference Harmer1923: 351.
?not Scrupocellaria marsupiata Jullien: d'Hondt, Reference d'Hondt1973: 1212.
?part Scrupocellaria marsupiata Jullien: d'Hondt, Reference d'Hondt1975: 556, figs 14–16.
Notoplites clausus (Busk): Souto et al., Reference Souto, Reverter-Gil and Fernández-Pulpeiro2011: 38, figs 16–20.
Type material
Lectotype (designated by Souto et al., Reference Souto, Reverter-Gil and Fernández-Pulpeiro2011): NHMUK 1887.12.9.83, Busk collection, Station 70, a single specimen of ~1.6 cm length with rootlet base, broken into several pieces.
Remarks
This species, which was considered to be a junior synonym of Notoplites marsupiatus (Jullien, Reference Jullien1882) since shortly after its introduction, has recently been resurrected and imaged by Souto et al. (Reference Souto, Reverter-Gil and Fernández-Pulpeiro2011: figs 16–20). It is possible that part or all of the material d'Hondt (Reference d'Hondt1975: 556) recorded from a wide region around the Azores Archipelago as Scrupocellaria marsupiata may belong to N. clausus (but see Souto et al., Reference Souto, Reverter-Gil and Fernández-Pulpeiro2011: 39; Berning, Reference Berning2013: 5). Similarly, the material d'Hondt (Reference d'Hondt1973) reported from south of the Azores may belong to N. saojorgensis. In the absence of images, these records have to be considered doubtful, and the type locality ~400 km W of the Azores, at a depth of ~3000 m, remains the only verified occurrence.
Sharing the fimbriated scutum that covers the entire opesia, Notoplites marsupiatus from the continental slope of NW Spain as well as N. clausus and N. saojorgensis from the Azores region seem to form a distinct clade within Notoplites.
Superfamily bifaxarioidea Busk, Reference Busk1884
Family bifaxariidae Busk, Reference Busk1884
Genus Raxifabia Gordon, 1988
Raxifabia minuta (Busk, Reference Busk1884)
(Figure 4)
Bifaxaria minuta Busk, Reference Busk1884: 81, pl. 13, fig. 5, 5a

Figure 4. Raxifabia minuta (Busk, Reference Busk1884), holotype, NHMUK 1887.12.9.380: (A) overview of colony with base rooted in foraminiferal sand; (B) colony in lateral view, note that the two distal zooids show evidence of repair by means of intramural budding (a secondary orifice rim is visible in one zooid), as well as by re-formation of the spinocysts, thus distorting the spinocystal characters in these zooids; (C) colony in frontal view; (D) oblique view on orifice and distal avicularia. Scale bars: A, 1 mm; B, C, 200 μm; D, 100 μm.
?not Sclerodomus minutus Busk: d'Hondt & Schopf, Reference d'Hondt and Schopf1984: 940.
Type material
Holotype (by monotypy): NHMUK 1887.12.9.380, Busk coll., Station 70, colony base with rootlets fixed in Globigerina sand, mounted on slide.
Description
Colony erect, rooted, biserial (Figure 4A, B), branching not observed, forming a single rod (~4.3 mm in length) with a lensoid cross section, tapering proximally, maximum width of lateral face ~350 μm, maximum width of frontal face ~220 μm. Zooids alternating back to back (Figure 4B), extremely elongate rectangular in frontal view (L: 751 ± 16, 735–777, 5; W: 217 ± 8, 210–227, 4), laterally compressed (i.e. the height of a zooid is greater than its width: H: 231 ± 21, 198–256, 8), resulting in narrower frontal colony faces (Figure 4C), separated by a groove on raised ridges; frontal shield smooth, with a median suture that is produced by merging of the pair of spinocysts, a distinct row of pores running along the suture with others scattered on the remaining shield, another pair of larger pores in the most proximal part via which the spinocysts and possibly also the rhizoids may be formed. Orifice suborbicular (Figure 4C, D), slightly wider than long (L: 123 ± 2, 119–125, 6; W: 132 ± 4, 127–136, 4), proximal margin shallowly concave, potential condyles not seen as obscured by the opercula.
Avicularia presumably interzooidal, paired, distolateral to every orifice and tightly framed by the spinocysts of the proximal and distal zooids (Figure 4D), oriented at almost 90° angle to zooidal frontal surface, i.e. with the frontal plane directing distally; outline oval, rostrum semi-elliptical (L: 62 ± 2, 60–65, 5; W: 51 ± 4, 46–56, 5), directing slightly outwards and toward zooidal frontal surface; the presence of a crossbar could not be determined in this unbleached specimen.
Ovicells and ancestrula not recognizable.
Remarks
The only specimen, from ~400 km W of the Azores at 3000 m depth, is a complete but small and immature colony with the basal rhizoidal part preserved. As the holotype could not be bleached, and the proximal part of the colony surface is completely covered by rhizoids, it was not possible to examine the potential ancestrula and early astogenetic colony. The same applies to the details of the orificial condyles and the avicularia as these are covered by the opercula and mandibles, respectively.
Gordon (Reference Gordon1988: 287) included Bifaxaria minuta in the genus Raxifabia, which he newly introduced for bifaxariid species with a subcircular to schizoporelloid (i.e. sinusoidal) autozooidal orifice and a frontal shield that is formed by a single pair of spines. Raxifabia minuta is so far the only representative of the genus in the Atlantic while all other congenerics occur in the Pacific. Considering the distance between localities, however, the specimens subsequently recorded by d'Hondt & Schopf (Reference d'Hondt and Schopf1984) from the equatorial Atlantic off West Africa and Brazil as R. minuta are very likely to belong to one or more distinct species.
Superfamily lepralielloidea Vigneaux, Reference Vigneaux1949
Family romancheinidae Jullien, Reference Jullien1888
Genus Hemicyclopora Norman, Reference Norman1894
Hemicyclopora canalifera (Busk, Reference Busk1884)
(Figure 5)
Mucronella (Phylactella?) canalifera Busk, Reference Busk1884: 159, pl. 22, fig. 2.

Figure 5. Hemicyclopora canalifera (Busk, Reference Busk1884): (A) overview of lectotype (NHMUK 1887.12.9.634); (B) a specimen of Lepralia labiosa from the Calvet collection with the oral spines preserved (MOM INV-22496); (C) orifice (MNHN-IB-2008-2436), (D) maternal zooid and kenozooidal ovicell in lateral view showing the umbo on the proximal ooecial margin (MNHN-IB-2008-2436); (E) ancestrula (partly obscured by another ancestrula of an unknown species) and early astogenetic zooids with eight oral spines (MNHN-IB-2008-2436). Scale bars: A, B, 500 μm; C, 100 μm; D, 200 μm; E, 300 μm.
Mucronella canalifera Busk: Waters, Reference Waters1888: 24, pl. 3, fig. 44; Calvet, Reference Calvet1931: 93.
?part Lepralia labiosa (Jullien): Calvet in Jullien & Calvet, Reference Jullien and Calvet1903: 134.
Lepralia labiosa (Jullien): Calvet, Reference Calvet1907: 410.
Lepralia canalifera (Busk): Calvet, Reference Calvet1907: 410.
?part Hippoporina? labiosa (Jullien): d'Hondt, Reference d'Hondt1975: 577.
Type material
Lectotype of Mucronella canalifera (here designated): NHMUK 1887.12.9.634, Busk coll., Station 75, from sand, figured specimen (Busk, Reference Busk1884: pl. 22, fig. 2).
Description
Colony encrusting, unilaminar, multiserial, forming an irregular patch (Figure 5A, B). Autozooids oval to hexagonal (L: 650 ± 63, 557–784, 20; W: 460 ± 34, 421–528, 20), separated by distinct grooves (Figure 5B); frontal shield strongly and evenly convex, surface finely granular, with a single row of small marginal pores invisible from directly above, two rows lateral of the orifice, frontal shield abruptly rising proximal of orifice to form a thick prominent lip that is variably curved and flared, highest usually proximally (Figure 5A–D); vertical walls much reduced, numerous basal pore-chambers present (Figure 5D), 4–7 combined into elongated areas framed by gymnocystal calcification, one area distally, two distolaterally and two laterally; basal wall only marginally calcified. Orifice suboval (Figure 5C), slightly longer than wide (L: 159 ± 12, 138–178, 20; W: 138 ± 10, 123–159, 20), proximal border variably concave, condyles thick and bluntly triangular, orifice margin usually with 6 (rarely 8) long outwardly bent spines (Figure 5B), the proximal-most pair just distal to condyles, early astogenetic zooids with 8 spines (Figure 5E).
Ovicellate zooids slightly dimorphic, differing in the presence of a gap between the distal-most pair of oral spines in which the ovicell is accommodated; ovicell produced by an encrusting kenozooid (Figure 5D), ooecium prominent, globular, wider than long (L: 263 ± 27, 226–306, 14; W: 332 ± 15, 314–360, 14), endooecium entirely calcified, imperforate, surface finely granular as zooidal frontal shield, proximomedian margin with a distinctly raised triangular umbo with smooth proximal gymnocystal calcification; ovicell closed by the operculum (cleithral).
Ancestrula oval, presumably with extensive proximolateral gymnocystal calcification, opesia confined to distal part of frontal area, almost lenticular in outline, framed by a raised gymnocystal rim surrounded by ?8–10 spines, proximal cryptocystal margin smooth and shallowly concave, proximally passing into an extensive shelf with a granular surface; first autozooid budded distally, second zooid distolaterally (Figure 5E).
Remarks
When introducing Mucronella canalifera, Busk (Reference Busk1884: 159) also treated specimens from Madeira as synonymous, which he previously reported as Lepralia mangnevilla Audouin, Reference Audouin and Panckoucke1826 (Busk, Reference Busk1860: 284). It has recently been shown, however, that these Madeiran colonies are actually quite different and belong to Saevitella peristomata (Waters, Reference Waters1899) (see Berning, Reference Berning2012: 44).
Waters (Reference Waters1925: 543, pl. 29, fig. 10) described and figured the ancestrula of Hemicyclopora canalifera as normal tatiform, i.e. with a large oval opesia. The (albeit poorly preserved) ancestrulae observed in the material studied during this work suggest, however, that the opesia is restricted to the distal third of the frontal by an extensive immersed cryptocystal shelf, just like in the other species of the genus (Harmelin & Rosso, Reference Harmelin and Rossoin press). Cook (Reference Cook1968: 217) already suggested that the proximal cryptocystal shelf may have been broken in Waters' specimen. Cook (1968: 216) further synonymized H. canalifera with Hemicyclopora multispinata (Busk, Reference Busk1861) from Madeira but the latter is supposed to have 8–10 spines in adult zooids. Until the types of H. multispinata have been analysed based on SEM images this decision has to be rejected owing to the great distance between localities, and we here regard Hemicyclopora canalifera as a distinct entity. The species has also been cited from other North Atlantic regions (Calvet, Reference Calvet1931: Bay of Biscay, Norway, Spitsbergen), which most probably represent distinct species as well.
Most, or possibly even all, of the specimens referred to Lepralia labiosa Jullien, 1903 belong to H. canalifera (both having the same type locality, Pico Island). The status and morphology of the species is unclear as the syntypes from Hirondelle Station 247 (Jullien in Jullien & Calvet, Reference Jullien and Calvet1903: 69) are lost (d'Hondt, Reference d'Hondt1975: 577; Tricart & d'Hondt, Reference Tricart and d'Hondt2009). The case is complicated by the fact that the specimen figured in Jullien & Calvet (Reference Jullien and Calvet1903: pl. 9, fig. 6) as L. labiosa, which is kept in Calvet's collection at Monaco (MOM INV-22582, marked as ‘Type’), is from Hirondelle Station 226. It can, therefore, not be regarded as a syntype because Jullien explicitly mentions only specimens from Station 247 as part of the type series. Calvet's action of choosing a figured specimen that Jullien has not seen when describing the species in the first part of their 1903 work represents another case in which he substantially interfered with the taxonomic decisions (e.g. Berning et al., Reference Berning, Spencer Jones and Vieira2021: 344), in contrast to what he stated in the preface (Jullien & Calvet, Reference Jullien and Calvet1903: 3).
Unfortunately, the specimen is also severely affected by Bynesian decay, which masks morphological details, and it could not be imaged using SEM. Jullien's original description agrees with the figure in that the ovicell is forming a little tubular peristome, while the distinct proximal umbo that is characteristic of H. canalifera is absent. All additional specimens from Station 226 available in the MOM collection, however, belong to H. canalifera (Figure 5B), as do all examined specimens from Talisman Station 125 (Faial-Pico Channel) that were later reported by Calvet (Reference Calvet1907) as Lepralia canalifera and Lepralia labiosa.
In the Azores H. canalifera was recovered from 80–820 m depth. Whereas downslope transport may be responsible for the occurrence at great depths (see Discussion), the colonies from ‘Challenger’ Station 75 (820 m) were relatively well-preserved, while showing no morphological differences to specimens from shallower waters. Apart from its type locality, the Faial-Pico Channel, which also marks the shallowest record of the species, H. canalifera was recovered from Graciosa, another island of the central group, at ~400 m depth (as Hippoporina labiosa by d'Hondt, Reference d'Hondt1975). The Hemicyclopora species present in São Miguel in the eastern group of islands, which was recorded as Hippoporina discrepans (Jullien, 1903) by d'Hondt (Reference d'Hondt1975: 561), is distinct from both the nominal species and H. canalifera, and has recently been referred to as Hemicyclopora sp. 1 by Harmelin & Rosso (Reference Harmelin and Rossoin press).
Superfamily smittinoidea Levinsen, Reference Levinsen1909
Family smittinidae Levinsen, Reference Levinsen1909
Genus Smittoidea Osburn, Reference Osburn1952
Smittoidea oratavensis (Busk, Reference Busk1884) comb. nov.
(Figure 6)
Smittia oratavensis Busk, Reference Busk1884: 153, pl. 22, fig. 1.
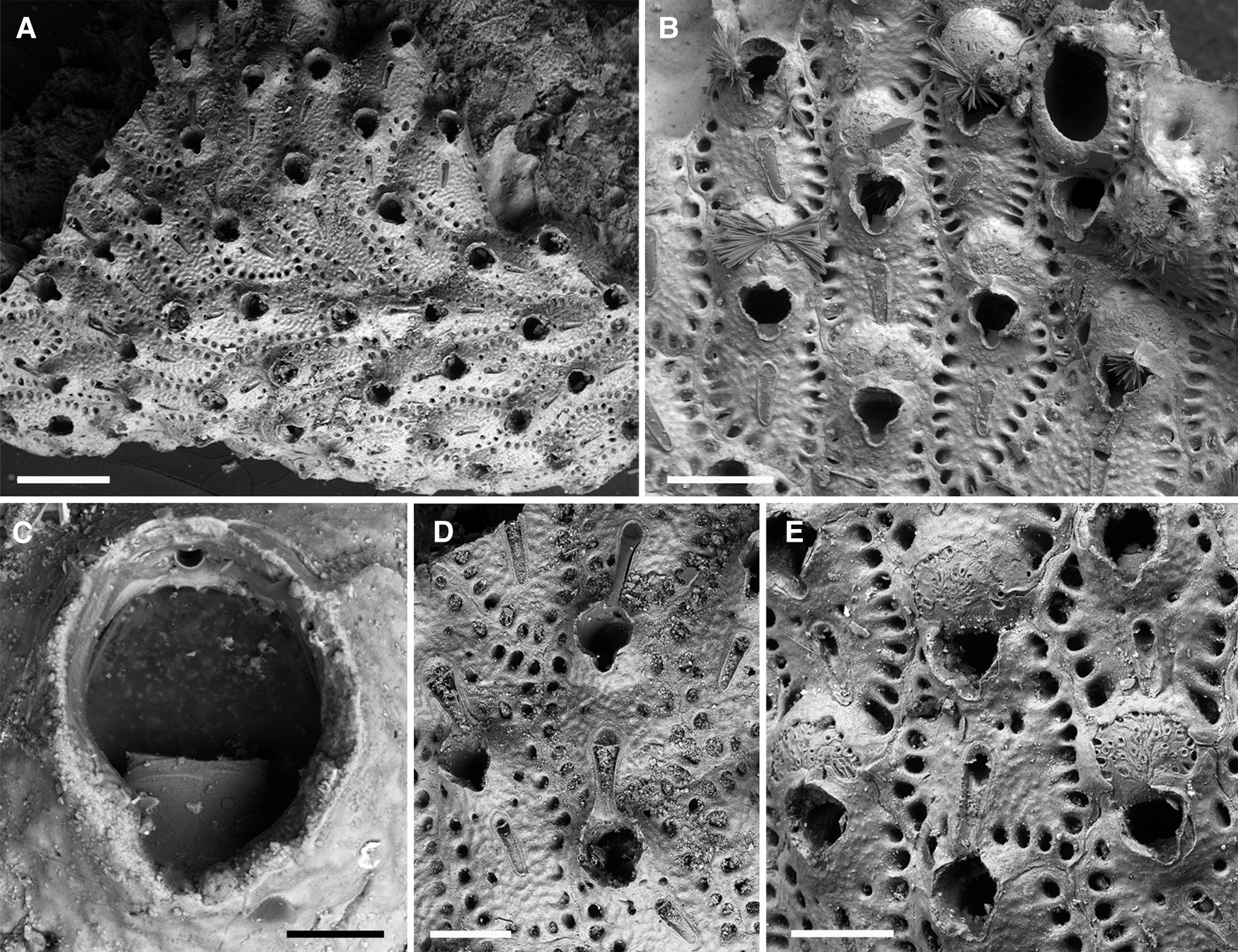
Figure 6. Smittoidea oratavensis (Busk, Reference Busk1884): (A) overview of lectotype (NHMUK 1887.12.9.609); (B) lectotype of Smittia ensifera Jullien, 1903 (MOM INV-22514); (C) close up of orifice, note the toothed distal margin and the single oral spine base (lectotype, NHMUK 1887.12.9.609); (D) both types of avicularia (lectotype, NHMUK 1887.12.9.609); (E) ovicellate zooids (paralectotype, NHMUK 1944.1.8.293). Scale bars: A, 500 μm; B, 300 μm; C, 50 μm; D, E, 200 μm.
Smittia ensifera Jullien in Jullien & Calvet, Reference Jullien and Calvet1903: 102, pl. 12, fig. 4; Calvet in Jullien & Calvet, Reference Jullien and Calvet1903: 149, pl. 17, fig. 5.
Smittia ophidiana Waters: Calvet, Reference Calvet1907: 433;
Smittina ophidiana Waters: Calvet, Reference Calvet1931: 91; d'Hondt, Reference d'Hondt1975: 557 (listed).
Smittoidea ensifera Jullien: Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015: 95 (listed).
Type material
Lectotype of Smittia oratavensis (here designated): NHMUK 1887.12.9.609, Busk coll., Station 75, sand, one colony on bioclast, ?figured specimen (Busk, Reference Busk1884: pl. 22, fig. 1).
Lectotype of Smittia ensifera (here designated): MOM INV-22514, Calvet coll., ‘Hirondelle’. 14/8/1888, Station 226, 38°31.32′N 28°34.52′W, Faial-Pico Channel, 130 m, colony on bioclast with free growth margin, on slide.
Description
Colony encrusting unilaminar, multiserial (Figure 6A). Zooids elongated pentagonal to hexagonal, widest usually at mid-distance (L: 564 ± 48, 464–634, 20; W: 356 ± 36, 277–438, 20), separated by shallow grooves or sutures (Figure 6B, E); frontal shield flat to slightly convex, rising abruptly around orifice to form a peristome with a deep U-shaped proximal sinus and notch, surface faintly nodular, large central area imperforate, a single row of ~15–20 densely spaced round areolar pores along zooecial margins and 2 pores distolateral to central avicularium (Figure 6B, D, E); vertical walls extensive, with 3 or 4 uniporous septula per neighbouring zooid. Orifice suborbicular (Figure 6C), obscured by a peristome, distal rim with few fine teeth, a single or two distal oral spines in early ontogeny are quickly lost, the bases being incorporated into the peristome, condyles indistinct if present, lyrula large, forming a broad square about half of total orifice width; peristomial aperture slightly longer than wide (L: 168 ± 13, 145–197, 20; W: 139 ± 7, 124–154, 20).
Ovicell hyperstomial (Figure 6B, E), globular with a slightly flattened front (L: 227 ± 14, 203–266, 20; W: 264 ± 22, 231–300, 20), ectooecium smooth with numerous rimmed pseudopores of usually round shape, proximal margin rather straight and slightly overarching the distal orifice, the peristome encroaching the proximolateral margins while the distolateral ectooecium is marginally covered by secondary calcification of the distal zooid(s).
Avicularia dimorphic, always adventitious, single, central and proximally or slightly proximolaterally directed (Figure 6B, D, E); rostrum in the smaller avicularia extremely elongated triangular or even with parallel lateral margins and a rounded tip (L: 167 ± 20, 133–205, 20; W: 46 ± 6, 38–58, 20), mandible of same shape and length as rostrum; rostrum in larger avicularia distally flared and open, downcurved and incorporated into distal peristomial margin of proximal zooid (L: 244 ± 20, 207–280, 20; W: 73 ± 9, 54–95, 20), mandible with downcurved bifid tip, exceeding length of rostrum and reaching into the peristome when close; both types having a palate with a depressed calcified shelf apart from a small semicircular opesia distal to crossbar and a suborbicular proximal window, crossbar thin and without columella.
Ancestrula tatiform, longer than wide (L: 360; W: 260), opesia reduced to distal half, suboval, only slightly longer than wide (L: 150; W:130), framed by a thin raised rim abutting against 9 stout spines grouped in 3 relatively narrowly spaced distal pairs, and 3 more widely spaced proximal spines, with the most proximal spine being slightly thicker and presumably overarching the opesia; cryptocyst reduced to a narrow proximal shelf; proximal gymnocyst extensive, convex, surface smooth to slightly pitted.
Remarks
Although Busk (Reference Busk1884) named the species after the Port of Orotava (Tenerife Island, from where he had previously received specimens of apparently the same species), the type locality given in the original description is clearly meant to be Pico Island in the Azores (‘Challenger’ Station 75), while Orotava is merely listed in brackets subsequently. The syntypes present in the collection are also exclusively from the Azores, and the specimen that was presumably figured by Busk (Reference Busk1884: pl. 22, fig. 1) is here designated as lectotype (NHMUK 1887.12.9.609). Note that, while Busk correctly gave the name of the town Orotava, he termed the species oratavensis. As the same spelling was given in the figure caption, however, it cannot be regarded as a lapsus calami and is therefore considered as correct original spelling.
Smittoidea oratavensis has never been reported again after its discovery, and is unrecognized today (cf. Bock & Gordon, Reference Bock and Gordon2021a), presumably because it was synonymized with Smittoidea marmorea (Hincks, Reference Hincks1877a) soon after its establishment (Jelly, Reference Jelly1889: 249). Smittoidea oratavensis differs from that species, however, in producing a larger avicularium in some zooids, the tips of which are incorporated into the distal peristome of the proximal autozooid. Moreover, in S. oratavensis a single or a pair of distal oral spines are present during early ontogeny, while spines are absent altogether in S. marmorea (cf. Hayward & Ryland, Reference Hayward and Ryland1999: 268), and the distal rim of the primary orifice bears several small denticles that are not reported to occur in S. marmorea.
The morphologically most closely related species of S. oratavensis rather seems to be the Mediterranean Smittoidea ophidiana (Waters, Reference Waters1879) as all of the above mentioned characters are shared. Calvet (Reference Calvet1907, Reference Calvet1931) and d'Hondt (Reference d'Hondt1975) already noted their close similarity and reported specimens from the Azores under this name. A specimen from its type locality (Naples), which was collected in 1875 by Waters and is presumably part of the syntype series, was studied for comparison (NHMUK 1899.7.1.2345A). Distinct differences exist in that S. ophidiana has much larger autozooids (mean ZL 840 μm, ZW 530 μm), larger apertures (mean ApL 230 μm, ApW 170 μm), and more numerous areolar pores (~25). While the large frontal avicularia are also bigger (mean lAL 360 μm, lAW 110 μm) in S. ophidiana, the small avicularia are about the same size (mean sAL 50 μm, sAW 46 μm) as in S. oratavensis. Ovicells are unfortunately lacking in the S. ophidiana specimen studied, while the bifid tips of the mandible in the long avicularia seem to be longer in the Mediterranean species than in the Azorean population, in which, however, only a single mandible was preserved. These differences are, overall, important enough to maintain S. ophidiana and S. oratavensis as distinct species, and we therefore resurrect Smittoidea oratavensis (Busk, Reference Busk1884).
Another Smittoidea species from the Azores, Smittia ensifera Jullien, 1903, was introduced ~20 years later, without making reference to Busk's species (Jullien & Calvet, Reference Jullien and Calvet1903: 102, 149). Syntypes of S. ensifera from ‘Hirondelle’ Station 226 are absent from the MNHN (Tricart & d'Hondt, Reference Tricart and d'Hondt2009), which is housing Jullien's collection. To fix the status of S. ensifera, which is also unrecognized today (Bock & Gordon, Reference Bock and Gordon2021a), a syntype from the same station that is kept in the Calvet collection at the MOM is designated here as lectotype (MOM INV-22514). As there are no morphological differences between S. oratavensis and S. ensifera (see Figure 6A and B, respectively), the former is here treated as a junior subjective synonym of the latter.
As the peristome conceals much of the orifice, making it difficult to measure its exact size, the length and width of the peristomial aperture was measured instead in all specimens. The bathymetric distribution of S. oratavensis ranges from 70–820 m, although the specimens from the deep ‘Challenger’ Station 75 are not very well preserved and may have been transported downslope. Wisshak et al. (2015: 95) have recently recorded living specimens from settlement panels at 150 m (as S. ensifera). Apart from the type locality, Pico Island, S. oratavensis was also recorded from other Azorean islands (d'Hondt, Reference d'Hondt1975): Graciosa (76–220 m), Terceira (220 m), São Miguel (130–260 m) and the Formigas Islets south of São Miguel (70–130 m). As synonymies with species from the NE Atlantic continental shelf and the Mediterranean Sea are rejected here, the species is regarded as endemic to the Azores.
Superfamily schizoporelloidea Jullien, Reference Jullien1882
Family microporellidae Hincks, Reference Hincks1879
Genus Microporella Hincks, Reference Hincks1877b
Microporella hastigera (Busk, Reference Busk1884)
(Figure 7)
Flustramorpha hastigera Busk, Reference Busk1884: 136, text-fig. 40, pl. 21, figs 7, 7a–c.

Figure 7. Microporella hastigera (Busk, Reference Busk1884): Paralectotype (NHMUK 1887.12.9.548). Scale bar: 5 mm.
Diporula hastigera (Busk): Waters, Reference Waters1888: 23, pl. 3, figs 28 & 29; Jullien in Jullien & Calvet, Reference Jullien and Calvet1903: 51, pl. 1, fig. 3, pl. 6, fig. 3; Calvet in Jullien & Calvet, Reference Jullien and Calvet1903: 129; Calvet, Reference Calvet1907: 407.
Microporella hastigera (Busk): Calvet, Reference Calvet1931: 87; d'Hondt, Reference d'Hondt1975: 577 (listed); Di Martino et al., Reference Di Martino, Taylor and Gordon2020: 8, figs 4–5.
Type material
Lectotype (designated by Di Martino et al., Reference Di Martino, Taylor and Gordon2020): NHMUK 1887.12.9.547, Busk coll., Station 75, volcanic mud, large fragment (1.6 cm in length), figured specimen (Busk, Reference Busk1884: pl. 21, fig. 7).
Remarks
Microporella hastigera (Busk, Reference Busk1884) is one of the few erect species in this genus, its lectotype has recently been designated, revised and figured using SEM by Di Martino et al. (Reference Di Martino, Taylor and Gordon2020: 8, figs 4 & 5). Owing to its erect growth, large colonies and strong calcification (Figure 7), this species is among the most conspicuous and ubiquitous bryozoans in the Azores, particularly comprising a significant amount of the biogenic sediment around the islands, and numerous specimens exist in the historical collections. A complete list of all available specimens is therefore not provided here, and the material given represents only those samples that have personally been examined.
Previously also assigned to the genus Diporula Hincks, 1879, M. hastigera has originally been recorded from off western and northern Pico Island from depths between 80 and 820 m. Particularly during the French ‘Biaçores’ expedition (see d'Hondt, Reference d'Hondt1975), the species was also reported from around the islands of Flores (105–170 m), Graciosa (190–406 m), São Jorge (245 m), Terceira (90–220 m), São Miguel (61–550 m) and the Formigas Islets south of São Miguel (190–220 m). Curiously, however, M. hastigera was not recorded from settlement panels recently brought out in the Faial-Pico Channel in depths down to 500 m for up to two years, although other Microporella species were present on the panels (Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015). Calvet (Reference Calvet1931: 87) reported M. hastigera to also occur in the Cape Verde Islands at 52 m depth but, considering the distance between the archipelagos, that population is probably not conspecific.
A fossil species that is closely related to, and probably the direct ancestor of, M. hastigera was recently found in lower Pliocene sediments of Santa Maria (Ávila et al., Reference Ávila, Ramalho, Habermann, Quartau, Kroh, Berning, Johnson, Kirby, Zanon, Titschack, Goss, Rebelo, Melo, Madeira, Cordeiro, Meireles, Bagaço, Hipólito, Uchman, da Silva, Cachão and Madeira2015: 68; as Microporella sp. 2). The fossil differs from the modern species in a few aspects and will be described in more detail elsewhere.
Superfamily celleporoidea Johnston, Reference Johnston1838
Family celleporidae Johnston, Reference Johnston1838
Genus Buskea Heller, 1967
Buskea ovalis (Busk, 1881) comb. nov.
(Figure 8)
Cellepora ovalis Busk, Reference Busk1881a: 352; Busk, Reference Busk1884: 202, pl. 28, fig. 5; pl. 35, fig. 6.
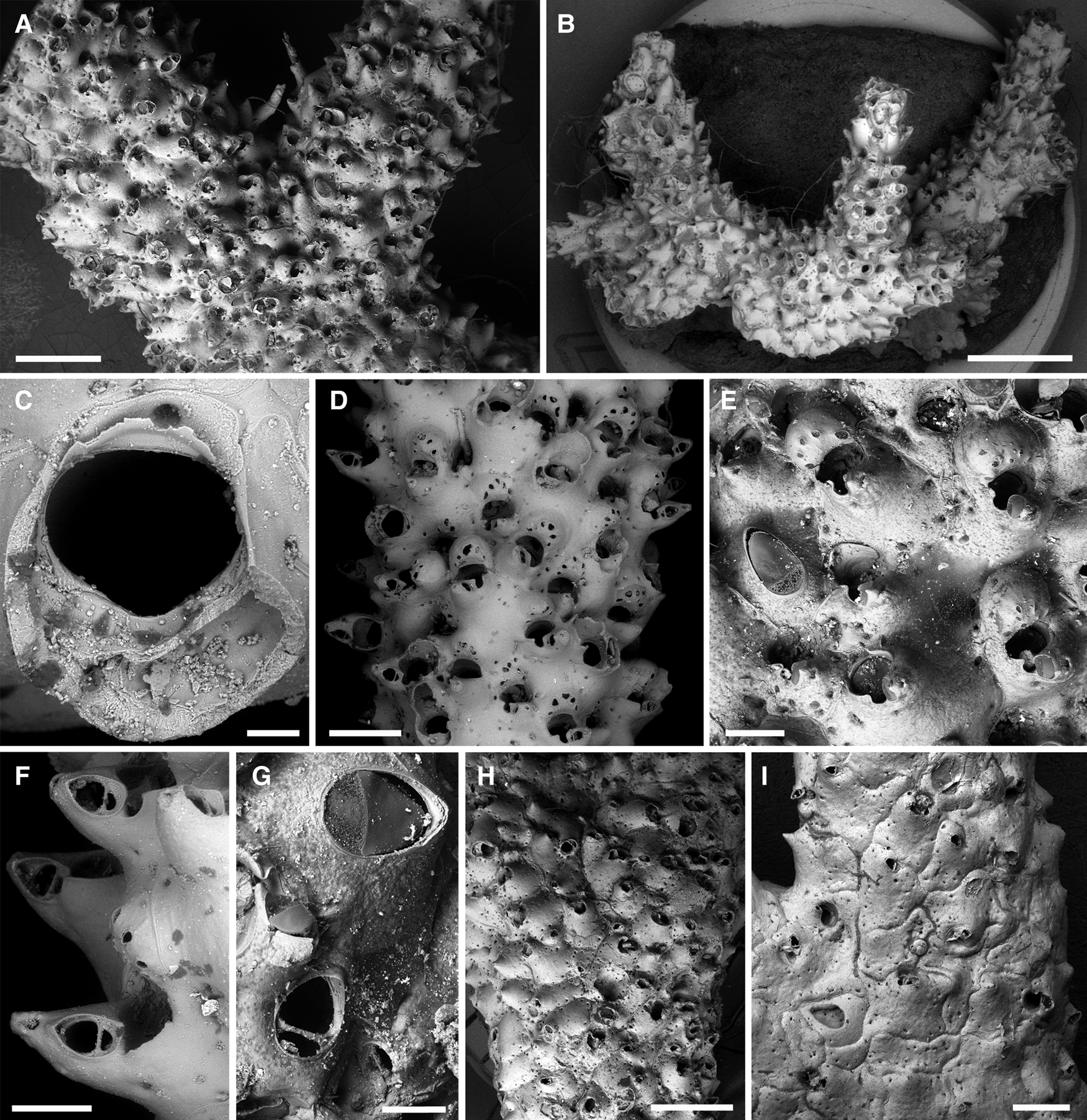
Figure 8. Buskea ovalis (Busk, 1881): (A) overview of lectotype (NHMUK 1887.12.9.793); (B) encrusting basal part of colony beginning to form erect branches (MNHN-IB-2008-2498); (C) orifice (MNHN-IB-2008-7449); (D) cylindrical branch with ovicellate zooids (MNHN-IB2008-7449); (E) ovicells and a large frontal avicularium (lectotype, NHMUK 1887.12.9.793); (F) close-up of suboral avicularia (MNHN-IB-2008-7449); (G) close-up of frontal avicularia, note that the crossbar in the lower avicularium is lacking a crossbar (lectotype, NHMUK 1887.12.9.793); (H) part of an older branch (NHMUK 1887.12.9.795); (I) basal part of an old colony with surface formed by kenozooids and frontal avicularia (NHMUK 1899.7.1.3454). Scale bars: A, H, 1 mm; B, 2 mm; C, 50 μm; D, I, 500 μm; E, F, 200 μm; G, 100 μm.
Part Palmicellaria skenei Ellis and Solander: Jullien & Calvet, Reference Jullien and Calvet1903: 154; Calvet, Reference Calvet1907: 431.
Harmerella dichotoma (Hincks): d'Hondt, Reference d'Hondt1975: 558 (listed).
Type material
Lectotype (here designated): NHMUK 1887.12.9.793, Station 75, figured specimen (Busk, Reference Busk1884: pl. 28, fig. 5), two colony fragments presumably of the same colony, one large branching fragment (1.3 cm long) and a small branch tip, from sand.
Description
Colony initially uni- to multilaminar encrusting, soon producing an erect colony with irregular conical branch tips that become massive and subcylindrical by means of frontal budding (Figure 8A, B), colony may reach several cm in size, branches up to 4 mm in diameter. Autozooids in younger parts of erect colony elongated oval in outline (L: 636 ± 44, 593–697, 4; W: 379 ± 24, 358–414, 4), frontal shield convex, thicker branches often covered by extremely broad and relatively flat polygonal autozooids or kenozooids during late astogeny (Figure 8H, I), zooids separated by fine sutures, that may be disguised by frontal thickening; frontal shield smooth with a single row of conspicuous areolar pores, suborally forming a broad pointed umbo on left or right (Figure 8D, E). Primary orifice suborbicular (Figure 8C), slightly broader than long (L: 134 ± 7, 125–142, 8; W: 154 ± 14, 129–171, 8), with a broad (~2/3 of proximal orifice width) and very shallow (~1/6 of total orifice length) sinus, condyles indistinct, very short and blunt; orifice becoming immersed by a peristome during ontogeny, aperture with a large drop-shaped pseudosinus or labial notch on one side, formed by a raised flap of the proximolateral peristomial margin and a blunt tooth produced by the proximolateral avicularian cystid (Figure 8D–F), pseudosinus occasionally closed in by these projections to form a circular foramen.
Ovicells hyperstomial during early ontogeny (L: 222 ± 23, 186–282, 16; W: 262 ± 20, 231–292, 16), later becoming subimmersed due to secondary calcification (Figure 8D, E), produced by the zooid distal to the maternal one; ectooecium smooth, proximomedially perforated by 5–7 large round, oval or drop-shaped pseudopores, proximal margin slightly concave, arched high above the orifice, not closed by the operculum.
Avicularia dimorphic (Figure 8E–G). Suboral adventitious avicularia single, positioned terminally on suboral umbo, directing distolaterally, oblique or even perpendicular to frontal zooecial surface, oval in outline (L: 102 ± 19, 62–138, 20; W: 77 ± 12, 51–97, 20); rostrum semielliptical, crossbar complete with or without short blunt columella. Frontally budded avicularia of variable size, occasionally almost as large as autozooid (L: 221 ± 43, 140–279, 17; W: 127 ± 19, 85–150, 17), oval in outline, often slightly narrower distally; rostrum slightly raised distally, pointing in various directions, crossbar complete, with or without short stout columella, uncalcified areas semicircular (proximally) and semielliptical (distally).
Ancestrula and early astogenetic encrusting part not observed.
Remarks
Although Buskea ovalis (Busk, 1881) is recognized in this combination today (Bock & Gordon, Reference Bock and Gordon2021b), to our knowledge the species was never cited again after its discovery by Busk (Reference Busk1881a, Reference Busk1884), and it has not been formally assigned to the genus Buskea before, which we do here. The specimen figured by Busk (Reference Busk1884: pl. 28, fig. 5; pl. 35, fig. 6) is designated as lectotype (NHMUK 1887.12.9.793). The material from the Faial-Pico Channel recorded as Palmicellaria skenei Ellis & Solander, Reference Ellis and Solander1786 by Calvet (Reference Calvet1907) is identical with Buskea ovalis. The same applies to the specimens that were collected during the ‘Biaçores’ cruise, which d'Hondt (Reference d'Hondt1975) recorded as Harmerella dichotoma (Hincks, Reference Hincks1862), a species that is today also placed in the genus Buskea.
In contrast to the type species of Buskea, B. nitida Heller, Reference Heller1867, the branches in B. ovalis are not composed of regular zooid series. Instead, frontal budding prevails just proximal to the young branch tips. Its colony growth form is thus rather reminiscent of species in the genus Turbicellepora Ryland, 1963, although B. dichotoma from the eastern Atlantic and Mediterranean Sea is also reported to produce frontally budded zooids, at least in proximal colony regions (Hayward & Ryland, Reference Hayward and Ryland1999: 348). It is therefore difficult to morphologically distinguish Buskea from Turbicellepora, while Galeopsis Jullien, 1903, though apparently encrusting, is another genus with a very similar colony morphology (see below).
Zooid length and width in B. ovalis were measured in early ontogenetic zooids at branch tips; frontally budded zooids in more proximal colony regions are distinctly broader. As most colonies available were either unbleached or poorly preserved, the crossbars of only a few suboral and frontally budded interzooidal avicularia could be observed. While a columella seems to be absent in some adventitious and interzooidal avicularia, in other avicularia it was present, being small and short in suboral avicularia, and distinctly larger and stout in interzooidal avicularia. More bleached material needs to be screened in order to determine the frequency of columella occurrences in the two avicularium types.
As already noted by Busk (Reference Busk1881a: 352, Reference Busk1884: 202), B. ovalis often uses the basal parts of thecate hydroids for settlement and support of its erect colony part, a habit shared with Celleporina ansata (Busk, Reference Busk1881a) described below. Buskea ovalis is endemic to the Azores, occurring from 57–1135 m depth. While most records were from 50–400 m depth, the specimens recovered from over 823 m (lectotype) and down to 1135 m (Jullien & Calvet, Reference Jullien and Calvet1903) are likely to be allochthonous. Colonies were reported from around the central Azorean islands of Pico, Faial, São Jorge, Terceira and Graciosa, as well as from around São Miguel, the Formigas islets and off W Flores (cf. d'Hondt, Reference d'Hondt1975), although material from the latter three localities could not be examined during this study and have to be regarded as uncertain.
Buskea fayalensis (Waters, Reference Waters1888) comb. nov.
(Figure 9)
Part Haswellia(?) auriculata Busk var. fayalensis Waters, Reference Waters1888: 31.
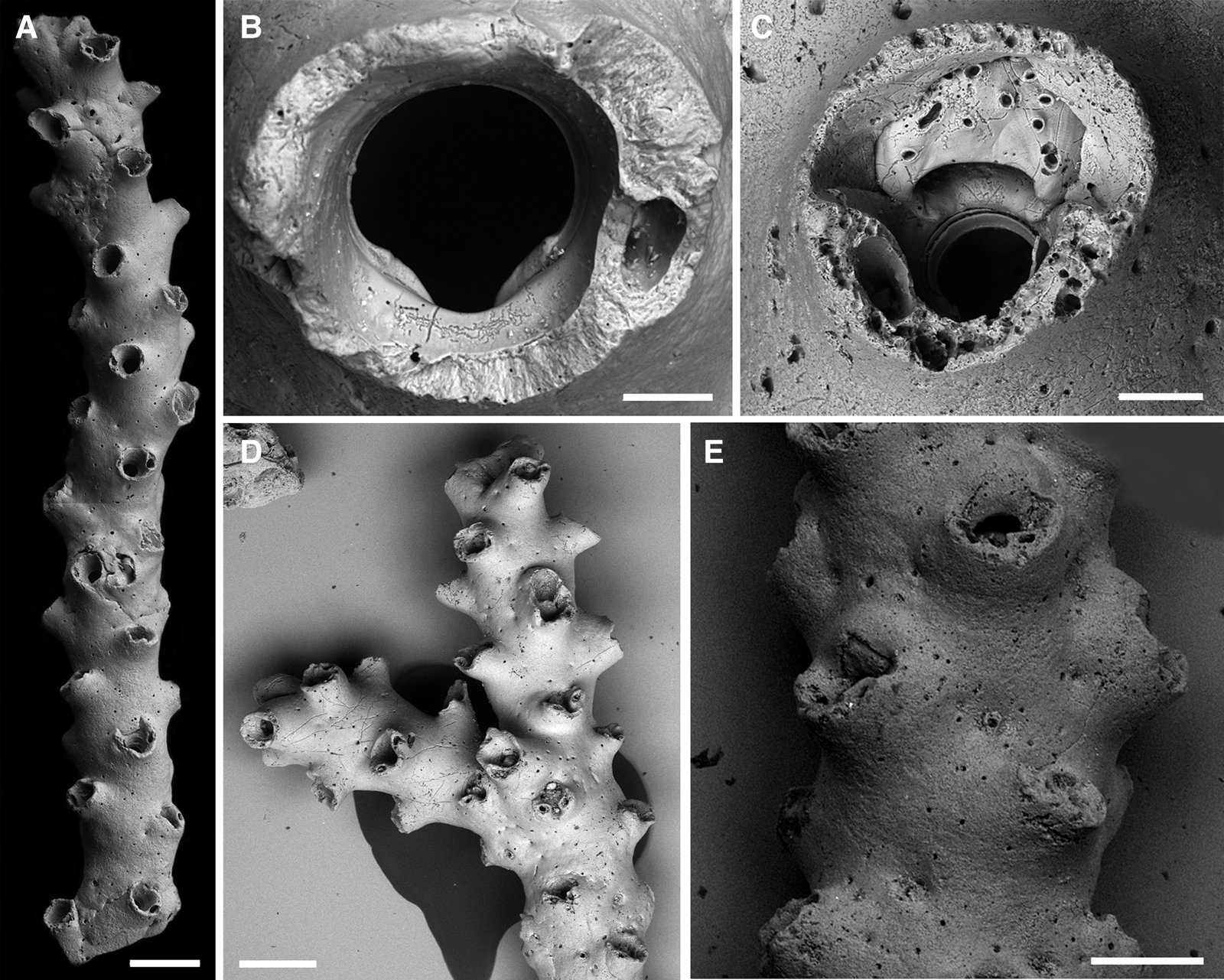
Figure 9. Buskea fayalensis (Waters, Reference Waters1888): (A) branch overview (lectotype, NHMUK 2022.8.5.1); (B) orifice with the base of a single peristomial avicularium (lectotype, NHMUK 2022.8.5.1); (C) ovicell (paralectotype, NHMUK 1934.2.12.4a); (D) branching colony, note that some of the zooids have paired avicularia on the peristome (paralectotype, MM 2768d); (E) older ovicellate branch, note that, while secondary calcification has thickened the branch, the peristomes are still protruding from the surface (paralectotype, MM 2769b). Scale bars: A, D, 500 μm; B, 50 μm; C, 100 μm; E, 300 μm.
Part Haswellia auriculata Busk: Jullien & Calvet, Reference Jullien and Calvet1903: 145, not pl. 17, fig. 3; Calvet, Reference Calvet1907: 446; Calvet, Reference Calvet1931: 77.
Not Buchneria fayalensis (Busk): Gautier, Reference Gautier1962: 217, fig. 19; Zabala & Maluquer, Reference Zabala and Maluquer1988: 155, text-fig. 421.
Type material
Lectotype (here designated): NHMUK 2022.8.5.1, Station FP, formerly part NHMUK 1934.2.12.4, of John Murray bequest, one colony fragment.
Paralectotypes: MM 2767, Waters coll., Station FP, sections of two colony fragments in Canada balsam, on slide. MM 2768, Waters coll., Station FP, five colony fragments (here sub-numbered a–e) on slide. MM 2769, Waters coll., Station FP, of the four colony fragments on a slide (here sub-numbered a–d), only specimens b and d are Buskea fayalensis while specimens a and c belong to a distinct Buskea species. NHMUK 1889.12.12.6, Waters coll., Station FP, four colony fragments in vial (sub-numbered a–d). NHMUK 1934.2.12.4, Station FP, John Murray bequest, three colony fragments in vial (here sub-numbered a–c).
Description
Colony erect, rigid, vinculariiform (i.e. with cylindrical branches, Figure 9A), branching irregular and at an angle between ~60–90° (Figure 9D), branches between bifurcations fairly long (>1 cm) and slender (~700 μm in diameter, not including the peristomes) and usually rather straight, zooids opening on all sides. Zooids arranged in 4 alternating longitudinal series, ideally back to back but often horizontally and/or vertically displaced (Figure 9A, D, E); zooecia distinctly longer than wide (L: 750 ± 64, 623–860, 20; W: 442 ± 45, 365–521, 18), frontal shield smooth, slightly convex, abruptly rising around orifice to form a prominent and thickly calcified peristome, entire peristome directing somewhat distally (Figure 9D); zooecial boundaries invisible, covered by secondary calcification, ~10 tiny pores faintly indicating the zooecial outline. Orifice only slightly longer than wide (L: 129 ± 5, 123–134, 7; W: 138 ± 1, 137–140, 7), anter transversely elliptical, poster usually with a rounded V-shaped sinus though occasionally wider U-shaped, framed by a pair of narrow but long and sloping condyles that disappear underneath the proximal orifice margin (Figure 9B).
Ovicell hyperstomial during early ontogeny, soon becoming incorporated into the peristome that is larger than in autozooids and which covers the distal part of the ooecium (Figure 9C), later further immersed by secondary calcification (Figure 9E), proximal half of ooecium exposed, showing the smooth ectooecium that is perforated by numerous pseudopores of varying size and shape, opening suborbicular, highly arched above orifice, not closed by the operculum.
Avicularia adventitious, most often single, occasionally absent or paired on (proximo)lateral peristomial rim (Figure 9C, D), directing distolaterally, crossbar complete, often with a distinct columella.
Ancestrula and early astogenetic encrusting part not observed.
Remarks
The histories of this and several superficially similar species are intricate. Busk (Reference Busk1884: 173) described Haswellia auriculata from the Tristan da Cunha Archipelago in the South Atlantic without any reference to specimens from the Azores. When later revising some of the ‘Challenger’ material, Waters (Reference Waters1888) vaguely attributed hitherto undetermined specimens from the Faial-Pico Channel to that species and suggested that this variety be termed H. auriculata var. fayalensis. In several subsequent works, Calvet (in Jullien & Calvet, Reference Jullien and Calvet1903; Calvet, Reference Calvet1907, Reference Calvet1931) recorded the species again from the Azores. Although referring to Busk's species H. auriculata and not to Water's subspecies in the synonymy, Calvet clarifies in the text that he accepts that the Azorean specimens belong to H. auriculata var. fayalensis (Jullien & Calvet, Reference Jullien and Calvet1903: 145). Not so Brown (Reference Brown1952: 216), who considered the Azores material identical to both H. auriculata and Vincularia pentagona d'Orbigny, Reference d'Orbigny1842 from the Malvinas (Falkland Islands), with the latter being the potential senior synonym of the three species. Considering the enormous distances between each of the three localities, however, the species are certainly all distinct. Whereas H. auriculata var. fayalensis has been treated as a distinct species since the second half of the 20th century, though temporarily assigned to the genus Buchneria Harmer, Reference Harmer1957 by e.g. Gautier (Reference Gautier1962) and Zabala & Maluquer (Reference Zabala and Maluquer1988), V. pentagona and H. auriculata are still regarded as synonymous (Gordon, Reference Gordon1984: 116; Bock & Gordon, Reference Bock and Gordon2021c).
Both Buchneria fayalensis (Waters) and V. pentagona are currently placed in the genus Galeopsis Jullien, 1903 (Gordon, Reference Gordon1984; Bock & Gordon, Reference Bock and Gordon2021d). This assignment is also problematic, however, and needs yet to be clarified, as the type species of Galeopsis, G. rabidus Jullien, 1903 from the Azores, is not well defined, while the holotype (by monotypy) seems to be lost (Tricart & d'Hondt, Reference Tricart and d'Hondt2009). According to Jullien & Calvet (Reference Jullien and Calvet1903: 145), the specimen is a unilaminar encrusting colony from ‘Hirondelle’ Station 247 that lacks ovicells and grows on a reteporiform bryozoan. Another specimen from the Paris collection (MNHN-IB-2008-3721), doubtfully from the same station and encrusting a coral, does not seem to be the figured colony, and may even belong to a different species (BB, pers. observ.). In any case, the preservation of this colony, as well as of another specimen from an unknown station filed under G. rabidus (MNHN-IB-2008-843), does not allow the species to be precisely identified and characterized. Additional specimens present at the MOM could, unfortunately, not be studied using SEM as a new directive, which became effective during the course of this work, does not permit to take on loan specimens from the Calvet collection any more.
The reason for the affiliation of the erect-growing B. fayalensis to the genus Galeopsis with encrusting colonies was the discovery by Gordon (Reference Gordon1984: 116, pl. 46, fig. C) of the transitional colony stage in a species from New Zealand he referred to V. pentagona. The zooids in both colony stages are similar to the erect B. fayalensis and the encrusting G. rabidus from the Azores, changing considerably in morphology from the encrusting to the erect part, and Gordon thus placed V. pentagona, B. fayalensis as well as another southern hemisphere species in Galeopsis. The problem with this decision is, however, that the southern hemisphere species (see also Moyano, Reference Moyano1985) are characterized by ovicells with an incompletely calcified ectooecium that centrally exposes the endooecium (i.e. producing a tabula as in Celleporina Gray, 1848 or Buffonellaria Canu & Bassler, Reference Canu and Bassler1927). In contrast, both Galeopsis fayalensis (Figure 9C) and presumably also G. rabidus (as identified by Calvet, Reference Calvet1931: 78) have an entirely calcified yet pseudoporous ectooecium as in Buskea (see above) and Turbicellepora Ryland, 1963. The southern hemisphere taxa should therefore not be congeneric with the Azorean species.
With its smittinid-type ooecium, the morphology of the orifice and suboral avicularium as well as its erect colony form and structure, G. fayalensis in fact conforms to the characters of the North Atlantic genus Buskea Heller, 1867. While the suboral pseudosinus or spiramen is lacking in the erect colony part, in contrast to the hitherto described Buskea species, a thick prominent peristome encircles the entire orifice instead, incorporating the suboral avicularium. This difference, however, may be regarded as only of minor systematic importance, and the sinus may be lacking only as a result of the avicularia being placed more distally on the peristome than in other Buskea species. Although the morphology of zooids in the encrusting parts of Buskea species is not known, there are no apparent character differences that justify the exclusion of the erect G. fayalensis from Buskea, and we here assign the species to that genus. In order to come to a conclusion concerning the synonymy of Buskea fayalensis and G. rabidus on the one hand, and the characterization of the genus Galeopsis on the other, new material showing a transition from the encrusting to the erect colony part needs to be collected from the Azores, a neotype with an ovicell should be selected for G. rabidus, and the morphology of encrusting zooids in other Buskea species needs to be determined.
When introducing the species, Waters (Reference Waters1888) considered Buskea fayalensis to also occur off Capri (Mediterranean Sea). Potential specimens from Capri in the Waters collection have thus to be regarded as syntypes. Based on (1) the geographic distance between the Azores and the Mediterranean Sea, (2) the fact that the species grows in depths where rafting of adult colonies is unlikely, and (3) the figures provided by Zabala & Maluquer (Reference Zabala and Maluquer1988) and Gautier (Reference Gautier1962), however, we regard the Mediterranean population as a distinct species.
Even in the Azores several other Buskea species are present: besides B. ovalis and B. fayalensis, d'Hondt (Reference d'Hondt1975) recorded Buskea billardi (Calvet, Reference Calvet1906), originally described from NW Africa, and the Mediterranean/NE Atlantic Buskea dichotoma (Hincks, Reference Hincks1862) was reported by Jullien & Calvet (Reference Jullien and Calvet1903) and Calvet (Reference Calvet1907, Reference Calvet1931). Several additional morphotypes, including encrusting colonies with smittinid-type ovicells, were observed among the historical and newly collected material during this study. In fact, in one sample among Waters' syntypes (MM 2769) two of the four colony fragments (specimens MM 2769a, c) belong to a distinct and as yet undescribed species, Buskea sp. (Figure 10): the branches are composed of up to six alternating zooidal series while the zooids are smaller, frontal spatulate avicularia occur, the peristomial avicularia are usually paired, the ooecium differently exposed, and the orificial sinus is distinctly wider. It may be that the image of, and remarks on, the specimens Jullien & Calvet (Reference Jullien and Calvet1903: 145, pl. 17, fig. 3) reported may also represent this species, while specimen MOM INV-22602 (identified as Haswellia auriculata) belongs to B. fayalensis.

Figure 10. Buskea sp.: An older branch fragment with ovicellate zooids, paired peristomial avicularia, and frontal spatulate avicularia (MM 2769a). Scale bar: 300 μm.
In the absence of images of the species in Waters (Reference Waters1888), we here select the most well-preserved specimen from the syntype series (NHMUK 2022.8.5.1) as lectotype of B. fayalensis. Althouth the specimen is from the Robert Murray bequest (NHMUK1934.2.12.4), which came to the museum later than Waters' bryozoan collections, Waters (Reference Waters1888: 1) stated that he had seen Murray's specimens in Edinburgh. The material thus has to be considered as syntypic.
Although Waters described the operculum, all of the Azorean colonies that remain in the collections today are dead, often physically damaged and bioeroded, with no organic tissue preserved. Branching occurs seldom and often at an angle of ~90°, which may suggest that the new branch budded from a fragment that was lying on the seafloor rather than representing a natural bifurcation of an undamaged colony. Zooecia with undamaged frontal shields often exhibit a second or more orifice rims, indicating the presence of one or more intramural buds, the formation of which are likely to have been induced following predation of the zooids (cf. Berning, Reference Berning2008). Owing to the damaged peristomes, none of the terminal avicularia are entirely preserved; measurements could therefore not be taken. Also, the larger part of the ovicells is covered by the peristome, obscuring their dimensions.
Buskea fayalensis is regarded as endemic to the Azores and was recorded from depths between 90–1260 m in the Faial-Pico Channel.
Genus Celleporina Gray, 1848
Celleporina ansata (Busk, Reference Busk1881a) comb. nov.
(Figure 11)
Part Cellepora ansata Busk, Reference Busk1881a: 356.

Figure 11. Celleporina ansata (Busk, Reference Busk1884): (A) overview of lectotype (NHMUK 1887.12.9.806b); (B) overview of lectotype of Lekythopora laciniosa Calvet, Reference Calvet1906 (MNHN IB-2008-55); (C) ovicellate zooids and interzooidal avicularia (MNHN-IB-2008-55); (D) orifice (MNHN-IB-2008-55); (E) close-up of autozooidal apertures (paralectotype, NHMUK 1887.12.9.806c); (F) ancestrula (NHMUK 1887.12.9.795). Scale bars: A, B, 500 μm; C, 300 μm; D, 50 μm; E, F, 100 μm.
Cellepora ansata Busk, Reference Busk1881b: pl. 27, fig. 1.
Part Cellepora ansata Busk: Busk, Reference Busk1884: 204, pl. 30, fig. 4; pl. 36, fig. 17.
Part Schizoporella costazii (Audouin): Jullien & Calvet, Reference Jullien and Calvet1903: 84, 137.
Lekythopora laciniosa Calvet, Reference Calvet1906: 166; Calvet, Reference Calvet1907: 445, pl. 29, figs 13 & 14; Souto et al., Reference Souto, Reverter-Gil and De Blauwe2014: fig. 3.
Part Costazzia costazzii [sic] (Audouin): Calvet, Reference Calvet1931: 115.
Osthimosia ansata Busk: Pouyet, Reference Pouyet1973: 46 (listed).
Celleporina laciniosa Calvet: Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015: 94 (listed).
Celleporina cf. laciniosa Calvet: Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015: 94 (listed).
Type material
Lectotype of Cellepora ansata (here designated): NHMUK 1887.12.9.806b, Station 75, figured specimen (Busk, Reference Busk1884: pl. 30, fig. 4), the central one of three colonies on thecate hydroid stem, from sand.
Lectotype of Lekythopora laciniosa (here designated): MNHN-IB-2008-55, Calvet coll., Talisman, 13/8/1883, Station 125, Faial-Pico Channel (no exact position provided), 80–115 m, one spindle-shaped colony on the stem of a thecate hydroid, on slide.
Description
Colony initially encrusting unilaminar and biserial (on hydroid stems) to multiserial, later developing either spindle-shaped or massive celleporiform to branching colonies owing to frontal budding (Figure 11A, B). Autozooids in encrusting parts of colony flask-shaped, distinctly longer than wide (L: 608 ± 70, 497–717, 20; W: 292 ± 51, 221–383, 20), with a rhomb-like proximal part and a narrower distal peristome with parallel lateral margins that comprises almost half of the total zooid length; peristome at an acute angle to frontal surface and pointing distally; in frontally budded zooids the proximal part is much reduced in length and the peristome is almost perpendicular to frontal surface (Figure 11C); zooids separated by narrow grooves, lateral walls with several basal pore chambers per neighbouring zooid, communication pores uniporous. Frontal shield slightly convex, surface faintly to distinctly nodular, initially with a single row of 4–8 small areolar pores that become more distinct during ontogeny. Peristome well developed, frontally compressed, aperture therefore distinctly wider than long, distal and proximal apertural margins usually with a variably developed bow framed by a pair of terminally positioned avicularia (Figure 11E); distal part of the peristome formed by the frontal shield of the distal autozooid or kenozooidal ooecium. Primary orifice suborbicular (Figure 11D), almost as broad as long (L: 120 ± 19, 105–164, 12; W: 116 ± 16, 99–143, 12), with a broad (~2/3 of total orifice width) and deep (almost 1/4 of total orifice length) sinus, condyles indistinct.
Ovicells prominent or subimmersed depending on position and age of the neighbouring zooids, produced by a much reduced kenozooid at the base of the peristome, ooecium hemispherical, almost twice as wide as long (L: 116 ± 16, 91–153, 14; W: 215 ± 26, 154–255, 14), tabula relatively flat and smooth with a single series of marginal pores and some scattered central ones when larger, proximal margin raised and occasionally forming a mucro (Figure 11C, E), ovicell opening arched high above the orifice, not closed by the operculum. Ovicells may already be produced by zooids in the encrusting part of the colony, and also during very early astogeny (from 4th generation zooids onwards).
Avicularia polymorphic (Figure 11C, E), adventitious avicularia paired, small (L: 60 ± 15, 35–87, 20; W: 46 ± 11, 29–61, 20), positioned terminally on proximolateral peristome, directing distolaterally or laterally, oblique to frontal zooecial surface, oval in outline; rostrum semielliptical, distally denticulate, crossbar complete, without columella, proximal uncalcified area semicircular, distal area semielliptical. Frontally budded avicularia of variable size and shape (L: 162 ± 47, 88–269, 20; W: 70 ± 19, 41–110, 20), either with parallel lateral margins or slightly spatulate; mandible relatively weakly sclerotized and somewhat shrivelled in dried specimens, rostrum distally raised and denticulate, pointing in various directions; crossbar complete, without columella but occasionally thickened centrally on its proximal side, an immersed calcified shelf comprising over half of palate length, distal uncalcified area suborbicular to elliptical, proximal area elliptical to reniform.
Ancestrula oval (L: 266 ± 18, 252–286, 3; W: 187 ± 39, 151–229, 3), convex, surface densely pitted (Figure 11F); opesia reduced to a distal D-shaped area entirely covered by an operculum, widest at about mid-distance (L: 67 ± 2, 64–69, 4; W: 96 ± 7, 90–106, 4), proximal margin straight, 7 spines surrounding the aperture with a distinct gap between the distalmost pair where the first generation autozooid is formed.
Remarks
Unfortunately, the only three-dimensionally preserved syntypes of Cellepora ansata Busk, 1881, which all encrust stems of thecate hydroids, are covered by glue, concealing many of the species' characters. The least affected colony has here been designated as lectotype.
The drawing Busk provided in the ‘Challenger’ report in 1884 (pl. 30, fig. 4), after having introduced C. ansata in 1881, does not bear much resemblance with the actual specimens. Unsurprisingly, Calvet (Reference Calvet1906) newly described the same species again ~20 years later, with his Lekythopora laciniosa being easily recognizable from his images (Calvet, Reference Calvet1907: pl. 29, figs 13 & 14). In his next work on Azorean bryozoans, Calvet (Reference Calvet1931: 115) then synonymized L. laciniosa with the ubiquitous Costazzia costazzii [sic] (Audouin, Reference Audouin and Panckoucke1826) (now regarded as Celleporina costazii), specimens of which he also reported from the Mediterranean Sea and Gorringe Bank in the eastern Atlantic besides the Azores. Celleporina costazii is a species complex, however, and the Azorean specimens are specifically distinct from the European ones assigned to that species. As no morphological differences were observed at zooidal level between the types of C. ansata and L. laciniosa (for which a lectotype has here been designated), we regard the latter as junior subjective synonym of the former. Pouyet (Reference Pouyet1973: 46) later transferred C. ansata to the genus Osthimosia Jullien, 1888, under which the species was known until today, but this decision is not upheld here as the ovicell is clearly that of a Celleporina, and the species is accordingly transferred to that genus.
While one specimen among material recorded as Schizoporella costazii (Audouin, Reference Audouin and Panckoucke1826) from the Fayal-Pico Channel by Jullien & Calvet (Reference Jullien and Calvet1903: 84, 137) proved to be Celleporina ansata, it is also likely that some or all of the specimens recorded as Costazia costazii by d'Hondt (Reference d'Hondt1975: 558), are conspecific with Busk's species. Among Busk's syntype specimens there is one misidentified colony (NHMUK 1899.7.1.3231, Station 75), however, demonstrating that there is at least another Celleporina species present in the central Azores. The zooids in this pisiform colony have, among other differences, a single lateral avicularium on the peristome, and the vicarious avicularia are distinctly spatulate.
Wisshak et al. (Reference Wisshak, Berning, Jakobsen and Freiwald2015: 94) recently reported C. ansata as Celleporina laciniosa from 60 and 150 m depth, and as C. cf. laciniosa from 150 m depth off Faial. The latter taxon, which formed biserial, vine-like colonies on thecate hydroid stems, was initially thought to differ slightly from the former, which produced massive branching colonies on hard substrata at 60 m after two years of exposure, as well as broad, encrusting unilaminar and multiserial colonies on the settlement panels at 150 m. Close inspection of the zooids, however, and comparison with the types of C. ansata, suggests that all specimens belong to Busk's species. The different colony growth forms may thus be a response to growth on different substrata and in different environments (i.e. nutrient regimes). The relatively short exposure times of up to two years was likely not enough for larger colonies to form at greater depths.
Measurements of zooid length and width were taken on zooids encrusting the flat substrate of settlement panels (Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015). Frontally budded zooids in celleporiform parts of the colony are significantly shorter and usually partly covered by neighbouring zooids, making it difficult to measure them. Most characters are highly variable in size, particularly the length of the frontal avicularia varies threefold, and the largest adventitious peristomial avicularium is more than twice the size of the smallest one recorded. Cellepora ansata has so far only been recorded in the central Azores from 60–820 m depth.
Family phidoloporidae Gabb & Horn, Reference Gabb and Horn1862
Genus Reteporella Busk, 1884
Reteporella atlantica (Busk, Reference Busk1884) comb. nov.
(Figure 12)
Retepora atlantica Busk, Reference Busk1884: 116, text-fig. 15, pl. 28, fig. 1, 1a, 1b; Waters, Reference Waters1888: 20; Calvet, Reference Calvet1907: 452.

Figure 12. Reteporella atlantica (Busk, Reference Busk1884): (A) overview of a colony (MNHN-IB-2008-4296); (B) overview of lectotype (NHMUK 1899.7.1.2904); (C) ovicellate zooids during relatively late ontogeny as evidenced by the presence of frontal avicularia (lectotype, NHMUK 1899.7.1.2904); (D) close-up of the different types of suboral and frontal avicularia (lectotype, NHMUK 1899.7.1.2904); (E) orifice (MNHN-IB-2008-4296); (F) spines in lateral view and a giant suboral avicularium at lower centre; (G) abfrontal side of lectotype (NHMUK 1899.7.1.2904); (H) close-up of abfrontal avicularia (lectotype, NHMUK 1899.7.1.2904). Scale bars: A, 5 mm; B, 500 μm; C, F, 200 μm; D, H, 100 μm; E, μm; G, 1 mm.
Retepora marsupiata Smitt: Calvet, Reference Calvet1931: 102.
?part Sertella marsupiata – d'Hondt 1975a: 580.
Reteporella sp.: Wisshak et al., Reference Wisshak, Berning, Jakobsen and Freiwald2015: 95 (listed).
Type material
Lectotype (here designated): NHMUK 2016.6.9.5, Station 75, one colony fragment, figured specimen (Busk, Reference Busk1884: pl. 28, fig. 1, erroneously given as Retepora cellulosa in the caption), formerly part of NHMUK 1899.7.1.2904.
Description
Colony erect, rigid, fenestrate (Figure 12A), irregularly cup-shaped with a widely lobed and infolded rim, >7 cm in diameter and height, of whitish/pearly colour at greater depth and bright orange in shallower water; outer abfrontal surface covered by kenozooids, inwards-facing surface bearing autozooids, trabeculae with 2–4 or occasionally 5 alternating longitudinal autozooid series (W: 460 ± 110, 297–788, 75) (Figure 12B), fenestrulae variably shaped but most often elongate oval (L: 1076 ± 177, 750–1540, 55; W: 440 ± 101, 203–667, 55). Autozooids initially elongate rectangular to polygonal (distance between midpoints of orifices of obliquely adjacent zooids: 265 ± 33, 192–321, 47), separated by narrow grooves but zooid boundaries soon obliterated owing to secondary calcification, zooecial surface relatively smooth in some parts but mostly nodular (Figure 12D), early ontogenetic zooids with a single row of small lateral areolar pores that become larger and more scattered on the surface during ontogeny; interzooidal communication via uniporous septula.
Orifice rounded D-shaped, broader than long (L: 72 ± 8, 63–85, 10; W: 97 ± 6, 89–107, 10), distal margin denticulate, proximal margin straight to shallowly concave (Figure 12E); condyles short but broad, blunt, situated at about mid-distance of orifice, lateral orifice margin in early ontogenetic zooids equipped with 4 long spines (Figure 12F), with a distinct gap between the distalmost pair while only the proximal pair persists in ontogenetically older auto- and ovicellate zooids. Peristome formed by two proximolateral flaps in early ontogeny, the larger one incorporating the suboral avicularium, the proximal end of which merging with the tip of the opposing flap to form a short suture-zone and a round or drop-shaped pseudosinus (L: 48 ± 8, 30–71, 40), the central avicularium cystid occasionally producing one or more short peristomial denticles distally.
Ovicells recumbent on frontal shield of the distal zooid, becoming (sub)immersed and almost completely covered by secondary calcification during ontogeny (Figure 12C), ooecium globular, slightly longer than wide (L: 197 ± 20, 163–231, 15; W: 169 ± 8, 155–182, 15), not closed by the operculum; ectooecium entirely calcified except for a narrow central fissure extending for more than half its length and staying exposed throughout ontogeny (L: 114 ± 14, 91–143, 28), a short proximal labellum with a straight edge overhanging the orifice.
Avicularia adventitious, polymorphic (Figure 12D, F), positioned suborally, frontally and abfrontally. Three morphotypes of single suboral avicularia present, all pointing proximolaterally and with the frontal plane obliquely positioned relative to colony surface; suboral avicularium in most zooids small (L: 58 ± 5, 47–64, 21; W: 57 ± 5, 42–63, 21) and round to oval with a semicircular rostrum, distal margin toothed and raised, crossbar complete with a very short, broad and blunt columella, palate a narrow immersed shelf, uncalcified area distal to crossbar D-shaped, proximal uncalcified area semicircular; semicircular mandible with a tiny round lucida. Other zooids produce a triangular suboral avicularium of intermediate size (L: 157 ± 20, 126–210, 21; W: 59 ± 5, 50–67, 21), rostrum very elongate, distally distinctly hooked, crossbar complete with a long straight columella, palate occupied by an immersed calcified shelf apart from a proximal triangular-trifoliate opesia, uncalcified area proximal to crossbar oval to semicircular; mandible very weakly sclerotized and with a round lucida at its proximal end. In yet a few other zooids the suboral avicularium is gigantic (L: 320 ± 19, 294–348, 9; W: 91 ± 7, 81–101, 9) while being similar in general morphology to the smaller triangular one; in most cases positioned distal to an ovicell with the cystid covering the entire frontal shield of the zooid, rostrum very elongate triangular, distally conspicuously hooked and distinctly projecting beyond the trabecular surface, mandible also very weakly sclerotized and with a small proximally placed lucida.
Another two types of adventitious avicularia, which are morphologically similar to the oval as well as the triangular suboral avicularia of intermediate size yet being smaller on average, may additionally be budded anywhere on the surface of the frontal autozooids during various ontogenetic stages (Figure 12C, D, H). The small round to oval avicularia may be obliquely positioned relative to the plane of the zooids (L: 44 ± 7, 36–54, 8; W: 42 ± 5, 36–49, 8). The larger elongate-triangular avicularia are parallel to the zooidal surface and very variable in size (L: 140 ± 35, 93–230, 30; W: 51 ± 9, 39–77, 30), the rostrum is only slightly hooked distally and the uncalcified area distal to the crossbar is more distinctly trifoliate than in the suboral avicularium; in shorter avicularia the lateral margins of the rostrum are often not straight but slightly incurved.
On the abfrontal surface these two types are present as well: while the small round to oval avicularium is of similar size than the frontal one (L: 44 ± 4, 39–47, 3; W: 39 ± 3, 36–42, 3), the triangular one is about the same size as the intermediate suboral one (L: 152 ± 39, 100–232, 26; W: 50 ± 9, 38–73, 26). Mandibles in all of the triangular avicularia very weakly sclerotized.
Abfrontal kenozooids of variable size and shape, irregularly positioned, separated by slightly raised and straight or undulating vibices, surface granular with several small scattered pores (Figure 12G).
Ancestrula and early astogeny not observed in the available material.
Remarks
With its large, erect and reticulate colonies, Reteporella atlantica is one of the most conspicuous and ubiquitous bryozoans in the Azores, and has been recorded several times after its discovery, though occasionally under the name Sertella marsupiata (Smitt, Reference Smitt1873), with which R. atlantica has once been synonymized. Smitt's species from Florida has recently been redescribed as Reteporellina marsupiata by Winston (Reference Winston2005: 115), however, and is clearly distinct. A comparison with other species of the genus is as yet difficult as most historical taxa have never been revised and figured using SEM.
Characteristic of R. atlantica and several other morphologically related species is the great variability in size and morphology of suborally and frontally budded adventitious avicularia. Most common is a small elliptical avicularium, the same type of which is also occurring on the frontal and abfrontal surfaces, though somewhat smaller in dimensions. Particularly, but not exclusively, in ovicellate regions of the colony, an elongated triangular suboral avicularium of variable size replaces the smaller elliptical one. This type is again budded also on both the frontal and abfrontal surfaces during ontogeny, with the frontally budded avicularium being slightly smaller in size than the suboral and abfrontal ones. In a few zooids in ovicellate parts of the colony, a gigantic, distally conspicuously hooked avicularium may be produced suborally (Figure 12F). In this type, the avicularian cystid covers the autozooid's entire frontal surface.
A similarly large avicularium has been reported to occur in Reteporella grimaldii Jullien, 1903 from the eastern Atlantic (Reverter-Gil & Fernández-Pulpeiro, Reference Reverter Gil and Fernández Pulpeiro1999: fig. 5D, E), as well as in species from the Mediterranean Sea reported under this name (Hayward & KcKinney, 2002: fig. 42D, and references therein), which are, however, very likely specifically distinct from the nominal species (see Baptista et al., Reference Baptista, Berning, Curto, Waeschenbach, Meimberg, Santos and Ávila2022). Several characters, such as the absence of frontal avicularia with pointed rostra in the R. grimaldii species complex, testify for the distinctness of these taxa and R. atlantica, however.
A curious fact concerning the mandibles in all of the suboral, frontal and abfrontal triangular avicularia in R. atlantica was already noted by Busk (Reference Busk1884: 117). Despite the often enormous size of the rostra, the mandibles are very weakly sclerotized and the occlusor muscles attach at their extreme proximal ends. This is in contrast to other Reteporella species in which strong muscles attach to the centre of the mandible, forming a distinct foramen for insertion. Thus, despite the large rostrum and avicularian cystid size, the mandibles in the Azorean species do not seem to be able to act as a forceful anti-predator defence. As with so many other avicularium types (e.g. the small, oval suboral ones), their function, as well as the reasons for the species to invest so much energy into such large structures, remain unknown to date.
Reteporella atlantica was originally reported from off northern Pico at ~820 m, and particularly from between the islands of Faial and Pico at depths of ~60–130 m (Waters, Reference Waters1888). D'Hondt (Reference d'Hondt1975) later recorded the species from all three island groups. Preliminary results of a molecular genetic study on Azorean Reteporella suggests that the species is indeed widespread in the archipelago (Baptista et al., Reference Baptista, Berning, Curto, Waeschenbach, Meimberg, Santos and Ávila2022). Without an analysis of the historical material, however, it is impossible to judge whether all or only part of the specimens belong to R. atlantica as several closely related species co-occur in each group of islands. Accordingly, we here regard as R. atlantica only those specimens from around the type locality, pending further studies.
Owing to the geographic distance to the Azores, it is highly unlikely that specimens reported as Retepora atlantica from Madeira (Waters, Reference Waters1899: 16), and as Sertella atlantica from Cape Verde (Cook, Reference Cook1968: 200), the Canary Islands (Cook, Reference Cook1968: 200; Arístegui Ruiz, Reference Arístegui Ruiz1984: 204), northern Iberia (d'Hondt, Reference d'Hondt1977: 61) and the Strait of Gibraltar (Harmelin & d'Hondt, Reference Harmelin and d'Hondt1992: 30), are indeed conspecific with the Azorean species.
Discussion
Of the 21 species recorded from the greater Azores region during the HMS ‘Challenger’ cruise (Table 1), which represent ~10% of the bryozoan species hitherto described from the archipelago, 12 are here considered to have been validly introduced as new taxa by Busk (Reference Busk1881a, Reference Busk1884) and Waters (Reference Waters1888), and are accordingly revised or reported above. The remaining nine species comprise taxa that were recorded under the names of previously established taxa from elsewhere, or were newly described based on a type from a distinct geographic locality but considered to also occur in the Azores (Table 1). As such, Membranipora galeata var. multifida Busk, Reference Busk1884 (nowadays regarded as Chaperiopsis multifida) was introduced for a specimen from the Cape of Good Hope (Busk, Reference Busk1884: 64). The figured specimen (and therefore the potential lectotype) of Retepora imperati Busk, Reference Busk1884, which is placed in the genus Schizoretepora Gregory, Reference Gregory1893 today, is from Cape Verde (Madurell et al., Reference Madurell, Spencer Jones and Zabala2019), while the Azorean specimens of the syntype series belong to the genus Phidolopora Gabb & Horn, Reference Gabb and Horn1862 (BB, pers. observ.). Comparison between colonies from the respective type localities and material from the Azores suggests that the non-Azorean populations are specifically distinct. The same applies to the Azorean taxa assigned to previously established species from the European continental shelf, such as Micropora coriacea (Esper, Reference Esper1791) and Hippothoa divaricata Lamouroux, Reference Lamouroux1821 (BB, pers. observ.). The Azorean representatives remain to be newly described and are accordingly given as ‘n.sp.’ in Table 1.
Table 1. Cheilostomatid and cyclostomatid bryozoan species collected on the ‘Challenger’ cruise from the greater Azores region, and cited from the archipelago thereafter. Species investigated here in bold

FP, unnamed ‘Challenger’ station in the Faial-Pico Channel.
One result of this revision, adding to other previous taxonomic works on the archipelago's bryozoan fauna (e.g. Berning, Reference Berning2013; Berning et al., Reference Berning, Achilleos and Wisshak2019, Reference Berning, Spencer Jones and Vieira2021; Harmelin et al., Reference Harmelin, Bishop, Madurell, Souto, Spencer Jones and Zabala2019; Haugen et al., Reference Haugen, Novosel, Wisshak, Berning, Wyse Jackson and Zágoršek2020), is an increase in the number of species endemic to the Azores, further substantiating the biogeographic distinctness of the archipelago within the wider Macaronesian region that was recently highlighted by Freitas et al. (Reference Freitas, Romeiras, Silva, Cordeiro, Madeira, González, Wirtz, Falcón, Brito, Floeter, Afonso, Porteiro, Viera-Rodríguez, Neto, Haroun, Farminhão, Rebelo, Baptista, Melo, Martínez, Núñez, Berning, Johnson and Ávila2019). In an ongoing study, the remaining historical species as well as newly collected material (George et al., Reference George, Arndt, Wehrmann, Baptista, Berning, Bruhn, Carvalho, Cordeiro, Creemers, Defise, Domingues, Hermanns, Hohlfeld, Iwan, Janßen, Jeskulke, Kagerer, Kaufmann, Kieneke, Loureiro, Madeira, Meyer, Narciso, Ostmann, Pieper, Pointner, Raeke, Silva, Springer and Wilsenack2018) will be examined in order to more comprehensively understand the archipelago's bryozoan diversity (e.g. Baptista et al., Reference Baptista, Berning, Curto, Waeschenbach, Meimberg, Santos and Ávila2022). Moreover, the presence of fossil bryozoans in early Pliocene strata in the island of Santa Maria (Ávila et al., Reference Ávila, Ramalho, Habermann, Quartau, Kroh, Berning, Johnson, Kirby, Zanon, Titschack, Goss, Rebelo, Melo, Madeira, Cordeiro, Meireles, Bagaço, Hipólito, Uchman, da Silva, Cachão and Madeira2015) will aid in comprehending the origin of the Azorean fauna. The recent taxonomic efforts are therefore a prelude to our comprehension of the true richness, geographic distribution, evolution and ecology of Azorean bryozoans.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000231.
Acknowledgements
We would like to acknowledge Michèle Bruni (MOM) for help with the collection at Monaco, as well as Pierre Lozouet and Jérôme Mainguy at the MNHN in Paris. Rachel Petts and Henry McGhie (MM) gratefully sent on loan the Waters material from Manchester. As usual, Dennis Gordon (NIWA) and Phil Bock are thanked for sharing their bryozoan wisdom, and BB is grateful for Sérgio Ávila's (Universidade dos Açores) long-term support in his quest to better understand the Azorean bryozoans, Recent and fossil.
Author contributions
BB formulated the research question, designed the study, carried out the study, analysed the data, interpreted the findings, and wrote the article. MESJ formulated the research question, designed the study, carried out the study, and wrote the article.
Financial support
This study was funded by the SYNTHESYS Project (http://www.synthesys.info/), which is financed by the European Community Research Infrastructure Action under the FP7 ‘Capacities’ Program, and which enabled BB to study Azorean type material at the NHMUK (GB-TAF-3347) and the MNHN (FR-TAF-1902, −5579). Additional financial support was provided by the Portuguese Funds through FCT – Foundation for Science and Technology under the UID/BIA/50027/2013 and POCI-01-0145-FEDER-006821.
Competing interests
The authors declare none.















