Introduction
Deforestation and fragmentation are among the factors that introduce illnesses into wild communities (Gilbert and Hubbell Reference Gilbert and Hubbell1996). The deforestation of land occupied by rainforests has been associated with the emergence of diseases that attack mankind such as malaria, yellow fever, dengue and Chagas disease (Real Reference Real1996). Due to habitat loss, environments are fragmented and become saturated by species that are typical of deforested areas (Marini Reference Marini2001, Giraudo et al. Reference Giraudo, Matteucci, Alonso, Herrera and Abramson2008). Therefore, species that live in fragmented forest environments may be in contact with species of adjacent habitats or with exotic species, domestic or otherwise. This and other environmental disturbances may potentially lead to changes in the relationship between pathogens and hosts and intensify the infection probability of species (McCallum and Dobson Reference McCallum and Dobson2002, Morley Reference Morley2007).
Parasites can influence the temporal and spatial dynamics of populations of wild animals (Dobson and Hudson Reference Dobson, Hudson, Grenfell and Dobson1995, Hale and Briskie Reference Hale and Briskie2009) and the structure of communities (Holmes and Price Reference Holmes, Price, Anderson and Kikkawa1986) because infected individuals are more susceptible to predation (Anderson and May Reference Anderson and May1979). Besides that, individuals infected with blood parasites may reduce food consumption and lose body mass (Atkinson et al. Reference Atkinson, Robert, Karen and Iko2000), become sick and even die (Coatney et al. Reference Coatney, Cooper and Trembley1945). The introduction of an infection or disease from a resistant population to a susceptible one could have disastrous consequences (Dobson and May Reference Dobson, May and Soulé1986) and even lead species to extinction (McCallum and Dobson Reference McCallum and Dobson1995). Furthermore, an understanding of parasite-host relationships has important implications for conservation and management programmes (McCallum and Dobson Reference McCallum and Dobson2002).
The Atlantic Forest of tropical South America, which has long been isolated from other forest blocks, supports a very high degree of endemism and is considered one of the world’s biodiversity hotspots (Mittermeier et al. Reference Mittermeier, Myers, Gil and Mittermeier1999). It is a severely threatened habitat with less than 10% of its original extent remaining, only 1% of which is primary forest (Galindo-Leal and Câmara Reference Galindo-Leal, Câmara, Galindo-Leal and Câmara2003). Over 100 species of birds there are threatened by habitat loss, habitat fragmentation and increased disturbance of existing forest patches (Marini and Garcia Reference Marini and Garcia2005). In spite of its high bird species richness, endemism and the number of threatened species (Stotz et al. Reference Stotz, Fitzpatrick, Parker and Moskovits1996, Marini and Garcia Reference Marini and Garcia2005) the relationship between fragmentation and parasitism of Atlantic Forest birds is poorly known. Apparently, only one study with 275 birds of 44 species revealed that birds from two large forest fragments were more infected with Plasmodium than birds from two small forests (Ribeiro et al. Reference Ribeiro, Sebaio, Branquinho, Marini, Vago and Braga2005). As a matter of fact, the relationship between habitat fragmentation and parasitism remains largely unexplored either empirically (Püttker et al. Reference Püttker, Meyer-Lucht and Sommer2008) or theoretically (McCallum and Dobson Reference McCallum and Dobson2002). Forest management can cause physiological stress in birds and make them more susceptible to blood parasite infections (Suorsa et al. Reference Suorsa, Huhta, Nikula, Nikinmaa, Jäntti, Helle and Hakkarainen2003).
This study appears to be the first to compare the prevalence of several blood parasites in birds from small and large forest fragments. Here we test whether the prevalence of blood parasites in birds is affected by: 1) forest fragment size; and 2) degree of forest dependence. Overall prevalence of blood parasites, their values for each species and their occurrence and intensity at different seasons in these study sites are presented and analysed elsewhere (Sebaio et al. submitted).
Study area
We sampled birds in the following six areas of the Atlantic rainforest, state of Minas Gerais, south-eastern Brazil from August to October 2000 and from June to November 2001: (1) Reserva Particular do Patrimônio Natural Mata do Sossego (RPPN Mata do Sossego) located in Simonésia municipality (20°07′S 42°00′W), (2) Fazenda da Mata Escura located on the left bank of Rio Jequitinhonha (16o20’S 41o00′W), (3) Parque Estadual Serra do Brigadeiro (PESB) located within Serra da Mantiqueira (20o20’–21o00’S 42o20’- 42o40′W), (4) Reserva Particular do Patrimônio Natural Mata do Jambreiro (RPPN Mata do Jambreiro) in Nova Lima municipality (19°58’S 43°55′W), (5) Fazenda Santana located on the left bank of Rio Jequitinhonha (16o05’S 40o02′W), and (6) Reserva Particular do Patrimônio Natural de Caratinga (RPPN de Caratinga) in Caratinga municipality (20°06′S 41°21′W).
Methods
Field methods
To compare fragmentation effects, we captured 436 birds in six small (< 30 ha) and 489 birds in six large (> 1,000 ha) fragments. Pairs of small and large fragments in each area were isolated from each other or any other forest by at least 1 km, mostly by crops and pastureland. We erected mist nets (12 m long, 2.5 m high, 36 mm mesh) at ground level from 07h00 to 16h00, for 3–4 days in each fragment. Captured birds were identified with the help of field guides (Ridgely and Tudor Reference Ridgely and Tudor1989). Birds received a numbered metal band provided by CEMAVE/IBAMA to ensure we were not sampling the same individual twice and were released immediately after blood collection. Birds were classified according to the degree of forest dependency (independent, semi-independent or dependent) after Silva (Reference Silva1995).
Blood parasite collection and analysis
To collect blood samples we made a small puncture in the tarsal vein using a disposable lancet. We air-dried and fixed the thin blood smears with methanol in the field. We stained smears with 3% GIEMSA solution in buffered water (pH 7.2–7.4) diluted at 1:10 in the Malaria Laboratory of the ICB/UFMG. To inspect smears for parasites, well-trained microscopists (F.S. and F.B.) exhaustively examined 200 microscopic fields (approximately 150 erythrocytes/field) at 1,000 × magnification under oil-immersion.
Statistical analysis
We tested the effect of fragmentation and species forest dependence on the prevalence of parasites using a generalised linear mixed model (GLMM) with binomial error and logit link function (Paterson and Lello Reference Paterson and Lello2003). We performed one GLMM for each parasite genus (Plasmodium, Trypanosoma and Haemoproteus), since prevalence of parasites was not correlated (Trypanosoma and Plasmodium: r s = 0.46, P = 0.13; Trypanosoma and Haemoproteus: r s = -0.12, P = 0.70; and Plasmodium and Haemoproteus: r s = −0.11, P = 0.74). We used as a response variable the number of infected birds of each species per forest patch (binomial numerator) and the number of individuals sampled of the same species (binomial denominator). We included patch size (large or small) and species forest dependence (dependent, non-dependent and semi-dependent) as fixed factors. Species identity and patch nested within area were fitted as random effects. We also performed one GLMM with Poisson errors and log link function to test the effects of patch size and species forest dependence on the richness of parasites. Fixed and random factors in this model were the same as used in models above. In all procedures, GLMMs were fitted using Laplace approximation. This analysis allowed us to weight for effect of unequal and small sample sizes (< 15) when estimating parameters (Jovani and Tella Reference Jovani and Tella2006) and to account for unmeasured species-specific traits and differences between areas that could affect parasite prevalence and richness (Paterson and Lello Reference Paterson and Lello2003).
We followed a step-down procedure to select the final models using a likelihood ratio test. Initially, we fitted the full model including all variables and the interaction term of fixed effects. We then excluded sequentially non-significant interaction terms and explanatory variables. All models did not violate the assumptions of normality of random effects and colinearity among explanatory variables. We did not find over-dispersion in models (residual deviance divided by the residual degrees of freedom was < 1). All analyses were performed in R (http://www.r-project.org).
Results
We analysed 925 birds from 109 species and 11 families (Table 1). Among these, 47 % (n = 436 birds from 29 species) came from small fragments and 53% (n = 489 birds from 40 species) from large fragments (Table 1). A total of 147 birds (15.8%) from 62 species and all 11 families had at least one parasite of Plasmodium, Trypanosoma, Haemoproteus or microfilaria. Total prevalence values for these four parasites were 9.2%, 3.8%, 3.2% and 0.03%, respectively. Because microfilaria prevalence was too low we did not carry out statistical analysis of these data.
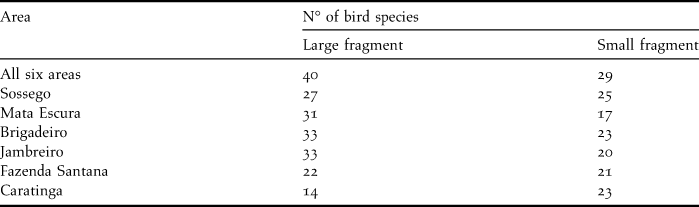
Table 1. Number of bird species in the six areas of Atlantic Forest sampled with mist-nets in the state of Minas Gerais in 2000 and 2001.
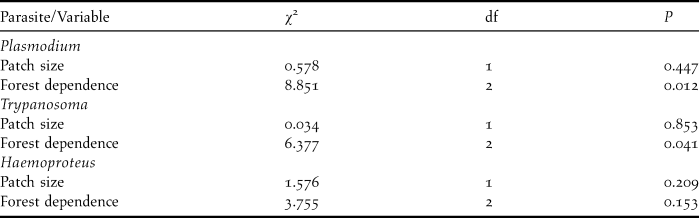
Prevalence of the three types of parasites (Table 2) did not differ between small and large forest patches (Table 3). The degree of forest-dependence, however, had a significant effect on the prevalence of Plasmodium and Trypanosoma on birds (Table 3). Birds not dependent on forest were more likely to be were usually infected with Plasmodium and Haemoproteus than other birds, but forest-dependent birds were more infected with Trypanosoma (Figure 1). Parasite richness on birds was not affected by patch size (![]() , P = 0.597) and species forest dependence (
, P = 0.597) and species forest dependence (![]() , P = 0.181).
, P = 0.181).
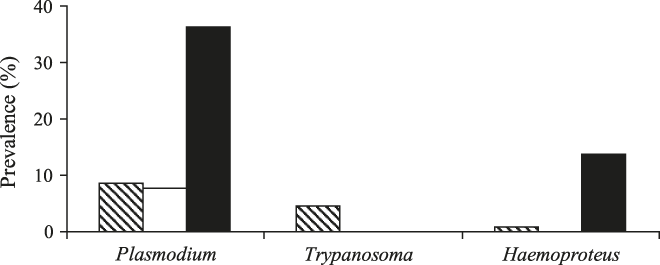
Figure 1. Prevalence of blood parasites in birds from Atlantic Forest fragments, Brazil, in relation to bird forest-dependence (Dependent = cross-hatched bars; Semi-dependent = white bars; Independent = black bars).
Table 2. Prevalence of blood parasites (%) in each large and small Atlantic Forest fragment in the state of Minas Gerais in 2000 and 2001.

Table 3. Likelihood ratio test results of generalised linear mixed model of prevalence of three blood parasites in relation to the patch forest size and species forest dependence.
Discussion
Our results did not support the hypothesis that forest fragmentation affects the prevalence of birds’ blood parasites. This is contrary to the expected and to a few empirical results, since habitat fragmentation and disturbance are expected to affect diversity and abundance of hosts and vectors. Contrary to our finding, the prevalence of avian blood parasites was greater in species reproducing in continuous than in non-continuous forests in Spain (Tella et al. Reference Tella, Guillermo, Foreros, Gajo and Donazar1999). These authors suggested that the abundance of vectors would be lower in habitats deforested or altered by man. Also, forest fragmentation increased the prevalence of gastrointestinal nematodes in specialist but not in generalist small Atlantic Forest mammals (Püttker et al. Reference Püttker, Meyer-Lucht and Sommer2008). More adverse environmental conditions, such as decreased air humidity, increase in air and soil temperature and in wind speed in disturbed habitats might affect the abundance of blood parasite vectors such as ceratopogonid, culicid and hippoboscid flies (Kettle Reference Kettle1968). This means that forest-dependent birds should have more chances of being infected than those with other types of habitat use. Our findings support this argument for Trypanosoma, but not for Plasmodium and Haemoproteus. However, forest-independent birds also from Minas Gerais had higher ectoparasite prevalence than other birds (Marini and Couto Reference Marini, Couto, Leite and Saito1997). Differences in vectors that transmit each of these blood parasites might help explain these apparently contradictory results. Plasmodium is transmitted by culicids, Haemoproteus by hippoboscids or ceratopogonids (Friend and Franson Reference Friend and Franson1999) and Trypanosoma can be transmitted either by culicids, hippoboscids, simulids or even ticks (Peirce Reference Peirce and Cooper1989). The distribution of habitat-dependent vectors, at micro and macro habitat scales might affect the hosts’ interspecific differences in blood parasite infection rates (Piersma Reference Piersma1997). Also, the movement of forest-independent birds among several habitats might increase their chances of being infected. Forest-independent birds in our study were almost four times more infected by Plasmodium than other birds. Overall, general cross-taxa explanations do not seem to explain satisfactorily all the variation in prevalence observed, as Merino et al. (Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008) also concluded.
The lack of a fragmentation effect may be related to the finding that blood parasite prevalence may be determined at a larger spatial scale than the fragment itself. The maintenance of this host-parasite cycle in a fragmented area may be related to the fact that several widely-distributed species are potential vectors of Plasmodium (Friend and Franson Reference Friend and Franson1999). Regional differences in the intensity of transmission were detected for Haemoproteus in the state of Florida, USA (Atkinson Reference Atkinson1988). Similarly, but at much larger geographic scale, the prevalence of Plasmodium and Haemoproteus was higher in Guyana than in Uruguay (Durrant et al. Reference Durrant, Beadell, Ishtiaq, Graves, Olson, Gering, Peirce, Milensky, Schmidt, Gebhard and Fleischer2006). Also, Leucocytozoon had a positive relationship and Haemoproteus and Plasmodium had a negative relationship with latitude in Chile (Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and Mcgehee2008). The lack of correlation between the infection rates of the three blood parasites we studied provides evidence that they have independent explanations for their infection rates. Environmental differences among regions may provide different opportunities for parasite-host associations and help explain differences in prevalence of blood parasites (Tella et al. Reference Tella, Guillermo, Foreros, Gajo and Donazar1999, Durrant et al. Reference Durrant, Beadell, Ishtiaq, Graves, Olson, Gering, Peirce, Milensky, Schmidt, Gebhard and Fleischer2006).
Natural sampling errors might also explain part of the variation in prevalence among fragments, areas or regions. Even though we controlled field methods and sample sizes of captured birds within each fragment, species composition and relative abundance of captured bird communities were usually not very similar between two fragments of a given area. Haemoproteus, for example, is specific to some bird families and is more common in certain species (Peirce Reference Peirce and Cooper1989). Thus, the prevalence of parasites could have been influenced by differences in the composition of species of the sampled families and not by the local characteristics of the analysed areas. Another criticism, the lower ability of visual examination of blood smears than PCR-based analyses to detect parasites (Ribeiro et al. Reference Ribeiro, Sebaio, Branquinho, Marini, Vago and Braga2005), might affect real estimates of prevalence values of Plasmodium or Haemoproteus, but not Trypanosoma. Also, since our approach was standardised, it reveals real relative differences among treatments. Lastly, seasonal differences in parasite prevalence (Cosgrove et al. Reference Cosgrove, Wood, Day and Sheldon2008) might explain part of the difference between areas, but not between fragment sizes, since the small and large fragments of an area were sampled during the same week.
Conclusions
This seems to be the first study testing the hypothesis that forest fragmentation affects the prevalence of avian blood parasites. We found no evidence that prevalence differs between large and small fragments, but that infection may be determined by hosts’ dependence on forests. A better understanding of the effects of habitat alteration on avian blood parasite prevalence should analyse each parasite separately, since different suites of hosts are affected more by different suites of parasites. Future research and conservation and management approaches considering avian blood parasite prevalence should also consider the ecology and behaviour of vectors.
Acknowledgements
CNPq granted a scholarship to F.S. and a research grant and fellowship to M.Â.M. Owners and administrators allowed our study in their properties or conservation units. IBAMA gave banding/collecting authorization. Prof. M. F. Ribeiro provided laboratory support. L. Lopes and A. Fernandes were invaluable during data collection in the field.






