1. Introduction
Lignin, a polyphenol compound, is found in plant cell wall, and is the second most abundant plant biopolymer after cellulose. It is found especially in the bark and wood fibers. Lignin is formed by variously organized phenyl-propane units, linked by alkyl-aryl ether and C–C bonds, and is derived chiefly from p-coumaryl, coniferyl, and sinapyl alcohols. The proportion of these aromatic units in the macromolecular structure depends on the taxonomic group of the plant and its anatomical part. The guaiacyl type is found predominantly in softwoods and guaiacyl-syringyl in hardwoods (Novaes et al., Reference Novaes, Kirst, Chiang, Winter-Sederoff and Sederoff2010; Santos et al., Reference Santos, Capanema, Balakshin, Chang and Jameel2012). The lignin in wood contributes to its mechanical strength, enabling the trees to support more than 2,000 metric tons of aboveground biomass (Fry & White, Reference Fry and White1938). Lignin is industrially utilized for making adhesives for composite wood products, such as particleboard and linoleum, and as an active ingredient of phenolic resins (Çetin & Özmen, Reference Çetin and Özmen2002). Higher lignin content of wood is known to yield greater quantity and quality (calorific value) of biochar for improving farm soil quality (Cagnon et al., Reference Cagnon, Py, Guillot, Stoeckli and Chambat2009; Novotny et al., Reference Novotny, Maia, Carvalho and Madari2015). For its importance in production of biochar (Deb et al., Reference Deb, Kloft, Lässig and Walsh2016; Spokas et al., Reference Spokas, Cantrell, Novak, Archer, Ippolito, Collins and Nichols2012) and industrially valued products (Chung & Washburn, Reference Chung and Washburn2012), information of lignin content in different species would facilitate appropriate feedstock selection.
2. Objective
Tropical deciduous forests constitute the most widespread forest type in India (Champion & Seth, Reference Champion and Seth1968), and is the habitat of at least 70 indigenous hardwood tree species. Wood lignin (WL) profiles of these trees are almost invisible in the available wood chemistry literature, except for only three species, namely, Shorea robusta Gaertn., Tectona grandis L.f., and Dalbergia sissoo Roxb. Ex DC from central India (Narayanamurti & Das, Reference Narayanamurti and Das1955). We intend to fill this gap in knowledge of lignin content of major trees of India’s deciduous forests, covering 68% of the total forest cover in India (Reddy et al., Reference Reddy, Jha, Diwakar and Dadhwal2015). We further intend to examine if the tropical Asian hardwood lignin contents conform the generalizations about WL and bark lignin (BL) contents, built primarily on tree lignin data from neotropical, temperate, and alpine zones of the world.
3. Materials and methods
3.1. Site selection
Since lignin content of hardwood trees (except S. robusta, T. grandis, and D. sissoo) from deciduous forests of India are yet unknown, we selected a dry deciduous forest of Bankura district in West Bengal and a moist mixed deciduous forest of Rayagada district in Odisha (Figure 1). The forest type and species composition of the sampling sites are given in Table 1.

Figure 1. Map showing locations in two States of India. Dots indicate the locations in the districts (yellow) from which wood samples were collected.
Table 1. Deciduous forest types in Odisha and West Bengal with dominant series

a Point samples with 100 m radius.
b Based on each species density estimated in 5 × 1,000 m2 transects.
3.2. Sample collection
A total of 48 species of native hardwood trees were sampled. Mature trees older than 10 years, with girth of >25 cm at 1.2 m stem height were selected for sampling. To avoid felling of any live tree, we did not collect samples of mature stem wood; instead, ca. 15 cm pieces of wood of ca. 5 cm diameter with intact bark were collected from matured branches.
3.3. Chemical analyses
Six samples of wood and bark of each species from each habitat were examined. The samples were prepared using C-TAB, following Van Soest (Reference Van Soest1963). Acid insoluble (Klason) lignin was estimated following Obst et al. (Reference Obst, Sachs and Kuster1988); 0.3 g of powdered sample was treated with 3 ml 72% sulfuric acid for 1 hr at 30°C. Subsequently, the solution was diluted to 4% for acid hydrolysis in an autoclave for 1 hr, followed by filtration using Whatman-1 filter paper to separate the soluble and insoluble fraction. To remove interference from different pigment compounds from the wood of Diospyros and Terminalia spp., which showed reddish to black filtrates after acid hydrolysis, we modified the extraction method by repeated washing of the filtrate with 1 L hot water (approx. 50°C), followed by 10–15 ml acetone and 1 L water, successively, before drying the filter paper and the precipitate, weighing and preparing the substance for gravimetric analysis.
The acid soluble lignin content (S) was estimated using UV–vis spectrophotometer (JASCO V-730 Bio, Tokyo, Japan) at 215 and 280 nm, and calculated from the following formula:
derived from the simultaneous resolution of two equations, as given in Moreira-Vilar et al. (Reference Moreira-Vilar, de Cássia Siqueira-Soares, Finger-Teixeira, de Oliveira, Ferro, da Rocha, Ferrarese, dos Santos and Ferrarese-Filho2014):
where A280 and A215 are the absorbance values at 280 and 215 nm, respectively; and F is the furfural concentration (g L−1). Ash content was determined gravimetrically after combustion of the insoluble part of wood and bark at 550°C for 4 hr in a Muffle furnace.
3.4. Statistical analysis
Lignin content of each species from two types of forest was compared. Because our dataset is small (six replications for each species from each location), test of normality is inapplicable, and nonparametric tests are of low power. We therefore calculated the Bayesian credibility interval, equivalent to confidence intervals (Eberly & Casella, Reference Eberly and Casella2003), to estimate the significance of difference between means of lignin levels.
4. Results and discussion
The Klason lignin, acid soluble lignin, and ash contents of the wood and bark of 48 species are presented in Supplementary Table S1. The data corroborate the established fact (Novaes et al., Reference Novaes, Kirst, Chiang, Winter-Sederoff and Sederoff2010) that lignin content is fairly invariant within a species, but varies considerably between different species. The lignin content of certain species may vary, albeit slightly, with the sampling locale (Supplementary Table S1) with different edapho-climatic conditions. Lignin content of most of the species appear to be invariant. The difference between means of WL and BL contents from two habitats is within Bayesian credibility interval only for six species presented in Table 2. The difference in WL contents between two habitats is credible for four species, whereas the difference in BL contents is credible for five species.
Table 2. The six hardwood species showing Bayesian credible difference between means of wood and bark lignin contents from Odisha (OD) and West Bengal (WB) forests
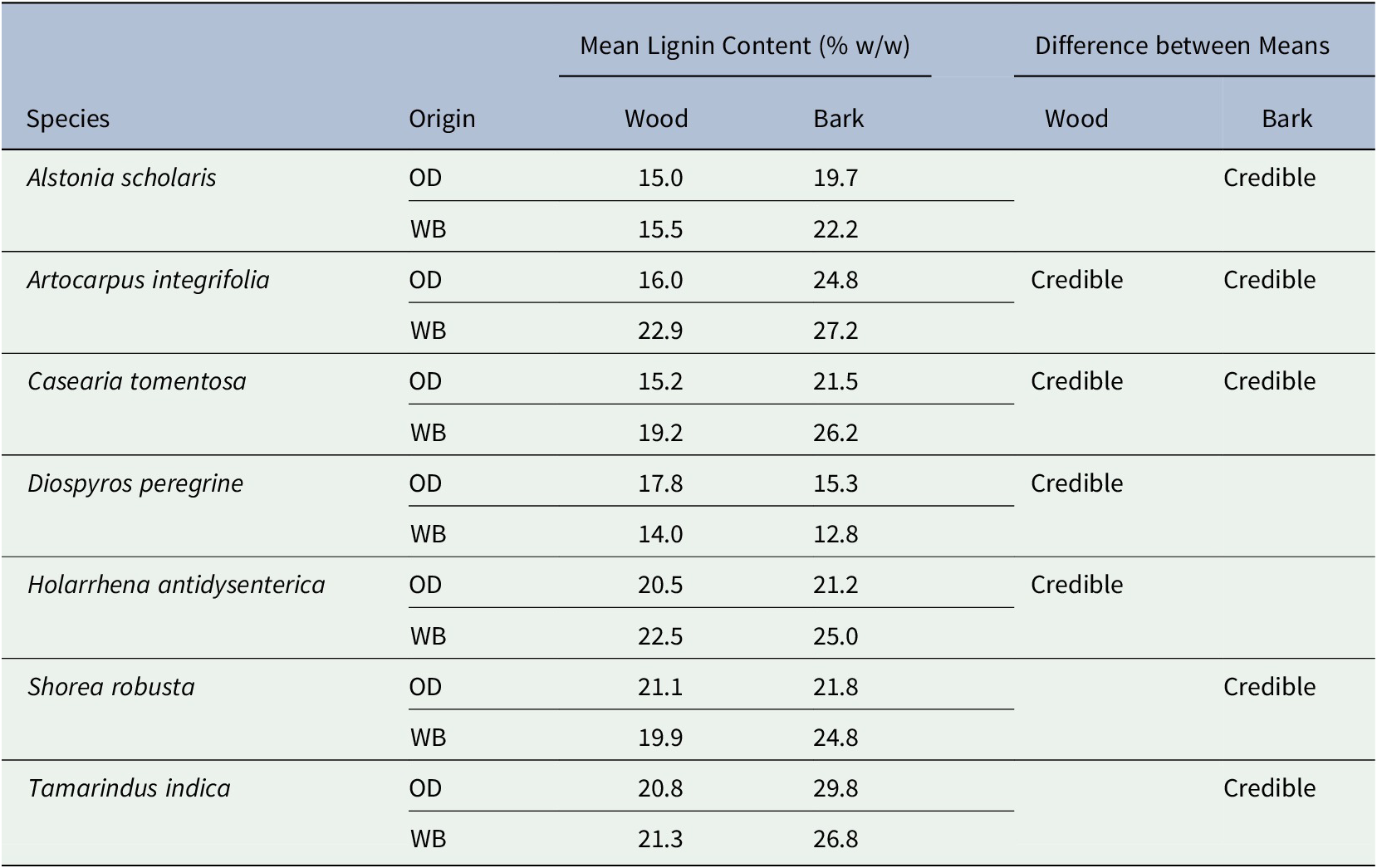
The WL contents range from 10.8% (Syzygium cumini) to 28.2% (Moringa oleifera). Considerably high (>20%) lignin content is found in the wood samples of Alangium salvifolium, Albizzia spp., Artocarpus heterophylla, Buchanania lanzan, Careya arborea, Cassia fistula, Diospyros melanoxylon, Ficus racemosa, Gmelia arborea, Holarrhena pubescens, Mytragyna parviflora, Pterocarpus marsupium, Tamarindus indica, and Terminalia spp. The WL content in S. robusta is considerably less (19.9–21.1%) in our samples compared with the heartwood samples (29.3%) reported in Narayanamurti and Das (Reference Narayanamurti and Das1955). BL contents of our samples range from 12.0 (Haldina cordifolia) to 36.8 (Neolamarckia cadamba). Pyrolysis of these high-lignin feedstock may produce better quality of biochar and resin than from other trees. Figure 2 shows the mean values of the wood and bark of the trees from two forest types.
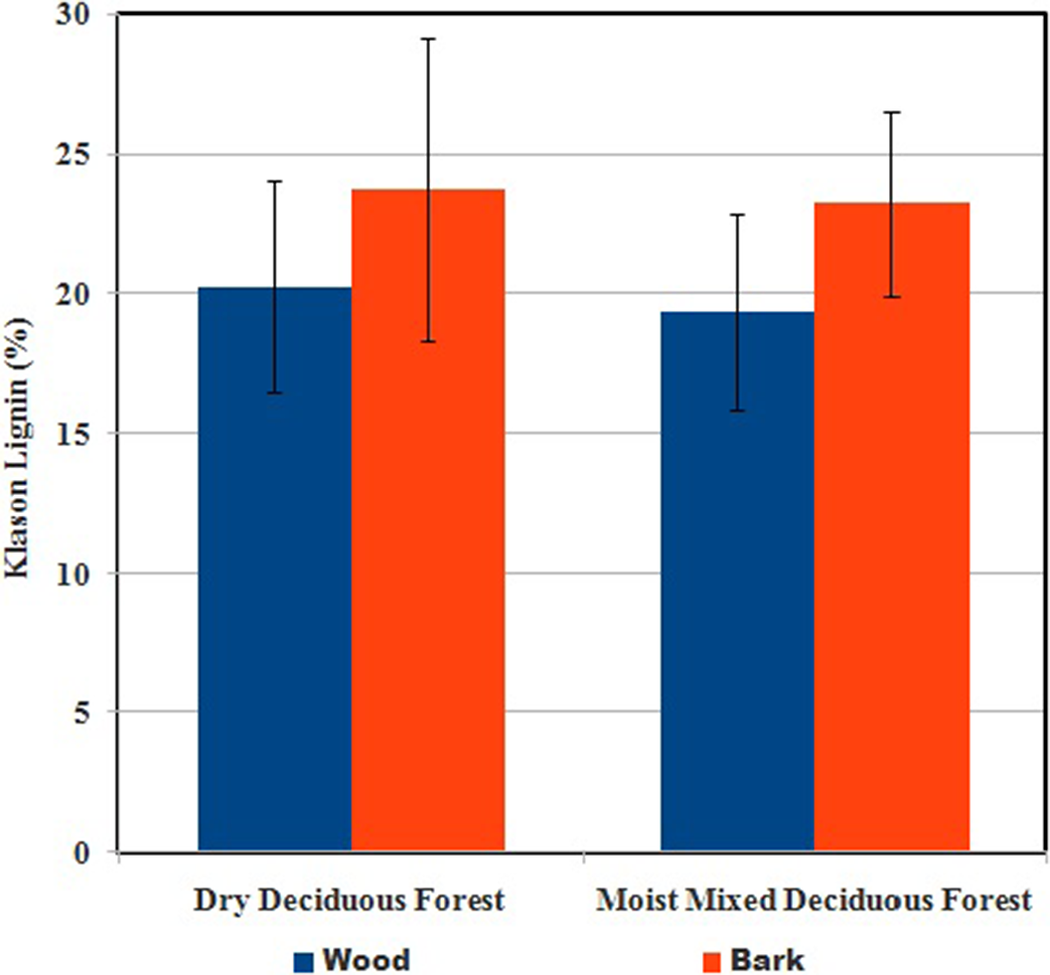
Figure 2. Mean % contents of Klason lignin in wood and bark of trees sampled from dry deciduous and moist deciduous forests.
The inner BL is chemically similar to the WL, but the outer (dead) bark contains different forms of it (Sjostrom, Reference Sjostrom1981). The BL content of hardwood trees is generally higher than WL (USDA, 1971). However, this contention, built primarily on the data from temperate, neotropical, and alpine trees, does not hold for some tropical deciduous trees. While the BL to WL ratio is >1.0 in 79% of our samples, 12 species have this ratio of <1.0. Kalita and Saikia (Reference Kalita and Saikia2004) also reported that the evergreen trees Mimusops elengii and Plumeria alba from northeastern India have BL < WL. In our study, the lowest ratio (0.54) is for M. parviflora.
The BL/WL ratio seems to be unrelated to the phylogenetic relatedness between taxa, with different BL/WL values for different species within the same genera, and is predictably, inversely related (slope = −0.057, R2 = 0.43, t = 1.98) to the corresponding WL content (Figure 3). We further note that BL/WL < 1.0 corresponds to low quality (Class III) timber, (e.g., D. malabarica, M. parviflora, and T. Chebula), whereas trees with stronger and high quality (Classes I and II) timber (Sundararaj et al., Reference Sundararaj, Shanbhag, Nagaveni and Vijayalakshm2015) tend to have a higher BL/WL ratio in our study. However, this apparent correspondence has a few exceptions; Anogeissus latifolia and N. Cadamba, known to belong in Class III timber class (Sundararaj et al., Reference Sundararaj, Shanbhag, Nagaveni and Vijayalakshm2015), have the value of BL/WL > 1.4 (Table S1).

Figure 3. Regression of BL/WL ratio with wood lignin contents of 67 samples. Slope = −0.057, R 2 = 0.43, p < 0.001.
5. Conclusion
Our study of 48 species reports a range of species-specific lignin contents, and reveal that some tropical trees are characterized by WL > BL, contrary to most hardwood trees. Further investigation is required to establish a predictive relationship between the mechanical properties of wood and their lignin profiles.
Limitations of the study
1. With limited resources, we were unable to collect samples from more locations than two.
2. Owing to legal restrictions, and in conformity with conservation principles, we collected samples of tree branches instead of mature stem.
Acknowledgments
We are grateful to Prof. Subhas C. Santra of Department of Environmental Science, University of Kalyani, for permitting us access to his laboratory facilities for ash content analysis. We are grateful to Debdulal Bhattacharjee and Mahendra Nauri for collection of wood samples, Dr. Sanjib Chattopadhyay for assistance in laboratory work, and Prof. Sabyasachi Chakraborty of Indian Statistical Institute, Kolkata for statistical analysis.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/exp.2021.18.
Data availability statement
All data are freely available from authors upon request.
Authorship contributions
Conceptualization, D.D.; Investigation, D.D.; Design, D.D.; Writing-original draft, D.D.; Chemical analyses, P.R.
Conflict of interest
The authors declare no conflict of interest.
Funding statement
This research received financial support from Mr. Avik Saha of Kolkata.








Comments
Comments to the Author: Because this manuscript presents a kind of basic research of wood science, presenting lignin content as many as wood species mentioned will be perfect. However, it is clear now after the authors added the limitation of the study.