Vancomycin-resistant enterococci (VRE) are bacteria present in the gastrointestinal tract that have developed resistance to antibiotics, namely vancomycin. Reference Gravel, Archibal and Pelude1 The Public Health Agency of Canada reported that bloodstream infections due to VRE (VRE-BSI) acquired in the hospital has more than doubled from 2014 to 2018, with 31% of patients dying within 30 days of diagnosis. 2 This mortality rate underscores the importance of preventing and limiting the spread of VRE and reducing the incidence of VRE-BSI in the hospital setting.
Hand hygiene, environmental cleaning, and antimicrobial stewardship are key to limiting the spread of antibiotic-resistant organisms. 3 Active screening to detect VRE colonization and use of contact precautions for patients colonized or infected has been found to limit VRE transmission. However, the efficacy of using contact precautions to prevent the spread of antibiotic-resistant organisms has been questioned. Reference Talbot4 Previous studies evaluating the effectiveness of screening and contact isolation practice on VRE infection have produced mixed results, Reference Talbot4–Reference Bryce, Grant, Scharf, Dempster, Lau, Laing, Shajari and Forrester11 and infection control practices to prevent the spread of VRE vary in Canada.
In 2015, based on recommendations from the Canadian Consensus Development Conference on Surveillance and Screening for Antimicrobial Resistant Organisms, new VRE screening protocols were implemented for all acute-care facilities in Alberta, which eliminated routine admission screening and focused on high-risk patient care units. 12 The purpose of this study was to determine whether discontinuing active screening for VRE in Alberta acute-care facilities had an associated impact on the rate of rise of hospital-acquired (HA) VRE-BSI.
Methods
Data source for IPC surveillance
Alberta Health Services (AHS) and its contracted partner Covenant Health (COV) provide all acute-care services in Alberta, Canada. 13 All acute-care facilities in Alberta participate in a single Infection Prevention and Control (IPC) surveillance program. 13 All patients who were admitted to AHS or COV acute-care facilities between January 1, 2013, and March 31, 2020, and who met definition for HA VRE-BSI were included in the analyses. Facilities under surveillance include acute and acute–tertiary rehabilitation facilities, including pediatric hospitals.
Definition of hospital-acquired VRE bloodstream infection
Rates of VRE-BSI have been reported since January 2013 by IPC in Alberta Health Services (AHS) and Covenant Health acute and acute–tertiary rehabilitation care facilities. AHS IPC surveillance defines HA VRE-BSI as all individuals admitted to AHS or COV acute-care and acute–tertiary rehabilitation facilities who had a positive blood culture identified with VRE and that represented a new episode for the patient and occurred on or after the calendar day 3 of admission. 13
VRE screening practice
Prior to 2015, all AHS and Covenant Health acute-care facilities performed routine VRE screening by rectal swab on all new hospital admissions, including elective, direct, labor and delivery, neonatal intensive care unit (NICU) and pediatrics. In 2015, new VRE screening protocols were implemented in AHS and COV acute-care facilities, eliminating routine admission screening and focusing on high-risk patient care units, such as intensive care, transplant, vascular and hematology–oncology units. Patients who had any history of a VRE positive colonization or infection and had not tested negative in 3 consecutive screening samples continued to be managed with contact precautions. These precautions included gown and gloves for all contact with the patient or patient environment, and a single room (or bed space if unavailable).
Statistical analysis
The study period comprised 87 months from January 1, 2013, to March 31, 2020. The primary outcome was the slope change in the incidence rate of VRE-BSI before the intervention (prior to October 2015) compared to the period after the intervention (after October 2015). Data were reported as the number of HA VRE-positive blood cultures divided by the patient days per month. All sites included for analysis had at least 1 HA VRE-BSI during the study period. We stratified the data into 3 separate cohorts for analysis (Table 1 1). Group 1 was defined as those sites that stopped VRE screening in October 2015 and identified as a low-risk patient population. Group 1 was not analyzed at the patient level; thus, a high-risk patient not admitted to any of the units in groups 2 and 3 was included in group 1. Group 2 was defined as all adult ICUs provincially where screening stopped in October 2015; these ICU patients were considered high risk. Group 3 was defined as 3 units (hematology, solid-organ transplant, and bone-marrow transplant) that continued to screen for VRE throughout the study period; these patients were considered high risk. For group 3, a hypothetical intervention period was applied using the same intervention start date as group 1, although there was no change in practice. An intervention time series Poisson regression was used to determine the slope change in VRE bloodstream infection incidence between the pre- and postintervention periods. For all groups, a level- and slope-change model was used. Reference Bernal, Cummins and Gasparrini14 The slope change (β3) was reported as an incidence rate ratio (IRR). The number of HA VRE-BSI cases was the outcome, and the log of the number of patient days was used as an offset. For all analyses, autocorrelation was assessed using a plot of the autocorrelation and partial autocorrelation functions. The presence of a seasonal affect and overdispersion was also investigated.
Table 1. Description of Study Cohorts
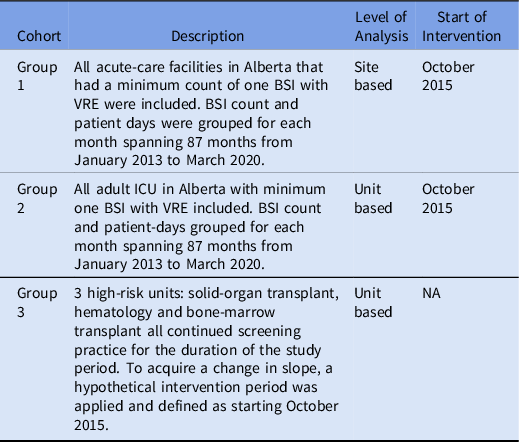
Note. BSI, bloodstream infection; VRE, vancomycin-resistant Enterococcus; ICU, intensive care unit; NA, not available.
A sensitivity analysis of lagged intervention effects was performed at the 3-month and 6-month marks for all cohorts following the intervention start date. This analysis was performed to detect any changes to the magnitude of the intervention effect for those sites that discontinued screening because these changes would become more apparent over time.
A 2-tailed P value of <.05 was deemed significant for all analyses. R version 3.6.0 software (R Foundation for Statistical Computing, Vienna, Austria) was used to analyze the data.
Results
In total, 202 HA VRE-BSIs were identified among all 3 groups by IPC surveillance from January 2013 to March 2020. Moreover, 129 cases (64%) occurred in group 1 (low risk, stopped screening).
Group 1
In total, 129 HA VRE-BSIs were identified in group 1 (n = 23 facilities) throughout the study period. Prior to the start of the intervention when routine screening still occurred, 49 HA VRE-BSIs were identified with a rate of 0.08 per 10,000 patient days. Also, 80 HA VRE-BSIs were identified in the postintervention period, with an identical rate of 0.08 per 10,000 patient days (P = .99) (Table 2). The slope change was not statistically significant with a 2% increase in cases each year (IRR, 1.015; 95% CI, 0.982–1.049; P = .37) (Table 3 and Fig. 1). No change was observed after introducing 3- and 6-month lags after the intervention start date, with IRRs of 1.022 (95% CI, 0.991–1.054; P = .17) and 1.025 (95% CI, 0.996–1.056; P = .09), respectively (Table 3).
Table 2. Count and Rate of HA VRE-BSI Before and After the Intervention by Cohort

Note. HA, hospital acquired; VRE-BSI: vancomycin-resistant Enterococcus bloodstream infection.
Table 3. Slope Change of the Incidence Rate of HA VRE-BSI After Discontinuation of VRE Screening and Contact Precautions in Stopped Screening and Screening Cohorts Incorporating Lagged Effects of 3 and 6 Months

Note. CI, confidence interval; HA, hospital acquired; VRE-BSI: vancomycin-resistant Enterococcus bloodstream infection.

Fig. 1. Scatter plot of HA VRE-BSI in Group 1. Grey area represents the postintervention period. Dashed line represents a counter-factual scenario. Solid line represents the actual scenario.
Group 2
In total, 46 HA VRE-BSIs were identified in group 2 (high-risk adult ICU, stopped screening, n = 9 ICUs) throughout the study period, with 32 cases in the preintervention period (1.07 per 10,000 patient days) and 14 cases in the postintervention period (1.16 per 10,000 patient days; P = .98) (Table 2). No statistically significant difference was detected in the slope change or rate of rise in VRE-BSI between the preintervention and postintervention periods, with an IRR of 1.025 (95% CI, 0.967–1.086; P = .40) (Table 3 and Fig. 2). No change was observed after introducing 3- and 6-month intervention lags.

Fig. 2. Scatter plot of HA VRE-BSI in Group 2. Grey area represents the postintervention period. Dashed line represents a counter-factual scenario. Solid line represents the actual scenario.
Group 3
In total, 27 HA VRE-BSIs were identified in group 3 (high-risk, continued screening, n = 3 units) throughout the study period, with 12 cases in the preintervention period (1.63 per 10,000 patient days) and 15 cases in the postintervention period (1.28 per 10,000 patient days, P = .95) (Table 2). Similar to the group 2 analyses, no significant differences were detected in the slope change or rate of rise in VRE-BSI between the hypothetical pre- and postintervention periods, with an IRR of 0.989 (95% CI, 0.924–1.059; P = .75) (Table 3). No change was detected after introducing 3- and 6-month intervention lags with IRRs of 0.987 (95% CI, 0.925–1.052; P = .68) and 0.986 (95% CI, 0.925–1.051; P = .66), respectively (Table 3 and Fig. 3).

Fig. 3. Scatter plot of HA VRE-BSI in Group 3. Grey area represents the postintervention period (hypothetical). Dashed line represents a counter-factual scenario. Solid line represents the actual scenario.
Discussion
Recent data from the Canadian Nosocomial Infection Surveillance Program show that VRE-BSI has been steadily increasing in Canada, and in the period from 2013 to 2018, the rate of VRE-BSI significantly increased from 0.16 to 0.34 cases per 10,000 patient days. Reference McCracken, Mitchell and Smith15 In this analysis, the observational comparison of rates per 10,000 patient days between the preintervention and postintervention periods across all cohorts demonstrated no statistically significant change in the rate of hospital-acquired VRE-BSI in Alberta. Furthermore, the intervention time-series Poisson regression analyses did not demonstrate a statistically significant change in the rate of rise of HA VRE-BSI in any group after discontinuation of screening in both high-risk (ie, ICU) and low-risk (general population) cohorts. ICU-only interventions, such as hand hygiene interventions, central-line insertion bundles, chlorhexidine gluconate (CHG) bathing, and antibiotic stewardship programs, may have played a role in finding no difference in the rate of rise of VRE-BSI in these ICU patients following a change in VRE screening protocols.
Contrary to our findings, recent results from Ontario suggest that VRE-BSI has increased in hospitals that stopped routine VRE screening. Reference Johnstone, Policarpio and Lam5,Reference Johnstone, Shing and Saedi6 The interrupted time-series regression analysis from Johnstone et al Reference Johnstone, Policarpio and Lam5 showed a significant increase in the rate of rise of VRE-positive blood cultures in nonscreening hospitals, with a slope change IRR of 1.25 (95% CI, 1.01–1.54; P = .04). Conversely, hospitals in Ontario that continued to screen did not see a significant increase, with a slope change IRR of 0.81 (95% CI, 0.56–1.15; P = .20). This effect remained after introducing lagged effects of 3 and 6 months and adjusting for cases occurring only at acute-care teaching hospitals. Reference Johnstone, Policarpio and Lam5 In contrast, our sensitivity analysis of lagged effects did not reach significance for those hospitals where screening stopped or where screening continued, though our smaller sample size might have underpowered these results. However, a strength of our analysis was that it was restricted to only hospital-acquired cases. A more prudent comparison to the results identified in Ontario by Johnstone et al Reference Johnstone, Policarpio and Lam5 would be to only include cases attributed to the reporting facility. After adjusting for cases attributable to the reporting facility, Johnstone et al Reference Johnstone, Policarpio and Lam5 found that the cohort for which screening stopped did not show a significant increase in VRE-BSIs at the no lag and the 3-month time points, but VRE-BSIs increased at the 6-month mark. These researchers acknowledged that misclassification bias may have occurred in that a patient could have developed VRE colonization at a screening hospital and had a positive blood culture at a hospital that stopped screening and vice versa. Reference Johnstone, Policarpio and Lam5
Our findings are inconsistent with several observational studies reporting that screening for VRE colonization is associated with reduced rates of VRE-BSI. Reference Price, Paule and Noskin16–Reference Mikulska, Del Bono and Raiola18 However, a previous observational study, which used some of the same data in the cohort that stopped screening as Johnstone et al, Reference Johnstone, Policarpio and Lam5 showed no significant difference in VRE-BSI in screening versus nonscreening hospitals. Reference Lemieux, Gardam and Evans7 However, the follow-up time or postintervention period in that study was half that of Johnstone et al Reference Johnstone, Policarpio and Lam5 with fewer data points, and their results did not reach statistical significance. Reference Lemieux, Gardam and Evans7
The strengths of our study include comprehensive and accurate provincial data collection from IPC surveillance covering slightly more than 7 years of data from 102 participating acute-care facilities. A single provincial surveillance system was used, including a single data platform, protocols for case identification and entry, and consistent data quality practices. All VRE-BSI cases are routinely checked for data-entry errors by IPC surveillance analysts, reducing potential for misclassification. IPC surveillance also compares surveillance cases to laboratory data to ensure complete case capture. We stratified our analyses by risk of VRE-BSI in the patient population according to national guidelines, providing more reliable conclusions regarding the effect of an intervention on specific patient risk groups.
This study had several limitations. First, we did not include data on potential confounders such as provincial hand hygiene compliance or antibiotic use within each acute-care facility. Second, this quasi-experimental design was susceptible to bias, including regression to the mean. However, we did include a sensitivity analysis of lagged effects that would have amplified the results observed in the cohorts that stopped screening. Finally, our results may not have reached significance with the small sample size of each group, though the longevity of this provincial study suggests that it would be difficult for any study to analyze a larger sample with similar data quality.
In conclusion, the evidence suggests that VRE-BSI is increasing in Canada, and patients with VRE-BSI have an increased risk of death and longer hospital stay. Whether routine screening to prevent the transmission of VRE in hospital is effective at reducing the rate of VRE-BSI remains unclear. In Alberta, the rate of HA VRE-BSI has remained consistent, and the findings of this study indicated that there has been no increase in the rate of rise of HA VRE-BSI in those sites or units that discontinued screening for VRE, regardless of patient risk group.
Acknowledgments
The authors thank individuals from the AHS Infection Prevention and Control Program, especially members of the IPC Data Quality Working Group, for their assistance and guidance with this research project. We especially thank Dr Stephanie Smith, Dr Bonita Lee, Dr Alex McFarlane, Dr Uma Chandran, Tiffany Herrick, Maureen Buchanan-Chell, and Craig Pearce for their feedback throughout this project.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.









