Introduction
Correct positioning of the endotracheal tube tip is very important in both preterm and term babies for symmetrical ventilation of the lungs. Reference Thayyil, Nagakumar, Gowers and Sinha1 There are several methods for estimating the depth of endotracheal tube insertion in neonates. Chest X-ray is still preferred as the standard method for confirming the location of the endotracheal tube in the trachea in neonatal ICUs. The location and depth of the endotracheal tube can be determined by direct laryngoscopy and observing clinical findings. However, each of these methods has their own limitations.
Our neonatal ICU serves as a “Congenital Heart Center” for newborns with CHD. Critical CHD is defined as a CHD requiring surgical or transcatheter cardiac intervention for the baby to survive. Reference Jenkins, Gauvreau, Newburger, Spray, Moller and Iezzoni2 The patients with critical CHD usually require intubation before and after surgery because of cardiorespiratory instability during their stay in the neonatal ICU. Currently in our unit, all intubated patients with critical CHD undergo routine daily chest X-rays to assess ventilation and determine endotracheal tube position. Considering the potential disadvantages of radiography, we decided to use ultrasound, which we frequently use for other purposes in this patient group, to detect endotracheal tube localisation.
Many studies have reported a close relationship between the ultrasonographic and chest X-ray measurements of endotracheal tube localisation in infants. It is noteworthy that various measurement points have been used in these studies aiming to determine the endotracheal tube location with ultrasound. Reference Slovis and Poland3–Reference Najib, Pishva, Amoozegar, Pishdad and Fallahzadeh6
In this study, we aimed to determine the localisation of the endotracheal tube by point-of-care ultrasound with having the carina as a landmark instead of aortic arch since vascular anomalies were often accompanied by critical CHD. In the imaging of the carina, the cross-section of the right pulmonary artery and the aortic arch was taken into account, as they were in the same anatomical plane.
Materials and Methods
This prospective observational clinical trial was conducted in neonatal ICU from September 2020 to October 2021. Between these dates, all newborns with critical CHD who were hospitalised in the neonatal ICU and required intubation were included in the study. During the study period, 86 newborns with critical CHD were followed up in our cardiac neonatal ICU. Among them, seven patients were not included as they did not require intubation. Newborns with upper respiratory tract anomaly, poor quality X-rays, and without parental consent were excluded from the study. 14 patients were dropped out according to the exclusion criteria. Finally, the study continued with 65 patients who provided the inclusion criteria. Written and signed informed consent was obtained from the parents. The study was approved by the local human research ethics committee (E-20/11-035, 2020).
A portable chest radiograph was taken for each patient 1 hour after primary intubation or reintubation. In our neonatal ICU, a body weight-based calculation method was used for determining endotracheal tube depth: “Endotracheal tube value at the rim (in cm) = 6 + body weight (in kg).” Endotracheal tube tip-carina distance was measured with portable ultrasound (GE/LOGIQ E) in all infants approximately 2 hours after chest X-ray to obtain a more reliable sonographic-radiographic relationship. There was no planned change in the endotracheal tube position during the period from the chest X-ray to the ultrasound. The infant’s head was held in a slight extension and in the midline while chest X-ray was taken and ultrasound was performed. A high-frequency (13 MHz) 12L linear transducer was used for POCUS. The baby’s nurse was present at the bedside for unexpected events. Imaging was stopped when any deterioration in the baby was noticed during the procedure. Utmost care was taken to maintain the infant’s oxygenation and temperature regulation.
After the hot gel was applied, the probe was placed in the suprasternal notch in the transverse position with the pointer to the right and slide downwards on the sternum with less than 0.5 cm movement to view the lower end of the endotracheal tube in the trachea (Fig 1a and b).
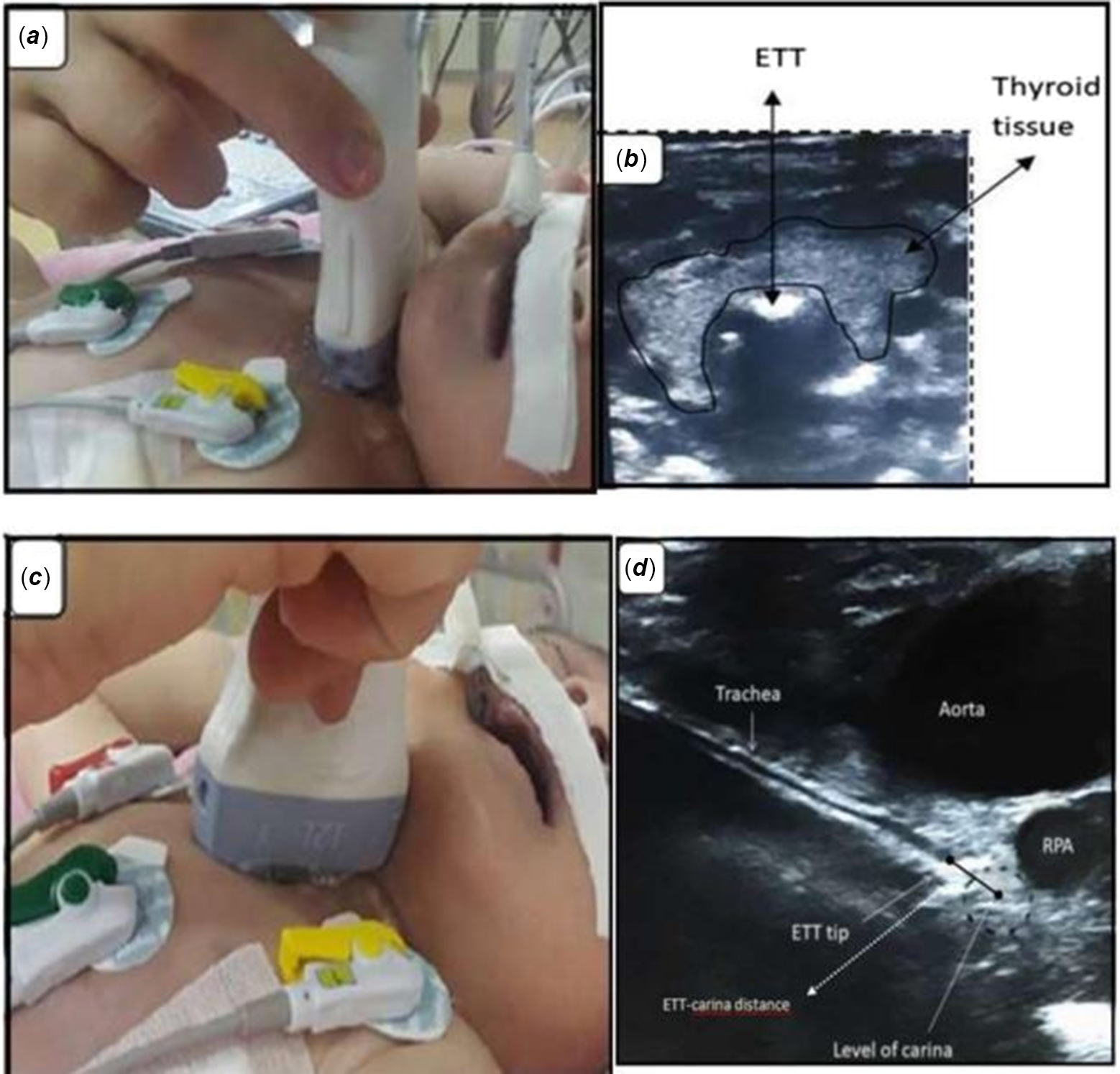
Figure 1. (a) Ultrasound probe placement for endotracheal tube localisation in a transverse position. ( b) Ultrasound view of an endotracheal tube in a transverse position (behind the thyroid tissue). (c) Ultrasound probe placement for endotracheal tube localisation in mid-sagittal position. (d) Ultrasound view of endotracheal tip-carina distance measurement in mid-sagittal position.
The probe was then rotated to the mid-sagittal plane with the pointer pointing up. After obtaining the right pulmonary artery and carina cross-section image under the aortic arch (Fig 1c and d), the probe was moved in-out slow motions of approximately 1.5 mm for clearer visualisation of the endotracheal tube tip. Reference Dennington, Vali, Finer and Kim5 Ultrasound was performed by a neonatology specialist (B.K.G) who had received ultrasonography training at least 6 months before the study and was unaware of the position of the endotracheal tube on the X-ray during the study.
Images were recorded during point-of-care ultrasound and stored in medical records for future viewing. The endotracheal tube tip-carina distance on the chest X-ray was evaluated by a radiologist (Y.S.) who was unaware of the ultrasound measurement. For the ideal position of endotracheal tube placement, endotracheal tube tip-carina distance was considered as 1–1.4 cm on ultrasonography. It was defined as 1 cm below clavicle in X-ray.
Demographic and clinical data of the study patients, including gestational age, birth weight, sex, mode of delivery, postnatal age at the time of the study, the types of critical CHD, and the time between chest X-ray and ultrasound imagination, were recorded.
Statistics
Statistical analyses were performed by using IBM SPSS 24.0 statistical programme. Kolmogorov–Smirnov test was used to determine the normality of data. Since data followed a normal distribution, arithmetic mean and standard deviation were given for the interpretation of demographic and clinical data. In comparison of endotracheal tube tip-carina distance in chest X-ray and ultrasound, Bland–Altman plot (Method of data plotting used in analysing the agreement between two different assays) and linear correlation tests were performed. A p value less than 0.05 was considered statistically significant.
Results
Siy-five intubated newborns with critical CHD whose endotracheal tube position was evaluated by both ultrasound and chest X-ray were included in the analyses; each patient was evaluated once. Demographic and clinical characteristics of the patients are shown in Tables 1 and 2. Mean gestational age was 37.8 ± 2.19 weeks, and birth weight was 2888 ± 595 g. The time between chest X-ray and ultrasound was 2.08 ± 1.6 hours. Bedside ultrasound imaging (imaging the endotracheal tube tip and measuring the endotracheal tube tip-carina distance) took an average of 5 minutes for each baby. Overall, they tolerated imaging without clinical worsening in infants. The procedure was interrupted due to desaturation in only two infants. Among all critical CHDs included in the study, only in pulmonary atresia type C (without pulmonary artery), a branch of the aortopulmonary collateral arteries below the aortic arch was used as the reference point to locate the carina. Only two of the patients included in the study were pulmonary atresia Type C. Right pulmonary artery was detected in the area where it should have been in other patients.
Table 1. Clinical characteristics of the study patients.

*POCUS: Point-of-care ultrasound.
*Mean ± standard deviation.
Table 2. The types of critical CHD.

The ideal position of endotracheal tube placement, endotracheal tube tip-carina distance was considered as 1–1.4 cm on ultrasonography. When the endotracheal tube was seen at the carina level (ETT tip-carina distance <0.5 cm) on ultrasound, the endotracheal tube was pulled up 1–1.5 cm by the nurse. When the endotracheal tube-carina distance was measured >1.5 cm on ultrasound, it was reported to the nurses that the endotracheal tube should not be held tight against the risk of dislocation. In addition, the presence of hyperechoic lines in the endotracheal tube during ultrasound has guided the estimation of the baby’s aspiration need.
Although the end of the endotracheal tube varied between the C6-T5 vertebrae on chest radiographs, 80% of the babies were located in the T2-T4 vertebrae. Endotracheal tube tip-to-carina distance on chest X-ray and ultrasound were 1.33 ± 0.64 cm and 1.43 ± 0.67 cm, respectively. There was no significant difference between chest X-ray and ultrasound measurements in endotracheal tube end-carina distance values evaluated with the Bland–Altman method (mean difference 0.10 cm, p = 0.068) (Fig 2). We observed a linear correlation between the endotracheal tube tip-carina distance on ultrasound and radiography (r2 = 0.60, p < 0.001).

Figure 2. Bland–Altman graphic*. Plot of distance measured by ultrasonography (ETTUS) versus radiography (ETT-XR) between endotracheal tube tip and carina. Differences between two measurements were not significant (mean difference 0.10 cm, p = 0.068). *Method of data plotting used in analysing the agreement between two different assays.
Discussion
Today, point-of-care ultrasound as an imaging modality continues to gain acceptance in different areas of neonatal medicine. Ultrasound is widely used in many situations and has an advantage over X-rays in terms of radiation exposure. There are no adequate ultrasonographic studies to determine the endotracheal tube position in neonatal critical CHD. Therefore, in this study, we evaluated the effectiveness of ultrasonographic measurement of endotracheal tube tip-carina distance in infants with CHD. In our study, it was aimed to reduce radiation exposure (at the beginning of life) and to prevent the development of radiation-related conditions in the later stages of life, as the most important reason for choosing newborns with critical CHD with long-term intensive care stays.
There was a concordance between ultrasonography and radiographic measurements in previous studies in evaluating ETT position. Reference Dennington, Vali, Finer and Kim5,Reference Jaeel, Sheth and Nguyen8 Historically, for the first time, Slovis et al. Reference Slovis and Poland3 examined the relationship between the aortic arch and endotracheal tube tip and ETT position in neonates. They found that the ETT tip position measured by ultrasound correlated well with the ETT tip-to-carina distance on radiographs (r2 = 0.80, p < 0.005).
In their largest study on newborns, Chowdhry et al. Reference Chowdhry, Dangman and Pinheiro4 defined by ultrasound as a “deeply located endotracheal tube” if the end-aortic arch apex distance of the endotracheal tube is <1 cm and the endotracheal tube tip is below the body of the third thoracic vertebra. However, newborns with critical CHD were excluded in this study Reference Chowdhry, Dangman and Pinheiro4 . In another study, Dennington et al Reference Dennington, Vali, Finer and Kim5 , Najip et al. Reference Najib, Pishva, Amoozegar, Pishdad and Fallahzadeh6 preferred to measure the distance between the endotracheal tube tip to the carina and confirmed a close relationship between the ultrasonographic and X-ray measurements. They used the superior portion of the right pulmonary artery, the anatomic equivalent of the carina as a landmark point to determine the endotracheal tube depth by ultrasound. The authors found that the mean of the measurements was not significantly different in both modalities.
In the current study, we measured the distance from the endotracheal tube tip to the carina in newborns with critical CHD. The section of the right pulmonary artery below the aortic arch was used as a guide for carina fixation. We compared our ultrasound findings with X-ray measurements and showed good correlation between the two imaging modalities.
Sethi et al. Reference Sethi, Nimbalkar, Patel, Kungwani and Nimbalkar9 reported the duration of imaging and interpretation time as an average of 19 minutes for ultrasound and 47 minutes for X-ray. In other studies, Saul et al. Reference Saul, Ajayi, Schutzman and Horrow10 reported the average ultrasound scan time as 7 minutes, while Dennington et al. Reference Dennington, Vali, Finer and Kim5 noted that ultrasound examinations by a neonatologist took less than 5 minutes. In our study, imaging time by ultrasound was 5 minutes.
Early and repeated exposure to radiation poses a special risk for infants. Reference Alzen and Benz-Bohm11 It is particularly important that children with critical CHD are exposed to cumulative radiation (diagnostic angiography, CT, and routine chest X-rays) during a long-term neonatal ICU stay, and this exposure can increase the risk of cancer.
In our study, the cross-section of right pulmonary artery (the same plane with the carina under the aortic arch in ultrasound) was determined as the guide point to determine the depth of endotracheal tube in newborns with critical CHD. We hypothesised the measurement of the distance between the endotracheal tube tip and the carina could be used for ultrasonographic evaluation. According to our previous data, we observed that 33.8% of the patients followed in the cardiac neonatal ICU had an aortic anomaly (unpublished data). Therefore, we thought that aortic arch apex-endotracheal tube tip measurement would not be appropriate in this patient group. In this study, it has been shown that the cross-section of right pulmonary artery can be used as a reference point for fast and easy determination of the carina in critical CHD.
In a recent study, Zaytseva et al. Reference Zaytseva, Kurepa, Ahn and Weinberger12 compared the efficacy of ultrasound with standard chest X-ray to confirm endotracheal tube position by formulating the optimal endotracheal tube depth distance from the gingival initiation as “body weight (kg) + 5.2 cm” in infants without critical CHD (intraclass correlation coefficient (0.95, 95% CI: 0.92, 0.98). In our study, we studied critical CHD patients, and we found a correlation between the two methods, but our correlation coefficient was lower than the study of Zaytseva et al. Reference Zaytseva, Kurepa, Ahn and Weinberger12 The possible reason for the difference was thought to be due to the absence of the right pulmonary artery in infants with pulmonary atresia Type C. Reference Mai, Isenburg and Canfield13 In these patients, the probability of detecting an aorta-pulmonary artery branch at the carina level instead of the right pulmonary artery was always low.
Our study has some strengths and weaknesses. Since the pulmonary artery does not develop in patients with type C pulmonary atresia, it is not appropriate to use the right pulmonary artery as a reference in the ultrasonographic measurement of the endotracheal tube end-carina distance only in these patients. In these patients, the sensitivity of this measurement method will be low. Patients with type C pulmonary atresia could be excluded. Additionally, the sample size of the study was somewhat small. However, this study is valuable because it is the first study to detect endotracheal tube placement ultrasonographically in critical CHD neonates. Furthermore, ultrasound procedures were performed by the same neonatologist.
As conclusion, critical CHDs are often accompanied by vascular anomalies. Therefore, endotracheal tube tip-aortic arch upper wall measurements in ultrasound are not always suitable methods in critical CHD. Endotracheal tube tip-right pulmonary artery upper wall (near the carina) measurements in ultrasound correlate with the measurements in X-ray. More sensitive studies are needed in the future.
Acknowledgements
We would like to express our sincere thanks to the intensive care nurses for their kind support.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The study was approved by the human research ethics committee (E-20/11-035, 2020).







