INTRODUCTION
Despite mild clinical manifestations, acute gastrointestinal infections (AGI) constitute an important burden to society [Reference Bhan1, Reference Kosek, Bern and Guerrant2]. Retrospective population-based surveys performed recently in different countries have estimated the incidence of symptomatic AGI from 0·33 to 1·40 per person-year [Reference Müller, Korsgaard and Ethelberg3–Reference Scavia6]. Additionally, AGI is a common reason for consulting a general practitioner (GP) and results in a significant loss of working days in the workforce.
Numerous recommendations exist for management of AGI in children and adults, but their implementation is rarely monitored. In Poland no national guidelines have been implemented to establish standard diagnosis and treatment of AGI patients. However, the 2008 guidelines from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition [Reference Guarino7] have been publicized and are likely to be adopted by physicians on a voluntary basis.
The available guidelines underline the crucial role of appropriate rehydration, realimentation and use of anti-emetic drugs in selected patients [Reference Guarino7–Reference Chow, Leung and Hon10]. Routine administration of antimicrobial drugs is not recommended and targeted antimicrobial therapy is only recommended for persons with selected confirmed bacterial or parasitic aetiology and for cases with severe outcomes and health conditions that are predisposed for serious complications, such as neonates, the immunosuppressed and those with inflammatory bowel disease or achlorhydria. Overuse of antibacterial prescriptions has been commonly reported in the treatment of upper respiratory tract infections in the outpatient setting [Reference Ferech11–Reference Grijalva, Nuorti and Griffin13], but few studies have addressed the inappropriate use of antibacterials specifically in relation to AGI treatment [Reference Osatakul and Puetpaiboon14–Reference Karras16]. To date, this problem has mostly been investigated in developing countries.
The aim of the present paper was to assess the population burden of antibacterial prescriptions in AGI treatment in Poland and to assess the determinants of antibacterial drug use in AGI patients.
METHODS
The present study was part of a larger prospective survey of AGI-related healthcare utilization and costs. The survey was conducted in 19 randomly selected healthcare facilities over 18 months (May 2008 to September 2009). Since this is the first report from this study, we present here the methods in detail.
Selection of study sites
Due to logistic reasons we restricted the study area to a region located close to the study coordination site in Warsaw. This region comprised six provinces inhabited by 14·4 million people (about 38% of Poland's population as of 31 December 2008). First, a random sample of outpatient units was selected from the list of all registered primary-health practices located in the six provinces. The number of sites in each province was selected proportionate to the number of its inhabitants. Similarly, a random sample of hospitals was selected, stratified according to the province's population. The selected units were screened for eligibility criteria: (i) location within 200 km of the coordination site; (ii) inclusion of at least one general practice or general medical ward; (iii) unit not to be discontinued within the following 12 months; (iv) unit manager's consent. Local investigators were trained in the study protocol. In order to estimate healthcare resources utilization and cost in all relevant age groups, each study site was assigned a patient quota in the following age groups: <4, 5–18, 19–65 and >65 years.
Recruitment of study participants
The investigators were responsible for recruitment of patients meeting the eligibility criteria (Table 1) during the study period until the inclusion limit was reached. The inclusion and exclusion criteria were compatible with the AGI definition proposed by the International Collaboration on Enteric Disease Burden of Illness [Reference Majowicz17] and used in the recent study of AGI prevalence in the community [Reference Baumann-Popczyk5].
Table 1. Inclusion and exclusion criteria for subjects used during the study
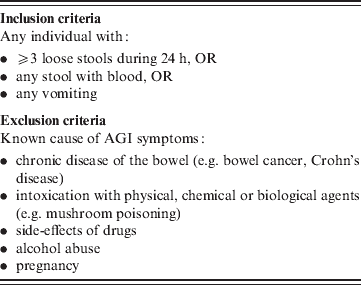
Informed consent was sought for follow-up interviews. In order to perform the follow-up interview, contact information was requested from consenting patients. The study was reviewed and approved by the Ethical Committee at the National Institute of Public Health in Warsaw.
Data collection
For each eligible patient, detailed information on healthcare resource utilization was collected using a standardized questionnaire. In GP practices the questionnaire consisted of 18 items, covering general demographics, clinical information, use of pharmaceuticals, diagnostic tests, materials, and specialist consultations. The hospital resource utilization questionnaire consisted of 26 items, addressing general demographics and clinical information, patient management at the admissions unit, and patient management in the hospital unit. A follow-up interview was conducted for all patients who agreed to be contacted, 2–4 weeks following the GP consultation or hospital discharge date. The structured telephone interview comprised of 16 questions relating to the patient's current occupation, impact of symptoms associated with AGI disease on their daily activities, further GP or specialist consultations, admissions to the hospital, use of prescriptions, and over-the-counter (OTC) medications and diagnostic procedures.
Data management and analysis
Healthcare resources were divided into the following categories: laboratory tests, imaging diagnostics, pharmacotherapy and specialist consultations. Pharmaceutical preparations were grouped according to Anatomical Therapeutic Chemical (ATC) classification. Use of pharmaceuticals having ATC codes was quantified using defined daily doses (DDD) according to WHO Collaborating Centre for Drug Statistics Methodology guidelines [18].
Estimation of the burden of antibacterial prescriptions in society
For estimation of antibacterial drug consumption in society a stochastic model was developed using @RISK 5.0 (Palisade Corporation, USA), a Monte Carlo simulation add-in to Microsoft Excel®:
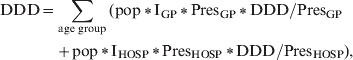 $$\eqalign{ {\rm DDD} \equals \tab \sum\limits_{{\rm age\ group}} {\lpar {\rm pop}\, \vskip2pt \ast \, \vskip-2pt \lowast {\rm I}_{{\rm GP}}\, \vskip2pt \ast\vskip-2pt \,\lowast {\rm Pres}_{{\rm GP}} \,\vskip2pt \ast\vskip-2pt \,\lowast {\rm DDD}\sol {\rm Pres}_{{\rm GP}} {\rm \ }} \cr \tab \plus {\rm pop}\,\vskip2pt \ast\vskip-2pt \,{\rm I}_{{\rm HOSP}} \, \vskip2pt \ast \, \vskip-2pt \lowast {\rm Pres}_{{\rm HOSP}} \, \vskip2pt \ast \, \vskip-2pt \lowast {\rm DDD}\sol {\rm Pres}_{{\rm HOSP}} \rpar \comma \cr} $$
$$\eqalign{ {\rm DDD} \equals \tab \sum\limits_{{\rm age\ group}} {\lpar {\rm pop}\, \vskip2pt \ast \, \vskip-2pt \lowast {\rm I}_{{\rm GP}}\, \vskip2pt \ast\vskip-2pt \,\lowast {\rm Pres}_{{\rm GP}} \,\vskip2pt \ast\vskip-2pt \,\lowast {\rm DDD}\sol {\rm Pres}_{{\rm GP}} {\rm \ }} \cr \tab \plus {\rm pop}\,\vskip2pt \ast\vskip-2pt \,{\rm I}_{{\rm HOSP}} \, \vskip2pt \ast \, \vskip-2pt \lowast {\rm Pres}_{{\rm HOSP}} \, \vskip2pt \ast \, \vskip-2pt \lowast {\rm DDD}\sol {\rm Pres}_{{\rm HOSP}} \rpar \comma \cr} $$where age group is either 0–18 or ⩾19 years, pop = population, IGP = rate of GP visits per person-year, IHOSP = hospital admission rate per person-year, PresGP = probability of antibacterial prescription by GP, PresHOSP = probability of antibacterial prescription during hospital admission; DDD/PresGP = mean daily defined dose of antibacterials prescribed by GP, DDD/PresGP = mean daily defined dose of antibacterials prescribed during hospital admission. Note that all parameters are age-group specific.
Annual rates of AGI-related GP visits and hospital admissions were obtained from a parallel population-based telephone survey [Reference Baumann-Popczyk5] and represented by beta distributions. The probability of antibiotic prescription was represented by beta distribution, and the mean DDD prescribed were represented by inverse Gaussian distribution. The distribution parameters were based on values from original datasets, estimated using maximum-likelihood estimators. The national annual antibacterial consumption was calculated using Monte Carlo simulation with the above-mentioned probability distributions and the 2009 population estimate for Poland. The model was run for 50 000 iterations to stabilize the output distributions. The mean and 95% uncertainty intervals are reported.
Determinants of antibacterial drug administration
A logistic regression model was fitted to assess factors associated with antibacterial drug administration. The response variable was administration of any antibacterial drug (ATC code: J01), and the explanatory variables were: month of onset, type of health unit (small outpatient clinic <5 physicians, large clinic >5 physicians, paediatric ward, general medical ward), age group and the following indicator variables: rural residence, presence of symptoms (diarrhoea, vomiting, fever, diarrhoea with presence of blood or mucus), specialist consultation and referral for microbiological investigation of AGI aetiology (i.e. any examination of stool or blood sample). To find the best model, backwards elimination was used, setting the significance level for removal from the model at 0·1. We assessed potential effect modification by two-way interactions between variables included in the model. Additionally, potential confounding by variables removed by backwards elimination was assessed by comparing the effect of adding them to the model. The fit of the model was assessed by the Hosmer–Lemeshow test. All analyses were performed in Stata v. 10 [19].
RESULTS
Selection of study sites and patient recruitment
The selection of study sites is summarized in Figure 1. As a result of the sampling procedure, 39 outpatient units meeting inclusion criteria were selected, of which 11 were finally included in the study. The response ratio was 6/13 (46%) in rural areas, 2/14 (14%) in towns with <100 000 inhabitants, and 3/12 (25%) in towns with >100 000 inhabitants. During a total of 77·4 unit-months of observation 393 patients were reported as meeting inclusion criteria. A total of 385 valid interviews on GP consultations were collected. For 115 patients a follow-up telephone interview was collected.

Fig. 1. Selection of units for healthcare resources utilization survey, Poland, May 2008 to September 2009.
Of 50 hospitals selected in six studied provinces 28 met the inclusion criteria. In 16 hospitals managers refused to participate in the study, and four hospitals withdrew from the study following the visit of the study coordinator due to lack of consent of unit personnel to the study procedures. The resulting response ratio was 4/11 (36%) in rural areas including small towns of <20 000 inhabitants, and 4/17 (24%) in urban areas. In the selected eight hospitals, nine hospital wards were recruited, including paediatric (n = 4), internal medicine (n = 4), and infectious disease unit (n = 1). Of 7226 patients hospitalized in the observed wards, 504 meeting the inclusion criteria were recruited and interviewed. For 145 patients a follow-up interview was collected. The demographic characteristics of the study population are described in Table 2.
Table 2. Demographic and clinical characteristics of patients enrolled in the study by healthcare unit type, Poland, May 2008 to September 2009

Patient management
Patient management in outpatient care is summarized in detail in Supplementary Table S1 (available online). Stool samples for microbiological investigation of AGI aetiology were collected from nine patients. No results of microbiological investigation were obtained due to lack of GP's follow-up. Thirteen patients were referred to the hospital, most commonly in the 5–18 years age group. Patient management of AGI cases admitted to hospital is summarized in Supplementary Table S2 (online). Microbiological investigation of AGI aetiology was attempted in 355 (70%) patients. For 313 patients admitted to the hospital only stool samples were investigated, in four patients only blood culture was performed, and in 38 patients both stool and blood cultures were attempted. During the course of hospitalization, aetiology was established for 73 patients (20·6% of all tested), of which 46 were bacterial, 26 viral, and one parasitic infection. In seven cases co-infection with two enteric pathogens was reported. Of bacterial pathogens the following were cultured: Salmonella spp. (25), Klebsiella spp. (5), Pseudomonas aeruginosa (4), Citrobacter spp. (4), enteropathogenic Escherichia coli (4), Staphylococcus spp. (3), Morganella morganii (2), Enterobacter spp. (2), Proteus spp. (2) and Enterococcus spp. (1). Among viral pathogens rotavirus (24) and adenovirus (2) infections were confirmed.
Antibacterial drug prescriptions
Table 3 lists the antibacterial drugs prescribed during the study period. GPs prescribed a total of 116·28 DDD of antibacterials for systemic use to 60 (15·6%) AGI cases. Hospital physicians prescribed a total of 389·00 DDD of antibacterial medications to 179 (35·5%) hospitalized AGI cases.
Table 3. Antibacterial preparations prescribed in the studied population, Poland, May 2008 to September 2009

INN, International non-prioprietary name; ATC, Anatomical Therapeutic Chemical (ATC) classification system; DDD, defined daily dose.
The overall antibacterial consumption estimated in the study population was 30·20 DDD/100 GP consultations and 77·18 DDD/100 hospital admissions. The estimated societal AGI-related consumption of antibacterials amounted to 5·48 million DDD (95% uncertainty interval 1·56–14·12 million DDD).
Determinants of antibacterial drug prescriptions
The results of the logistic model investigating the association between administration of antibacterials for systemic use and demographic, clinical and diagnostic factors are presented in Table 4. Season of AGI onset, presence of diarrhoea, vomiting and referral to a specialist were not significantly associated with antibacterial use and were therefore removed from the model. Prescription of antibacterial medications was more likely for adults aged >65 years compared to the <5 years age group (adjusted odds ratio 1·88). Inhabitants of rural areas were more likely to receive antibacterial drug prescriptions than inhabitants of urban areas. The presence of fever and blood or mucus in stool were independent predictors of antibacterial use. Physicians working in large outpatient clinics and physicians working in hospital wards were almost three times more likely than those in small outpatient clinics to prescribe antibacterials. Finally, referral for microbiological investigation was significantly associated with the decision to administer an antibacterial medication (Table 4).
Table 4. Factors associated with administration of any antibacterials for systemic use. Results of multivariable logistic regression model, Poland, May 2008 to September 2009

n, Number of patients in the estimation sample (i.e. observations included in the model); aOR, adjusted odds ratio; CI, confidence interval.
Hosmer–Lemeshow test: χ2 = 9·4, P value 0·309.
DISCUSSION
The present study is the first attempt to investigate antibacterial drug consumption in relation to AGI treatment, referring its results to the general population. This was possible due to the availability of results of a parallel study, which estimated incidence of self-reported AGI episodes in the community [Reference Baumann-Popczyk5]. Despite the fact that only a minority (27%) of patients were given antibiotics, the extremely high prevalence of AGI symptoms in the community, often leading to GP consultation, led to a high overall societal burden of antibacterial consumption. Antibacterial drug administration was more likely for patients: aged >65 years, with fever, mucus or blood in their stool, residing in rural areas, consulting physicians working in large outpatient clinics, admitted to hospital wards, and for those referred for microbiological investigation of AGI aetiology.
In most AGI cases, it is not necessary to prescribe antibacterial drugs. According to national surveillance, AGI caused by pathogens for which antibiotic treatment may be recommended (e.g. Shigella spp., Campylobacter spp., E. coli, Listeria spp.) constitute not more than 3% of reported AGI cases [Reference Baumann-Popczyk and Sadkowska-Todys20]. Use of unnecessary antibacterial drugs places a burden on society not only in monetary terms, but also by contributing to the development of antimicrobial resistance [Reference Caron and Mousa21]. Compared to figures on antibiotic use in Poland based on official reimbursement records from 2000 to 2005 [Reference Ferech11, Reference Muller22], AGI-related antibacterial consumption constitutes slightly less than 2% of prescribed antibacterials. Although the highest use of antibacterial medications is associated with upper respiratory tract infections, our results indicate that the amount of antibacterial medications used to treat AGI symptoms is not negligible.
A European multicentre study found that overuse of antibacterials in AGI management is more pronounced in central European countries than in other developed countries in Europe [Reference Szajewska, Hoekstra and Sandhu23]. On the other hand, the proportion of AGI cases that were prescribed antibacterials by GPs observed in Poland (16%) was considerably lower than in developing countries (37% in Mexico, 61% in Thailand, 66% in Pakistan, 71% in India) [Reference Osatakul and Puetpaiboon14, Reference Pathak15, Reference Bojalil and Calva24, Reference Nizami, Khan and Bhutta25]. This could be related to poor nutritional status of patients in the latter countries, as well as different aetiological agents leading to more severe outcomes of acute diarrhoea. As in several previous studies, antibacterial use was associated with fever and/or blood or mucus in stool [Reference Osatakul and Puetpaiboon14–Reference Karras16, Reference Bojalil and Calva24]. Our finding that physicians working in large outpatient clinics are more likely to prescribe antibacterials compared to colleagues from small practices could be an effect of confounding by urbanization status since the larger practices were often located in large cities. However, antibacterials were in fact more commonly prescribed to inhabitants of rural areas suggesting an opposite association with city size. Our observation therefore indicates that behaviour and practice-specific habits may play a more important role in the decision for antibacterial prescription than the presence of objective indications. The association between antibacterial prescription and rural residence has been identified in previous studies conducted in Poland, investigating use of broad-spectrum antibacterials for treatment of the common cold [Reference Chlabicz and Pytel-Krolczuk26, Reference Panasiuk27].
The observed association between antibacterial use and referral of patient samples for microbiological confirmation indicates that at least in some cases targeted antibacterial therapy was attempted. Microbiological investigation of aetiology was requested in 40% of AGI cases. The large difference observed between general practices (2%) and hospital wards (70%) reflects much wider availability of microbiological testing to hospital physicians and more severe clinical status of hospitalized patients. In recent European surveys it was observed that the proportion of AGI cases for which the consulting GPs requested a specimen for microbiological investigation ranged from 2% to 3% [Reference Müller, Korsgaard and Ethelberg3–Reference Scavia6]. Lack of confirmation of AGI aetiology prevents targeted antibacterial therapy. From a more general point of view, lack of valid information on AGI pathogens prevailing in the community limits the possibility of implementation and monitoring of efficient public health interventions, such as vaccination of humans or animals against enteric pathogens or Salmonella control programmes. In our study no diagnosis of Campylobacter spp. and norovirus infections, which are the most prevalent AGI pathogens in Europe were reported. The reason for this was non-inclusion of these pathogens in the routine investigation of AGI cases at the time of the study, not the actual epidemiological situation of the country [Reference Baumann-Popczyk and Sadkowska-Todys20].
Our study covered 38% of the population of Poland, potentially limiting the representativeness of our results. If regional differences had existed in AGI pathogen type occurrence or access to healthcare, the extrapolation of our results to the entire Polish population would be questionable. Although we did not sample healthcare units from the entire country, we believe that the stratified random sampling ensured a fair representation of the Polish population in terms of age distribution and different levels of urbanization. Restriction of our study to general hospitals is another important limitation of the study's representativeness. Inclusion of third-level reference hospitals, including university hospitals and institutes, would provide more insight into management of the most complicated cases. Nevertheless, due to self-limiting and mild symptoms in most AGI cases, the study probably constitutes a reliable snapshot of the management of acute diarrhoea in Poland. Another limitation relates to the difficulties in assessment of participation rate. Since local investigators were asked to collect information on each case meeting inclusion criteria, and informed consent was sought only for follow-up interview, we assumed that a systematic sample of cases was included in each age group relating to working hours of the investigators. Following a preliminary feasibility study we decided against a detailed monitoring of patients' registers, as most units did not have computerized registers, and our study had limited resources. We assumed that non-participants did not substantially differ from participants, as the decision to include patients depended to a great extent on the availability of the investigator, rather than on any factors influencing antibacterial use. The fact that we obtained follow-up interviews only from about 30% of studied subjects additionally limits the potential insight into adherence of patients to GPs' prescriptions, self-medication practices and additional use of ambulatory and private healthcare.
Despite the above-mentioned limitations, our study is the first Polish investigation of antibacterial consumption in the general community. We conclude that despite relatively uncommon antibacterial treatment of AGI by Polish GPs, the resulting antibacterial overuse places a high burden on society due to the high incidence of AGI symptoms, and their common referral to healthcare. Important differences appear in the approach to AGI antibacterial treatment between GPs, paediatricians and internists. We recommend revision of postgraduate training curricula and adoption as well as wide dissemination of national guidelines on AGI treatment. In the broader context, antibacterial prescriptions in AGI treatment should be more closely monitored at the outpatient and inpatient level. Further, synchronized monitoring of antimicrobial resistance at public health and veterinary health systems could improve early detection of emergence of resistant AGI pathogens, and aid efficient implementation of interventions.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268812001173.
ACKNOWLEDGEMENTS
The survey was jointly funded from two European Commission Sixth Framework Programme projects: POLYMOD (SSP22-CT-2004–502084) and the Network of Excellence MED-VET-NET (FOOD-CT-2004-506122), and co-financed by Polish Ministry of Science and Higher Education. The authors thank all study investigators for their contribution to the project and Anna Zielicka-Hardy for her helpful comments on the manuscript.
DECLARATION OF INTEREST
None.







