Introduction
Pycnogonida, or sea spiders, are almost exclusively free-living marine invertebrates. With more than 1300 named species (Appeltans et al., Reference Appeltans, Ahyong, Anderson, Angel, Artois, Bailly, Bamber, Barber, Bartsch, Berta, Błażewicz-Paszkowycz, Bock, Boxshall, Boyko, Brandão, Bray, Bruce, Cairns, Chan, Cheng, Collins, Cribb, Curini-Galletti, Dahdouh-Guebas, Davie, Dawson, De Clerck, Decock, de Grave, de Voogd, Domning, Emig, Erséus, Eschmeyer, Fauchald, Fautin, Feist, Fransen, Furuya, Garcia-Alvarez, Gerken, Gibson, Gittenberger, Gofas, Gómez-Daglio, Gordon, Guiry, Hernandez, Hoeksema, Hopcroft, Jaume, Kirk, Koedam, Koenemann, Kolb, Kristensen, Kroh, Lambert, Lazarus, Lemaitre, Longshaw, Lowry, Macpherson, Madin, Mah, Mapstone, McLaughlin, Mees, Meland, Messing, Mills, Molodtsova, Mooi, Neuhaus, Ng, Nielsen, Norenburg, Opresko, Osawa, Paulay, Perrin, Pilger, Poore, Pugh, Read, Reimer, Rius, Rocha, Saiz-Salinas, Scarabino, Schierwater, Schmidt-Rhaesa, Schnabel, Schotte, Schuchert, Schwabe, Segers, Self-Sullivan, Shenkar, Siegel, Sterrer, Stöhr, Swalla, Tasker, Thuesen, Timm, Todaro, Turon, Tyler, Uetz, van der Land, Vanhoorne, van Ofwegen, van Soest, Vanaverbeke, Walker-Smith, Walter, Warren, Williams, Wilson and Costello2012; Bamber et al., Reference Bamber, El Nagar and Arango2023), pycnogonids occur in all oceans and range in depth from 0 to 7370 m (Arnaud and Bamber, Reference Arnaud and Bamber1987). Although most studies deal with individuals collected from open marine environments, a few have reported sea spiders from caves (e.g. Akoumianaki and Hughes, Reference Akoumianaki and Hughes2004; Bamber, Reference Bamber2008; Onorato and Belmonte, Reference Onorato and Belmonte2017; Alvarez and Ojeda, Reference Alvarez and Ojeda2018; Gerovasileiou and Bianchi, Reference Gerovasileiou and Bianchi2021). Cave-dwelling sea spiders are generally not identified but just listed as Pycnogonida; exceptions are Pycnogonum coninsulum Bamber, Reference Bamber2008 from a submarine cave in Hong Kong and Anoplodactylus batangensis (Helfer, 1938) from an anchialine cave in Mexico (Bamber, Reference Bamber2008; Alvarez and Ojeda, Reference Alvarez and Ojeda2018). Having not yet been collected from open marine environments, Py. coninsulum appears to be an endemic cave species (Bamber et al., Reference Bamber, Evans and Robbins2008). Anoplodactylus batangensis is a cosmopolitan species also found outside the cave, suggesting that the individual in the cave may have been transported through passageways in the anchialine system (Alvarez and Ojeda, Reference Alvarez and Ojeda2018).
There are many submarine caves around the Ryukyu Islands, southwestern Japan, but knowledge of their invertebrate faunas is limited. Recent surveys of the invertebrate fauna of ‘Akumanoyakata’ submarine cave in Shimojijima Island have detected new or rare species among poriferans (e.g. Ise, Reference Ise2019), crustaceans (e.g. Saito and Fujita, Reference Saito and Fujita2022), polychaetous annelids (e.g. Worsaae et al., Reference Worsaae, Hansen, Axelsen, Kakui, Møller, Osborn, Martínez, Gonzalez, Miyamoto and Fujita2021), brittle stars (e.g. Okanishi and Fujita, Reference Okanishi and Fujita2019) and bivalves (e.g. Mizuyama et al., Reference Mizuyama, Kubo and Fujita2022), but there have been no records of sea spiders to date.
This paper reports one sea spider collected from the completely dark, anchialine zone at 10–20 m depth in the Akumanoyakata Cave. With a slender, pipette-shaped proboscis having distal annulation, it belongs to the family Austrodecidae Stock, 1954. Although the specimen is likely a juvenile female and it is unknown how many articles there are in the adult oviger (one character distinguishing between the austrodecid genera: Austrodecus Hodgson, 1907 with six or fewer articles [absent in males of several species] and Pantopipetta Stock, 1963 with ten articles and a terminal claw), we identified the specimen as Pantopipetta based on the very slender trunk without dorsomedian tubercles and the palp with three short distal articles (cf. Child, Reference Child1994; for details, see the ‘Discussion’ section). Pantopipetta pycnogonids are relatively rare (Hedgpeth and McCain, Reference Hedgpeth and McCain1971) and generally found at considerable depths (Child, Reference Child1994), with the shallowest record at 66 m (cf. Hosoda and Kakui, Reference Hosoda and Kakui2020). This is the first record of Pantopipetta from a submarine cave or anchialine environment, and at the shallowest depth record of 10–20 m. The specimen can be distinguished from all congeners (16 species; Hosoda and Kakui, Reference Hosoda and Kakui2020), and we describe it here as a new species. Additionally, we discuss the taxonomic significance of the number of palp articles in Pantopipetta species and cave pycnogonids in general.
Materials and methods
A pycnogonid was collected by scuba diving on 8 March 2021 in ‘Akumanoyakata’ Cave, located on a reef slope at Shimojijima Island, Miyako Island Group, Ryukyu Island, southwestern Japan (26°51.896′N, 128°14.732′E), with the entrance at about 35 m depth; see Osawa and Fujita (Reference Osawa and Fujita2019) for detailed information on the cave. From the second slope zone (Osawa and Fujita, Reference Osawa and Fujita2019; 80–100 m from the entrance, 10–20 m depth, no light, less than 28‰ salinity, rocky substrate), mud deposited around cnidarians and poriferans on the cave wall was collected with a commercially made aquatic suction sampler (yabby pump). The individual was sorted from the mud sample and preserved in 99% ethanol. The methods used for dissection, preparation of slides, light microscopy and drawing were as described by Kakui and Angsupanich (Reference Kakui and Angsupanich2012). Morphological terminology follows Child (Reference Child1979), except that the term ‘article’ is used instead of ‘segment’ for all appendages (Hosoda and Kakui, Reference Hosoda and Kakui2020). Measurements were made axially (dorsally for the trunk and abdomen; laterally for the palp, proboscis, ocular tubercle and legs) and are presented in millimetres. Measurements for congeners were obtained from original descriptions or measured from original illustrations. Trunk length was measured from the palp insertion to the base of the abdomen, and trunk width as the width of the segment at the narrowest portion of the trunk. The specimen studied was deposited in the Invertebrate Collection of the Hokkaido University Museum (ICHUM), Sapporo. To obtain information on male genital openings in Pantopipetta, we observed the type series of Pantopipetta lenis Hosoda & Kakui, Reference Hosoda and Kakui2020 (ICHUM6038, 6039).
Results
SYSTEMATICS
Family AUSTRODECIDAE Stock, 1954
Genus Pantopipetta Stock, Reference Stock1963
Pantopipetta hosodai sp. nov.
[New Japanese name: Dokutsu-suikuchi-umigumo]
(Figures 1, 2 and 3A–D)
Diagnosis (Juvenile Female)
Trunk segments 2 and 4 short (length/width ratios 1.3 and 2.6); ocular tubercle with swollen tip; lateral processes without dorsodistal tubercle; palp with three short distal articles; coxa 1 of legs 1–3 with long dorsal tubercle (longer than coxa-1 width); coxa 3 of legs 1–3 with long dorsal tubercle (as long as coxa-3 width); all femora with short dorsodistal tubercle (length 0.5 times femur width) bearing seta; auxiliary claws about 0.5 claw length.

Figure 1. Pantopipetta hosodai sp. nov., holotype, ICHUM8407, juvenile, ethanol-fixed specimen: (A) habitus, dorsal view; (B) habitus, left view; (C) cephalon, right view; (D) right oviger. lpc, lateral process of cephalon; ov, oviger; p1, palp article 1.
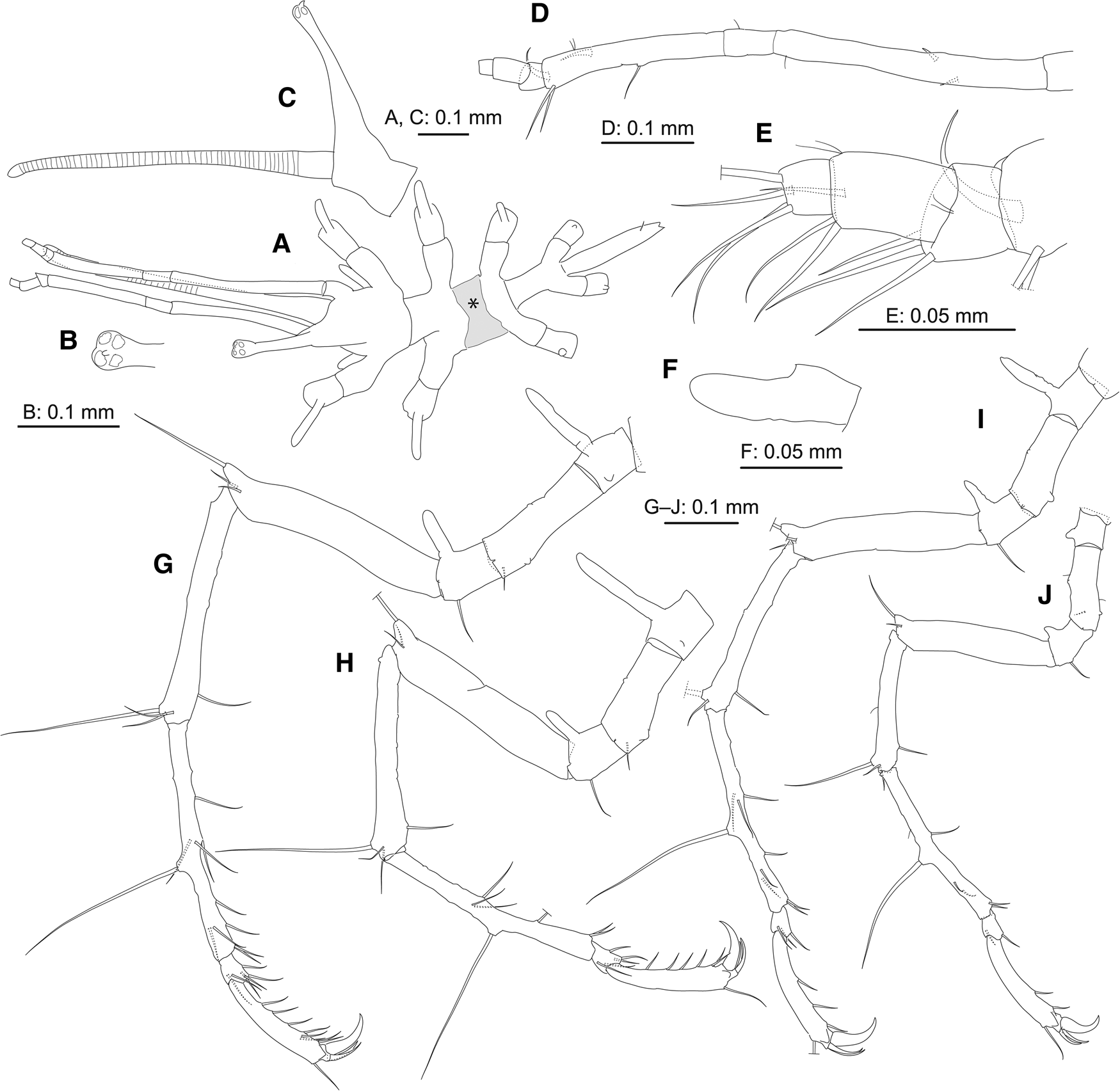
Figure 2. Pantopipetta hosodai sp. nov., holotype, ICHUM8407, juvenile: (A) habitus, dorsal view (grey-shaded area marked with asterisk indicates damaged area flattened by accidentally pinching with forceps); (B) distal tip of ocular tubercle, dorsal view; (C) cephalon, left view (lateral process of cephalon and leg 1 omitted); (D) left palp (ornamentation on short distal articles omitted); (E) distal portion of left palp; (F) right oviger; (G–J) left legs 1–4.

Figure 3. Genital openings of Pantopipetta: (A–D) P. hosodai sp. nov., holotype, juvenile female; (E) P. lenis Hosoda and Kakui, Reference Hosoda and Kakui2020, holotype, male. (A–E) left legs 1, 2, 3, 4 and 4, respectively. c2, c3, coxae 2 and 3. Arrowheads, genital opening.
Etymology
The specific name is a noun in the genitive case, honouring Yushi Hosoda, who has contributed to the taxonomy of Japanese pycnogonids.
Type Material
Holotype: juvenile female, ICHUM8407; three slides and one vial; second-slope zone in Akumanoyakata Cave (26°51.896′N, 128°14.732′E), 10–20 m depth, Shimojijima Island, Miyako Island Group, Ryukyu Islands, Japan, northwestern Pacific Ocean, mud; collected on 8 March 2021 by Yoshihisa Fujita.
Description of Holotype (Juvenile Female)
Trunk (Figures 1A, B and 2A–C) fully segmented, without dorsomedian tubercles; segments 2 and 4 short; segment 3 deformed, flattened by accidentally pinching with forceps (Figure 2A, grey-shaded area with asterisk). Lateral processes long, separated by about their basal diameter (trunk segments 1–3) or about twice their basal diameter (segments 3 and 4), without dorsodistal tubercle. Ocular tubercle (Figure 2B, C) tall, erect, with swollen tip bearing four tiny eyes; tiny distal process present. Proboscis pipette-like, annulated. Abdomen longer than trunk segment 4, with pair of subposterior setae (one broken).
Palp (Figures 1C and 2D, E) six-articulate. Article 1 longest, with two spines. Article 3 with one middle and two strong distal setae; two strong curved spines, one subdistal and one distal. Articles 4–6 ( = 3 short distal articles) with three, three and four distal setae.
Oviger (Figures 1D and 2F) with one article, naked.
Legs 1–3 (Figures 2G–I and 3A–C) slender. Coxa 1 with long dorsal tubercle (longer than coxa-1 width). Coxa 2 with tiny dorsal projection and tiny ventro-subdistal genital opening; subdistal seta on legs 1 and 2. Coxa 3 with long dorsal tubercle (longer than coxa-3 width) and ventrodistal seta. Femur with two distal setae and short dorsodistal tubercle (length half femur width) bearing seta. Tibia 1 with two (legs 1 and 2) or one (leg 3) dorsodistal and one ventro-subdistal setae, and dorsodistal robust seta. Tibia 2 with six (leg 1) or four (legs 2 and 3) ventral, two (legs 1 and 3) or one (leg 2) anterior, two (legs 1 and 3) or one (leg 2) posterior setae and mid-dorsal robust seta. Tarsus with two (legs 1 and 2) or one (leg 3) ventral, two (legs 1 and 2) or one (leg 3) anterior and two (leg 1) or one (legs 2 and 3) posterior setae. Propodus with one dorsodistal and six (legs 1 and 2) or three (leg 3) ventral setae, and two auxiliary claws; with one anterior and one posterior setae on leg 1; auxiliary claws similar in size, about half claw length. Cement gland opening not observed.
Leg 4 (Figures 2J and 3D) slender, much shorter than legs 1–3. Coxa 1 with short dorsal tubercle (shorter than half coxa-1 width). Coxa 2 similar to those in legs 1–3; tiny ventro-subdistal genital opening present. Coxa 3 with short dorsal tubercle (shorter than half coxa-3 width) and ventrodistal seta. Femur with distal seta and short dorsodistal tubercle (length half femur width) bearing seta. Tibia 1 similar to those in legs 1 and 2. Tibia 2 with three ventral, one anterior and one posterior setae, and mid-dorsal robust seta. Tarsus with one ventral and one anterior setae. Propodus with one dorsodistal and two ventral setae, and two auxiliary claws; auxiliary claws similar in size, about half claw length. Cement gland opening not observed.
Measurements: trunk length 0.46; width across second lateral processes 0.30; proboscis length 0.66; ocular tubercle length 0.33; abdomen length 0.22; length/width of trunk segments 2 and 4, 0.11/0.08, 0.12/0.05; length of palp articles 1–6, 0.30, 0.05, 0.20, 0.02, 0.04, 0.01 (0.62 in total); length of leg-1 articles (from coxa 1; including claw), 0.07, 0.18, 0.08, 0.30, 0.32, 0.33, 0.04, 0.16, 0.08 (1.55 in total); length of leg-2 articles (ditto), 0.08, 0.15, 0.07, 0.28, 0.27, 0.31, 0.03, 0.16, 0.08 (1.41 in total); length of leg-3 articles (ditto), 0.06, 0.14, 0.07, 0.26, 0.24, 0.28, 0.03, 0.17, 0.08 (1.33 in total); length of leg-4 articles (ditto), 0.05, 0.11, 0.05, 0.21, 0.18, 0.24, 0.03, 0.15, 0.07 (1.09 in total).
Pantopipetta lenis Hosoda & Kakui, Reference Hosoda and Kakui2020
(Figure 3E)
Material Examined
Holotype: male, ICHUM6038. Paratype: male, ICHUM6039.
Supplementary Information on Male Genital Openings
Coxa 2 of legs 1–3 without genital opening. Coxa 2 of leg 4 with ventro-subdistal genital opening (Figure 3E).
Discussion
Staging and sexing
Our specimen has leg 4 much shorter than leg 3, with a length of about 0.82 times of that of leg 3. Although Austrodecidae lacks information on ontogenetic development after the postlarval stage that bears unarticulated legs 4, the above condition was reported in non-adult individuals in several other families (e.g. Okuda, Reference Okuda1940; Brenneis et al., Reference Brenneis, Arango and Scholtz2011; Miyazaki and Hoshino, Reference Miyazaki and Hoshino2019). The oviger of our specimen comprises one naked article. Uniarticulate ovigers have been reported in three austrodecid species, namely, Austrodecus (Microdecus) fryi Child, Reference Child1994, Austrodecus palauense Child, Reference Child1983 and Austrodecus varum Child, Reference Child1994, but the latter two were species described based on juveniles (Child, Reference Child1983, Reference Child1994). The oviger of A. (M.) fryi bears setae, not naked. Naked uniarticulate ovigers were reported in non-adults of other families (e.g. Okuda, Reference Okuda1940; Brenneis et al., Reference Brenneis, Arango and Scholtz2011; Miyazaki and Hoshino, Reference Miyazaki and Hoshino2019).
We observed genital openings on the coxa 2 of legs 1–4 of our specimen, but they appeared to be smaller than those reported in confamilial adults (Loman, Reference Loman1908; Miyazaki, Reference Miyazaki2004), suggesting that they may not be fully formed. In Austrodecidae, genital openings were found on legs 1–4 in females (e.g. Loman, Reference Loman1908; Turpaeva, Reference Turpaeva1955; Miyazaki, Reference Miyazaki2004) and only on leg 4 in Austrodecus males (Miyazaki, Reference Miyazaki2004). Male genital openings had not been described in Pantopipetta until now. Here we showed that males of P. lenis bear genital openings only on coxa 2 of leg 4 as do Austrodecus males. Cement gland openings were not observed in our specimen.
Given the above, we concluded that our specimen is a juvenile female having immature leg 4 and oviger.
Generic affiliation
We identified our specimen as a member of Pantopipetta mainly based on that its palp has three short distal articles. All known Pantopipetta species have three or four short distal articles on the palp whereas all Austrodecus species have one or two, except for Austrodecus aconae (Hedgpeth and McCain, Reference Hedgpeth and McCain1971) having three short distal articles.
Austrodecus aconae was originally described as a member of Pantopipetta. Hedgpeth and McCain (Reference Hedgpeth and McCain1971) speculated their specimens that have four- or five-articulate oviger (but see below) may be immature and described them as a member of Pantopipetta. Hedgpeth and McCain (Reference Hedgpeth and McCain1971: 218) stated that ‘In all species of Austrodecus so far described the terminal joint [ = terminal short distal article] of the palp is set at an angle on the penultimate joint [ = penultimate short distal article]; this feature is not found in the species of Pantopipetta’, which appears to be the major reason why they put their species in Pantopipetta. It should be noted that, in the palp of Pantopipetta, the second short distal article is set at an angle on the first short distal article but not so between the terminal and penultimate short distal articles (e.g. Figure 2D, E; Hedgpeth and McCain, Reference Hedgpeth and McCain1971, Figure 6f; Child, Reference Child1994, Figure 15E).
Stock (Reference Stock1991: 270) wrote ‘A. [ = Austrodecus] aconae (Hedgpeth and McCain, Reference Hedgpeth and McCain1971), originally described as a species of Pantopipetta’ and transferred the species into Austrodecus without providing any reason. Child (Reference Child1994) followed this view and put the species in Austrodecus. The author observed its holotype and paratypes (two females and five males) and found that females bear four-articulate ovigers but males lack any trace of ovigers; a five-articulate oviger was not observed (note: Hedgpeth and McCain (Reference Hedgpeth and McCain1971) observed four females). Male austrodecids lacking ovigers have been reported only in two Austrodecus species, A. (Tubidecus) excelsum Stock, Reference Stock1991 and A. (T.) latum Stock, Reference Stock1991, but they have palps with two short distal articles (Stock, Reference Stock1991).
The generic affiliation of A. aconae can vary depending on whether researchers emphasize the number of short distal articles on the palp or that of the oviger. In this study, although we refrain from returning A. aconae into Pantopipetta, we deemed the number of short distal articles on the palp to be one of the diagnostic characters to distinguish Austrodecus (one or two) and Pantopipetta (three or four) and identified our specimen as a member of Pantopipetta.
Morphological comparisons
Because we concluded that our specimen was a juvenile female with immature oviger and leg 4, we did not use the character states for these two appendages to distinguish our species from congeners. In having auxiliary claws, P. hosodai sp. nov. resembles Pantopipetta auxiliata Stock, Reference Stock1968 from off the eastern coast of South Africa (68–69 m depth), P. lenis from Japan (140.7–151.5 m depth) and Pantopipetta oculata Stock, Reference Stock1968 from the Andaman Islands (66 m depth). It differs from the latter three species (character state in parentheses) in having the palp with three short distal articles (four) and in lacking a short palp article articulated with the cephalon (article present) (see the following section). In addition, P. hosodai sp. nov. differs from P. auxiliata in having lateral processes without dorsodistal tubercles (with one tall, knobby spur in P. auxiliata), coxa 1 of legs 1–3 with one dorsal tubercle (two in P. auxiliata) and the auxiliary claw on the legs about 1/2 claw length (about 1/3 in P. auxiliata); from P. lenis in having coxa 1 of legs 1–3 with one long dorsal tubercle (no tubercles in P. lenis), a long dorsal tubercle on coxa 3 of legs 1–3, as long as coxa-3 width (short, half coxa-3 width in P. lenis), and the femur of the legs with one short dorsodistal tubercle bearing a seta (no tubercle in P. lenis); and from P. oculata in having coxa 1 of legs 1–3 with one dorsal tubercle (four in P. oculata), the dorsodistal tubercle on the femur of the legs short, half femur width (long, longer than femur width in P. oculata), and the auxiliary claw on the legs about 1/2 claw length (about 1/3 in P. oculata).
Pantopipetta hosodai sp. nov. differs from A. aconae in having lateral processes without dorsodistal tubercles (with one short tubercle in A. aconae), coxa 1 of legs 1–3 with one dorsal tubercle (two in A. aconae), and auxiliary claws (no auxiliary claws in A. aconae). It also differs from three Austrodecus species having uniarticulate ovigers (A. (M.) fryi, A. palauense and A. varum) by the number of short distal articles on the palp.
Palp base and number of palp articles
Hosoda and Kakui (Reference Hosoda and Kakui2020) found that the palp base (the short article-like structure proximal to the longest palp article) is actually the first palp article in P. lenis. In P. hosodai sp. nov., however, the palp base is not articulated with the cephalon, but instead is a lateral process of the cephalon (Figure 1C); the long article (the first palp article in P. hosodai sp. nov.) that appears homologous to the second palp article in P. lenis articulates with the lateral process. The condition in P. hosodai sp. nov. is equivalent to Child's (Reference Child1994: 82) description, ‘no suture or segmentation lines at all around their [ = palp bases’] root’.
The connection between the lateral process of the cephalon and the palp has not generally been described in detail, but a short palp article distal to the process has been illustrated in the original descriptions or re-descriptions for eight species: P. auxiliata (Stock, Reference Stock1968, Figure 8b); ‘Pantopipetta brevicauda Stock, Reference Stock1963’ in Turpaeva (Reference Turpaeva1990, Figure 6-3; Child [Reference Child1982] synonymized this species with Pantopipetta longituberculata Turpaeva, Reference Turpaeva1955); Pantopipetta brevipilata Turpaeva, Reference Turpaeva1990 (Turpaeva, Reference Turpaeva1990, Figure 8-2); Pantopipetta capensis (Barnard, 1946) in Stock (Reference Stock1963, Figure 8a); Pantopipetta gracilis Turpaeva, Reference Turpaeva1993 (Turpaeva, Reference Turpaeva1993, Figure 4-1); P. oculata (Stock, Reference Stock1968, Figure 7b); Pantopipetta weberi (Loman, 1904) in Loman (Reference Loman1908, Figure 14-194 and 14-197) and P. lenis (Hosoda and Kakui, Reference Hosoda and Kakui2020, Figure 3B, C). All of these have a palp bearing four short distal articles. A short basal article has so far not been illustrated in the descriptions of Pantopipetta species that have a palp with three short distal articles (Pantopipetta armata Arnaud & Child, 1988; Pantopipetta armoricana Stock, 1978; Pantopipetta bilobata Arnaud & Child, 1988; Pantopipetta clavata Stock, 1994 and P. hosodai sp. nov.). This suggests that Pantopipetta species comprises two species groups: (i) species with an eight-articulate palp having a short article distal to the lateral process of the cephalon and four short distal articles and (ii) species with a six-articulate palp lacking a short article distal to the process and having three short distal articles. To confirm this hypothesis, the connection between the lateral process of the cephalon and the palp needs to be reexamined in known species.
Cave-dwelling pycnogonids
At least 15 pycnogonid species have been recorded from marine or anchialine caves in the Mediterranean (e.g. Gerovasileiou and Bianchi, Reference Gerovasileiou and Bianchi2021), Caribbean (Alvarez and Ojeda, Reference Alvarez and Ojeda2018) or northwestern Pacific (Bamber, Reference Bamber2008; this study). Among these species, only three were identified to the species level, each collected from a different environment. Pycnogonum coninsulum inhabited a submarine cave at 33‰ salinity, with no information on light provided (Bamber, Reference Bamber2008; Morton et al., Reference Morton, Bamber and Robbins2008). Anoplodactylus batangensis came from among vegetation in the illuminated anchialine pool of a cenote (1.63 salinity; Alvarez and Ojeda, Reference Alvarez and Ojeda2018). Pantopipetta hosodai sp. nov. came from a completely dark, anchialine environment inside a submarine cave (less than 28‰ salinity; cf. Osawa and Fujita, Reference Osawa and Fujita2019).
It is unknown whether pycnogonids inhabiting caves are troglobites (obligate cave-dwelling species). Two species, Py. coninsulum and P. hosodai sp. nov., were reported based on a single individual each from two different caves, and it is not known whether they also occur outside the caves. Faunal surveys have been conducted intermittently in Akumanoyakata Cave across a span of 10 years but our P. hosodai sp. nov. specimen is the first pycnogonid found. This suggests a very low abundance of pycnogonids in caves, or at least in Akumanoyakata Cave. More comprehensive sampling from both inside and outside caves is needed to ascertain the degree of their dependence on caves.
Acknowledgements
We thank Masaru Mizuyama (Meio University), Kyo Yunokawa (Ie-jima Diving Service), Atsuo Shioiri (Diving Service ‘COLORCODE’), Hiroki Ichi (Irabu-jima Fishermen's Association), Hirotaka Ichi, and Go Tomitani (Diving Service ‘Marines Pro Miyako’), Naoki Shirakawa (Diving Service ‘DOLPHIN KICK’) for assistance in scuba sampling in the submarine cave. We also thank Matthew H. Dick (Hokkaido University) for reviewing the manuscript and editing the English.
Author's contribution
KK conceived and designed the study, and made morphological observations; YF collected the pycnogonid; KK and YF wrote the manuscript, and read and approved the final draft.
Financial support
This study was supported in part by a KAKENHI grant (number JP20H03313) from the Japan Society for the Promotion of Science (JSPS).
Conflict of interest
The authors declare no conflict of interests.





