Introduction
Currently, the genus Echinorhinus includes two living species: the bramble shark Echinorhinus brucus (Bonnaterre, 1788) and the prickly shark E. cookei Pietschmann, 1928 (Bernardi and Powers, Reference Bernardi and Powers1992; Compagno et al., Reference Compagno, Dando and Fowler2005; Ebert et al., Reference Ebert, Dando and Fowler2021; Fricke et al., Reference Fricke, Eschmeyer and Van der Laan2022). The genus shows circumglobal distribution from cold-temperate to tropical seas occurring in continental and insular shelves and slopes near the bottom from 4 to 1214 m (Nelson et al., Reference Nelson, Grande and Wilson2016; Ebert et al., Reference Ebert, Dando and Fowler2021). The scant sighting and catching records show that both species co-occur in Australia, New Zealand and Japan (Taniuchi and Yanagisawa, Reference Taniuchi and Yanagisawa1983). However, E. cookei has been mostly reported along the eastern Pacific and Hawaii (Crow et al., Reference Crow, Lowe and Wetherbee1996; Long et al., Reference Long, McCosker, Blum and Klapfer2011; Calle-Morán and Béarez, Reference Calle-Morán and Béarez2020), whereas E. brucus is majorly reported in the Western (North Carolina and Gulf of Mexico), Caribbean Sea (Venezuela), South America (Brazil, Argentina, Colombia), and Eastern Atlantic (Europe, Africa), Mediterranean, both coasts of India and Oman (Barcellos and Pinedo, Reference Barcellos and Pinedo1980; Schwartz, Reference Schwartz1993; Caille and Olsen, Reference Caille and Olsen2000; Javadzadeh et al., Reference Javadzadeh, Vosoughi, Fatemi, Abdoli and Valinassab2011; Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014; Anguila et al., Reference Anguila, Nieto-Alvarado and Hernández-Beracasa2016; Ray and Mohapatra, Reference Ray and Mohapatra2020; Ebert et al., Reference Ebert, Dando and Fowler2021). The occurrence of a new species in the genus Echinorhinus aside from E. brucus distributed in the western Indian Ocean has been hypothesized based on the genetic distances of the NADH dehydrogenase subunit 2 (NADH2) (Henderson et al., Reference Henderson, Reeve, Jabado and Naylor2016). This work deals with a new geographic record, morphological and mitochondrial characterization of the bramble shark Echinorhinus cf. E. brucus in the Oman Sea.
Material and methods
One specimen of the bramble shark Echinorhinus cf. E. brucus was incidentally caught during the shark fishing season in January 2021 at Bandar Al Khairan, Muscat, Oman. The specimen was caught with a long line approximately at 80 m depth. The fisherman donated the dead shark to the authors (after arrival from the landing site to the fish market). Then, the shark was transported in a cool box to the facilities at the Fishery Quality Control Center (FQCC) in Muscat, Oman. The taxonomic identity to genus level of the specimen was corroborated following Compagno and Niem (Reference Compagno, Niem, Carpenter and Niem1998). Then, 52 morphometric and meristic characters were recorded (in centimeters, cm) and compared with previous published data (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014) (Table 1).
Table 1. Morphometry (cm) and its proportions (%) of Echinorhinus from Venezuela and Oman

Muscle tissue of the shark was biopsied and used for further DNA barcoding characterization. The genomic DNA was extracted with the phenol-chloroform isoamyl technique (Green and Sambrook, Reference Green and Sambrook2012). A fragment of cytochrome oxidase I (COI) was amplified through polymerase chain reaction (PCR) using the primer set FishF1 5′-TCA ACC AAC CAC AAA GAC ATT GGC AC-3′ and FishR1 5′-TAG ACT TCT GGG TGG CCA AAG AAT CA-3′ (Ward et al., Reference Ward, Zemlak, Innes, Last and Hebert2005). Each PCR reaction was performed with the PuReTaq Ready-To-Go (RTG) PCR Beads (GE Healthcare) in a 25 μl total final volume consisting of 22 μl of ultrapure water, 0.5 μl (10 μM) of each primer and 2 μl of gDNA template. Cycling conditions consisted of an initial step of 15 min at 95°C followed by 35 cycles of denaturation 60 s at 94°C, annealing 60 s at 60°C, and extension 120 s at 72°C followed by a final extension of 10 min at 72°C. The PCR assay was carried out using a thermal cycler Prolex PCR system (applied Biosystem). Both gDNA and PCR products were visualized in 1% agarose gel stained with ethidium bromide and documented with a Chemi XRS Gel Documentation System (Nu Genius). Both strands of the PCR products were sequenced. The ExPASY translate tool (proteomic server) (http://web.expasy.org/traslate/) was run using nucleotide sequences COI gene fragment to get amino acid sequences and open reading frame (ORF). Both nucleotide and amino acid sequences were further subjected to NCBI-BLASTX tool (http://blast.ncbi.nlm.nih.gov.Blast.cgi) for matching sequences and identity of the specimen. Then, the partial sequence of COI gene obtained (642 bp) was submitted to the NCBI database under Accession No. OP476452. The evolutionary relationship of the taxa was inferred using the neighbor-joining method and the optimal tree with the sum of branch length = 0.31842936 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) and only values ⩾70% are shown in the branches. The evolutionary distances were computed using the Tamura–Nei method and are in the units of the number of base substitutions per site. The variation rate among sites was modeled with a gamma distribution (shape parameter = 1). The analysis involved 11 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 606 positions in the final dataset. Evolutionary analyses were conducted in Molecular Evolutionary Genetics Analysis version 11 (MEGA-11 software) (Tamura et al., Reference Tamura, Stecher and Kumar2021). The p-distance values were compared between species.
Results
Description
The young male specimen showed cylindrical body, dorsal body surface dark purplish-gray to brown, ventral surface slightly paler; sides with scanty black spots disperse. Head moderately flattened; five pairs of gill slits. Lateral line running along upper half of body originating above the fourth gill opening and posteriorly passing through upper third of caudal peduncle curves upwards and runs to tip of upper caudal lobe. Snout short (length as mouth width, body proportion = 11%) and blunt, stout body, spiracles small (Figure 1A–C). Labial furrows very short, teeth wider than high (ratio = 1:0.44), multicuspid (20 in the upper jaw), on both jaws alike with a central oblique bladelike cusp with up to two very small cusplets on its side (Figure 1D). Darker fin margins, two small spineless dorsal fins. The second dorsal a little smaller than first one (ratio = 0.93:1), close together, the interspace between the first and second dorsal varying from about as long as base of first dorsal (Table 1). Allocated at posterior part of the body and originate behind pelvic fin origin. The posterior base of the first dorsal fin nearly aligned with the posterior base of the pelvic fin. The anterior base of the second dorsal fin overlaps with the end tips of the pelvic fin (and the claspers slightly overpass it) (Figure 1A and B). Pectoral fins short and angular. Anal fin and subterminal notch on caudal fin absent. Denticles sparse on the whole body surface (in dorsal and ventral side), yet irregularly distributed (Figure 1A–E), thorn-like shape (cusps) with smooth basal margins rather fine ridging radiate (not stellate). The cusps are angulated, centered or slightly displaced from the basal margin. Some bases (2–3) fused into compound plates giving a circular oval shape; size varied up to 12 mm in basal diameter (Figure 1E).
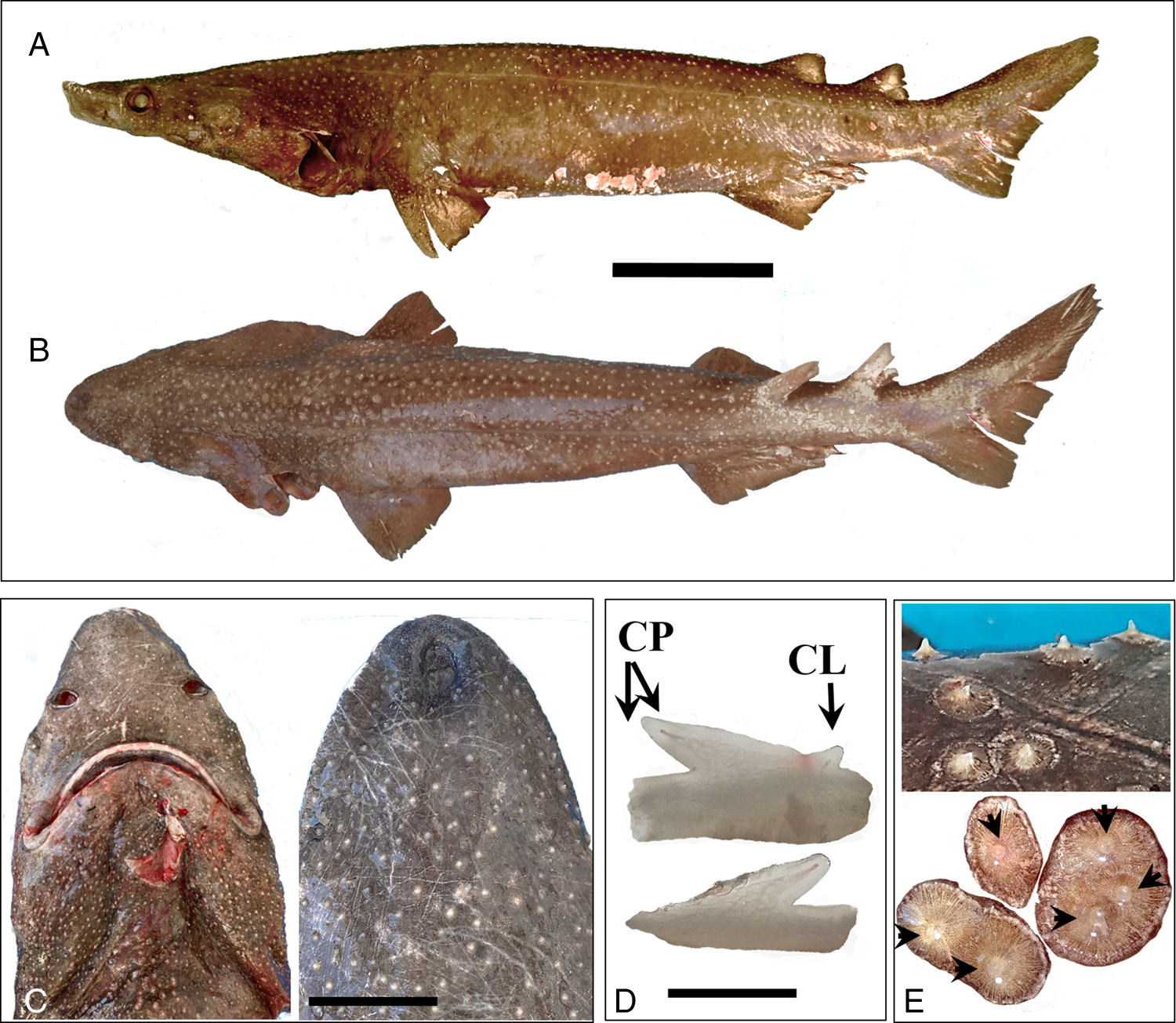
Figure. 1. Echinorhinus cf. E. brucus (fresh specimen). A, lateral view, B, dorsal view. C-D, ventral and dorsal view of the head. D, teeth shapes. E, modified scales 1–3 general view and fused dermal denticles. Cusp (CP) (CL) (A-B: 20 cm; C: 10 cm; D: 1 cm).
Morphometry
The morphometric data of the Echinorhinus specimen from Bandar (this work) and Salalah, Oman showed to be distinct to the specimen E. brucus from Venezuela. Comparison of the morphometric characters (in proportions) showed differences ranging from 0.1 to 9.1% between the specimens of Bandar and Venezuela (Table 1, Figure 2). The proportion values of 12 morphometric characters showed conspicuous differences ⩾ 3%. The more remarkable were the pre-caudal length, head length, pre-pelvic length, orbital-third gill slit space and pelvic posterior margin length showed the highest average proportion difference (Figure 2).

Figure. 2. Morphometric comparison (proportions) between Echinorhinus cf. E. brucus from Oman (Bandar Al Khairan and off Dhalkut, Oman) (▲) and E. brucus from Venezuela (□).
Molecular identification
The COI gene sequence of Echinorhinus cf. E. brucus from Oman represents a new haplotype and it is the third genetic identity known for the NW Indian Ocean. The phylogenetic relationships within the genus Echinorhinus showed three main clades. The Echinorhinus specimen from Oman was placed in a well-supported clade (bootstrap = 100) together with homologous sequences of the morphologically undescribed specimens from India. This group is sister to the specimen E. brucus from Venezuela, which formed its own clade though their relatedness was weakly supported (Bootstrap <70). The species E. cookei form an own well-resolved separated clade, and it is shown as the more distant relative (Figure 3).

Figure. 3. Neighbor-joining tree for 606 base pairs fragment of the COI gene for Echinorhinus cf. E. brucus from the present study (*) together with E. brucus and E. cookei.
Genetic distance
The genetic distance values (p-distance) at intraspecies level ranged between 0 and 0.05. Echinorhinus cf. E. brucus (i.e., Oman and India) showed very low average p-distance value = 0.004. A similar result was observed among E. cookei (average p-distance value = 0.005). Remarkably, the average genetic distance raised up to 0.017 when the specimen from Venezuela was included within the Echinorhinus cf. E. brucus group, which includes specimens from Oman and India (Table 2). Likewise, the p-distance values at interspecies level between E. cookei, Echinorhinus cf. E. brucus (Oman and India) and the specimen from Venezuela were very high ranging from 0.041 to 0.043, respectively (Table 2).
Table 2. Genetic distance based on COI gene fragment (p-distance values) in the genus Echinorhinus

The pairwise nucleotide differences at intraspecies level ranged from 0 to 5, whereas at the interspecies level, the range was remarkably high 22–27 pairwise differences. Particularly, the pairwise differences of the specimen from Venezuela varied from 22 to 23 nucleotides regarding E. cookei and Echinorhinus cf. E. brucus (Table 2).
Discussion
The identity of the species in the genus Echinorhinus is controversial and has been under debate.
The South African representatives of the genus, as well as the Australian-New Zealand and Hawaii received separate names as supposedly distinct from the species E. brucus of the North Atlantic (Bigelow and Schroeder, Reference Bigelow, Schroeder, Tee–Van, Breder, Hildebrand, Parr and Schroeder1948). So far Squalus brucus Bonnaterre, 1788, Squalus spinosus Gmelin, 1789, E. spinosus (Gmelin, 1789), E. obesus Smith, 1838 and E. mccoyi Whitley, 1931 are considered synonyms of E. brucus. Also, Echinorhinus brucus and E. cookei Pietschmann, 1928, were erratically classified as synonyms (Fowler, Reference Fowler1941; Bigelow and Schroeder, Reference Bigelow, Schroeder, Tee–Van, Breder, Hildebrand, Parr and Schroeder1948; Ramachandran et al., Reference Ramachandran, Ayoob and Koya2014).
Nowadays, E. brucus and E. cookei can be clearly distinguished from one another because the former presents spine-like non stellate dermal denticles, single or fused in plates with multiple cusps relatively big (⩾15 mm in diameter) spread on the body. Whereas E. cookei is uniformly covered with numerous small denticles (4–5 mm in diameter), stellate and not fused i.e. not forming plates with multiple cusps (Garrick, Reference Garrick1960; Compagno and Niem, Reference Compagno, Niem, Carpenter and Niem1998; Compagno et al., Reference Compagno, Dando and Fowler2005). Garrick (Reference Garrick1960) showed that juveniles (44–47 cm) and adults (198 cm) of E. cookei exhibit denticles consistently small and stellated.
The bramble shark, E. brucus is a poorly documented species known mainly from European Atlantic and Mediterranean waters. It has been reported occasionally throughout the Atlantic but appears very rare on the western seaboard (Iglésias and Mollen, Reference Iglésias and Mollen2020). Despite that the bramble shark has scarcely been reported in Omani waters, the species has not been confirmed. The first record of E. brucus occurred off Oman (Henderson et al., Reference Henderson, McIlwain, Al-Oufi and Al-Sheili2007). Then, an adult female of E. brucus was recorded off Dhalkut, southern Oman, Arabian Sea (Al-Shajibi et al., Reference Al-Shajibi, Chesalin and Al-Shagaa2014). Henderson et al. (Reference Henderson, Reeve, Jabado and Naylor2016) made the most recent records of these sharks in Oman, which was referred to as Echinorhinus sp. due to a divergent sequence of the NADH2 marker regarding specimens from Australia and the western Atlantic. The phylogenetic tree based on NADH2 marker shows that E. brucus, E. cookei and the specimens from Oman and Sri Lanka are distinct species. Thus, it was hypothesized that the specimens from Oman belong to an undescribed species (Henderson et al., Reference Henderson, Reeve, Jabado and Naylor2016; Fernando et al., Reference Fernando, Bown, Tanna, Gobiraj, Ralicki, Jockusch, Hebert, Jensen and Caira2019).
In the present work, the young adult shark from Oman exhibited single and fused denticles dispersed on the body, which formed plates with up to three multiple cusps fused (⩾10 mm). Such characteristics, as well as the general morphology of the body, is consistent with the diagnosis of the species E. brucus. Thus, Oman’ specimen can be distinguished from E. cookei based on the presence of fused denticles (which are not a feature for the latter) (Garrick, Reference Garrick1960). Remarkably, the specimen caught in Venezuela (reported as E. brucus) showed small dermal denticles (<5 mm) with intermediate morphology resembling both congeneric species i.e. fused denticles as in E. brucus, but stellated bases as in E. cookei (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014). In this context, the specimen from Venezuela could not be clearly assigned morphologically to E. brucus or E. cookei (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014). Comparatively, the dermal denticles of our specimen exhibit thinner and numerous trabecular fibers on their bases. However, such a characteristic is congruent with the diagnosis of E. brucus and consequently discriminates our specimen from both E. cookei and the specimen from Venezuela (referred as E. brucus) (Garrick, Reference Garrick1960; Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014). Likewise, male specimens of E. brucus occurring in India show morphological differences regarding the arrangement of denticles, the origin of lateral line and teeth (Silas et al., Reference Silas, Selvaraj and Regunathan1969; Nair and Lal Mohan, Reference Nair and Lal Mohan1971; Silas and Selvaraj, Reference Silas and Selvaraj1972). The specimen described in the present study is very similar to that one described by Nair and Lal Mohan (Reference Nair and Lal Mohan1971). In this regard, Iglésias and Mollen (Reference Iglésias and Mollen2020) inferred that the holotype of E. brucus, of the collections of the Muséum national d'Histoire naturelle in Paris (MNHN's), was destroyed, lost or preserved fragmented (skin, teeth or denticles). Thus, the holotype of the species is mentioned lost in all modern references (e.g. Iglésias and Mollen, Reference Iglésias and Mollen2020; Fricke et al., Reference Fricke, Eschmeyer and Van der Laan2022). Currently, the MNHN's ichthyology collections only include two stuffed whole specimens of moderate size and from the Mediterranean, an embryo as well as several fragments (skins, jaws, teeth, dermal loops, skeletal parts) often without associated information and none of which is likely to be reassigned to the lost holotype (Iglésias and Mollen, Reference Iglésias and Mollen2020). Independently, morphological comparison carried out within specimens referred to as E. brucus in this work (i.e., specimens from Venezuela and Oman), confirms the existence of a third living species in the genus, but also exhibits the need to reassign to the lost holotype of E. brucus.
Regarding the genetic diversity of the genus Echinorhinus, very little is known partly due to the fact that most specimens recorded worldwide lack genetic characterization. Paradoxically, the scarce public data of Echinorhinus deposited in both the Barcode of Life Data Systems (BOLD) and GenBank lack morphological descriptions. The mitochondrial COI and NADH2 regions are relatively more used to characterize these sharks and are essential to provide new information regarding the relatedness of shark populations of the genus Echinorhinus. In the present work, the new COI haplotype of our specimen reveals its conspecificity with those occurring in Western India. Likewise, the overall phylogenetic relationships at both the interspecific and intraspecific levels concurred with previous findings based on the COI gene fragment (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014). Indeed, the general topology of the tree showing three main clades is congruent with previous findings based on the NADH2 region. Besides, the genetic distance values at intraspecies and interspecies levels were congruent with those obtained with the COI and NADH2 (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014; Henderson et al., Reference Henderson, Reeve, Jabado and Naylor2016; Fernando et al., Reference Fernando, Bown, Tanna, Gobiraj, Ralicki, Jockusch, Hebert, Jensen and Caira2019). Thus, the hypothesis of the occurrence of a third species in the genus Echinorhinus is confirmed. Nevertheless, some considerations must be taken into account: 1) the specimen identified as E. brucus from Venezuela (Fariña et al., Reference Fariña, Quinteiro and Rey-Méndez2014) and North Carolina (USA) are conspecific (COI genetic identity = 99.68%, see his figure 3); 2) by integrating and comparing the morphology and genetics of the adult female from Venezuela with our specimen, it is observed that they are clearly different species, though not necessarily new; and 3) the interspecies relationships obtained with the COI and NADH2 regions need to be clarified. Specifically, in the analysis with the COI fragment E. cookei (i.e. specimens from Hawaii and Australia) is shown as the more distant taxon of the clade formed by Echinorhinus cf. E. brucus and E. brucus, whereas, with the NADH2, it is relocated as the more closely related to the ‘undescribed species’ (see Henderson et al Reference Henderson, Reeve, Jabado and Naylor2016; Fernando et al., Reference Fernando, Bown, Tanna, Gobiraj, Ralicki, Jockusch, Hebert, Jensen and Caira2019).
The findings regarding the genetic analysis with COI show that the morphological characteristics used to discriminate Echinorhinus spp. are very limited. Further contribution is required to contrast the relevance of other morphological characters of Echnorhinus cf. E. brucus examining previous descriptions in the literature in order to clearly diagnose Echinorhinus brucus (sensu stricto) or to resurrect previous synonymized names accordingly.
Acknowledgements
The authors express special thanks to Mr. Abdullah Said Al-Kindi for providing us the sample investigated, and the administration staff of the FQCC for facilitating the analyses conducted in the present work. We are grateful to the Ministry of Agriculture Fisheries and Water Resources (MAFWR), Sultanate of Oman and the Agriculture and Fisheries Development for the financial grant to the project number 71/3/1 ‘Investigations on aquatic parasites related to sea food safety and quality’. R.J.S.-M. acknowledges the Commission for the Operation and Promotion of Academic Activities (COFAA).
Author contributions
J.R.M.-A. and S-A.J. collected the data and were major contributors in writing the manuscript. N.A.-P. and R.J.S.-M. read and approved the final manuscript.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
Not applicable.
Data
All data generated or analyzed during this study are included in this published article.







