Historical background
The relationship between breath composition and health has been known for many centuries. More than 2500 years ago, the Greek physician, Hippocrates of Cos noted the importance of breath smell in the diagnosis of liver disease, using the term ‘foetor hepaticus’ to describe the characteristic breath odour associated with liver failure (Treatise on Breath Odour and Disease, 5th century BC).
The ancient Persian physician and philosopher, Ibn Sina (Avicenna) wrote that ‘...it is the role of the vital force (breath) to maintain a perfect equilibrium within the elements of the body, and between the elements of the body and the environment’ (The Canon of Medicine, 10th century). An important environmental determinant of breath composition is diet. Approximately 40 years ago, Pauling et al. ( Reference Pauling, Robinson and Teranish 1 ) investigated the relationship between breath composition and diet and recognised the potential impact of intestinal flora as a contributing factor to breath composition. Individuals were placed on a defined elemental diet, consisting almost entirely of small molecules that the authors assumed would be absorbed from the upper gastrointestinal tract, and that intestinal flora would be reduced in the lower gastrointestinal tract because of the lack of nutrients reaching them. Using temperature-programmed gas–liquid partition chromatography, the quantitative determination of about 250 substances in a sample of human breath was possible at that time.
Introduction
Today, using exquisitely sensitive analytical techniques, more than 500 compounds have been reproducibly identified in exhaled breath( Reference Risby and Solga 2 ), though as many as 3000 different compounds have been sporadically detected in breath of different individuals( Reference Phillips 3, Reference Phillips, Herrera and Krishnan 4 ). It is now possible to measure volatile organic compounds (VOC) in breath with great sensitivity (down to parts per billion by volume; ppbv) and specificity, using MS and related analytical methods. As a consequence, breath analysis now has a number of well-established clinical applications( Reference Španěl and Smith 5 ) (Table 1). It also has enormous potential value in metabolic research, particularly when combined with stable isotope labelling. It has, for example, been used in kinetic studies of amino acid metabolism( Reference Teraishi, Ozeki and Hori 6 ). Breath analysis may also be used for applications that would otherwise be difficult using other techniques, for example in the assessment of whole-body oxidative stress( Reference Louhelainen, Myllarniemi and Rahman 7 ), or cholesterol biosynthesis( Reference Salerno-Kennedy and Cashman 8 ). Breath analysis may also be a useful adjunct to blood and faecal analysis in the investigation of gut microbiota( Reference Hamer, De Preter and Windey 9 ). The present review briefly outlines the physiological and dietary factors that may have an important impact on breath compounds and the methods used for assessing them, together with the reported concentrations of these compounds in health and disease.
Table 1. Established and emerging clinical applications of breath analysis

Sources of volatile metabolites in exhaled breath
Volatile metabolites in exhaled breath are derived from several sources: they may be derived from the environmental inspired air, from cells, including micro-organisms that are located throughout the oral/nasal cavities and the pulmonary system, the upper and lower gastrointestinal tracts and from general human metabolism (Fig. 1)( Reference Popov 10– Reference Zhang and Li 12 ). For example, NO is present in trace amounts in atmospheric air and is therefore present in the inspired air; it is also an important biological mediator in the vasculature( Reference Singh and Evans 13 ), being derived from the action of NO synthase on the amino acid arginine, and is elaborated at high concentrations by activated inflammatory cells( Reference Weinberg 14 ). Its concentrations in expired air can therefore be considerably higher than in inspired air, and may be derived from several sources.

Fig. 1. The complex interactions between diet and expired breath metabolites.
Physiological variations in breath composition
Site of exhaled breath sampling
The measured concentrations of several exhaled breath constituents differ significantly depending on their site of breath sampling; whether from the mouth, nose, or the static oral cavity. Some of these compounds, for example NH3, ethanol and hydrogen cyanide, are predominantly generated in the oral cavity in healthy subjects( Reference Wang, Pysanenko and Dryahina 15, Reference Smith, Wang and Pysanenko 16 ). Hence oral health, including periodontal and dental disease, are potential confounding factors( Reference van den Broek, Feenstra and de Baat 17 ). It has been shown that concentrations of some compounds in the exhaled breath, for example NH3 and ethanol, can be increased by sugar and urea mouth washes( Reference Španěl, Turner and Wang 18 ). Hence, without careful preparation, mouth production of these and other compounds can compromise the quantification of endogenous trace compounds present in the alveolar breath. However, the concentrations of both the urea and sucrose solutions used in these latter studies that proved the enhancement of NH3 and ethanol levels were greater than normally present in food and beverages; thus in most situations such severe enhancements will not occur( Reference Španěl, Turner and Wang 18 ). It is also possible to simultaneously monitor mouth and nasal concentrations of breath compounds to elucidate their source( Reference Hodgson, Linforth and Taylor 19 ). Furthermore, it is possible to sample end-tidal gas only( Reference Herbig, Titzmann and Beauchamp 20 ) by physically filtering out gas from the oral cavity (for example, by using buffered end-tidal sampling), or by data processing post hoc ( Reference Herbig, Titzmann and Beauchamp 20, Reference Birken, Schubert and Miekisch 21 ).
Determinants of inter-individual and intra-individual variation
Intra-individual studies that have been carried out over about 30 d have revealed the temporal variations in the concentrations of several common breath metabolites for several individuals, including: NH3, acetone, isoprene, ethanol and acetaldehyde( Reference Smith, Turner and Španěl 11, Reference Diskin, Španěl and Smith 22 ). Breath NH3, acetone and isoprene concentrations were reported to have CV of typically 0·3 over this period. No obvious correlations were found in the distributions of these particular metabolites, except that the NH3 levels were greatest in the breath of the oldest subjects( Reference Diskin, Španěl and Smith 22 ). In population (inter-individual) studies over a longer time-frame (6 months), breath methanol levels appeared to have a log-normal distribution for the study population, and did not correlate with age, breath ethanol or ethanol consumed in the previous 24 h; however, there was an inverse correlation with BMI( Reference Turner, Španěl and Smith 23 ). Breath NH3 increased with age, and a weak but significant correlation between breath propanol and acetone levels was reported( Reference Turner, Španěl and Smith 24 ). Breath isoprene concentrations have been studied in healthy schoolchildren between 7 and 18 years of age, and in this group there was a strong positive association with age( Reference Smith, Španěl and Enderby 25 ), possibly related to growth, or steroid hormone biosynthesis.
Fasting and the acute effects of feeding on breath content
Effects of the fasted or fed state on breath constituents are complex. Breath acetone, NH3, ethanol, isoprene and methanol have been measured during single exhalations whilst fasting and following feeding with a liquid protein-energy meal( Reference Smith, Španěl and Davies 26 ). Breath acetone concentrations fell from a maximum during fasting, reaching their nadir between 4 and 5 h after feeding. Changes in breath NH3 concentrations were biphasic, possibly related to changes in portal blood flow, with a rapid fall to approximately 50 % of their fasting levels before rising to two or three times their baseline values at 5 h( Reference Smith, Španěl and Davies 26 ). A brief increase in breath ethanol concentrations was found after feeding, and this is probably related to the ethanol content of some foods. Subsequently, breath ethanol levels remained low throughout the experimental period. Isoprene concentrations did not change significantly( Reference Smith, Španěl and Davies 26 ). Levels of breath ethanol increased if a sweet drink or food had been consumed within 2 h before providing a breath sample, but surprisingly no increase in breath ethanol was apparent when modest alcohol consumption had occurred the previous evening. Endogenous breath ethanol and acetaldehyde levels were not significantly correlated with each other( Reference Turner, Španěl and Smith 27 ). It has recently been reported that breath hydrogen cyanide may rise following the consumption of food or drink( Reference Schmidt, Metsala and Vaittinen 28 ).
Effect of exercise and the breath cycle on breath content
Alveolar breath isoprene and methyl acetate have been reported to increase immediately after moderate exercise, returning to baseline soon thereafter( Reference King, Mochalski and Kupferthaler 29, Reference Turner, Španěl and Smith 30 ). We have recently reported that breath isoprene concentrations rise rapidly after commencing exercise, and then decrease during the period of exercise. Plasma cholesterol levels were not obviously correlated with isoprene concentration in breath. Also, isoprene levels were not found to be directly related to sex, age or BMI in this study of adults( Reference Turner, Španěl and Smith 30 ). The changes in breath isoprene during exercise have been attributed to changes in tissue fractional perfusion( Reference King, Koc and Unterkofler 31 ), and the changes in expiratory breath NO observed during exercise have been reported to be due to changes in air flow rate, rather than increased NO production( Reference St Croix, Wetter and Pegelow 32 ). The exercise-related changes in NH3 concentrations in breath exhaled via the nose appear to vary with age, with a several-fold increase in concentrations persisting into the post-exercise period( Reference King, Koc and Unterkofler 31 ). These changes may be dependent on renal function. Breath composition changes during the breathing cycle. For example, the variation observed in breath acetone appears to be dependent on exhaled volume, but not flow( Reference Dummer, Storer and Hu 33 ).
Molecules directly or indirectly derived from food, beverages and medicines
Following the ingestion of some compounds, there are wide inter-individual variations in their appearance in the breath. For example, following the ingestion of eucalyptol, a constituent of proprietary medications, its appearance in breath varies between 1 and 5 h after ingestion, showing wide inter-subject variations too( Reference Beauchamp, Kirsch and Buettner 34 ). Green tea was very effective in reducing volatile sulfur compounds (hydrogen sulfide and methyl sulfide) in mouth breath, this being attributed to its disinfectant properties( Reference Lodhia, Yaegaki and Khakbaznejad 35 ). The kinetics of the acute release of aromas from food or beverages is complex, being dependent on the physiological processes involved in swallowing, the lipid content of the food( Reference Hodgson, Langridge and Linforth 36 ) and the vapour pressure of the compound( Reference Buffo, Rapp and Krick 37 ). There are a number of volatile compounds in food that may rapidly appear in the breath following their consumption( Reference Cheng 38 – Reference Varlet and Fernandez 40 ).
Breath alkanes, smoking, other causes of oxidant stress and dietary antioxidants
Smoking is known to induce a state of oxidative stress that is associated with lipid peroxidation( Reference Lowe and Cemeli 41, Reference Comandini, Marzano and Curradi 42 ) and has been shown to be associated with substantial changes in breath composition( Reference Filipiak, Ruzsanyi and Mochalski 43 ). Oxidative stress has the potential to damage cells, tissues and organs via the production of reactive oxygen species such as superoxide, H2O2 and the hydroxyl radical( Reference Yao, Rahman, Basu and Wiklund 44 ). Oxidative stress may be estimated through breath measurements of biomarkers that include ethane, ethylene and pentane( Reference Aghdassi and Allard 45– Reference Andreoni, Kazui and Cameron 52 ). Although these hydrocarbons only represent a small and possibly variable proportion of the total amount of peroxidised PUFA, their determination in exhaled breath enables an assessment of oxidative stress in vivo ( Reference Kneepkens, Lepage and Roy 53 ). Do et al. ( Reference Do, Garewal and Clements 54 ) have previously reported that non-smokers have very low baseline levels of ethane, whilst ethane production correlated with active (packs per d) and lifelong (pack-years) tobacco consumption. Miller et al. ( Reference Miller III, Appel and Jiang 55 ) have reported similar findings and also report that breath ethane concentrations are related to the time interval between the last cigarette smoked and breath sampling. Do et al. ( Reference Do, Garewal and Clements 54 ) have also shown that antioxidant vitamin supplementation resulted in attenuation of smoking-related lipid peroxidation with a significant decrease in breath ethane production. Aghdassi & Allard( Reference Aghdassi and Allard 45 ) assessed oxidative stress using breath alkane output and other markers of lipid peroxidation in several conditions associated with inflammation, including smoking. Lipid peroxidation was significantly higher and antioxidant vitamin status significantly lower in smokers compared with non-smokers. β-Carotene or vitamin E supplementation significantly reduced lipid peroxidation, whilst vitamin C supplementation had no significant effect. These findings are consistent with those of Hoshino et al. ( Reference Hoshino, Shariff and Vangossum 56 ) and Allard et al. ( Reference Allard, Royall and Kurian 57 ).
In an animal model of vitamin E deficiency, the increased peroxidation of tissue lipids leads to an increased level of breath pentane( Reference Gelmont, Stein and Mead 58 ). However, in their paper, Gelmont et al. ( Reference Gelmont, Stein and Mead 58 ) reported that pentane production was also dependent on dietary linoleate. Breath pentane in the study animals was reduced by removal of linoleate from their diet, by starvation, antibiotic treatment or the addition of vitamin C to their food or water. Breath pentane was increased by the removal of vitamin E from the diet. The authors concluded that intestinal bacteria were a major source of breath pentane in addition to endogenous membrane lipid peroxidation( Reference Gelmont, Stein and Mead 58 ). In recent studies we have found, using selected ion flow tube (SIFT)-MS, that breath pentane is elevated in patients with inflammatory bowel disease such as ulcerative colitis( Reference Hrdlicka, Dryahina and Spanel 59 ).
The effects of a restricted-energy diet have also been investigated in the rat model( Reference Fritz, Kasper and Siebert 60 ). A significant decrease in ethane generation was found in the rats receiving an energy-restricted diet compared with those fed ad libitum, supporting the hypothesis that energy restriction reduces the level of oxidative stress( Reference Sohal, Ku and Agarwal 61 ).
Breath pentane is derived from the oxidation of n-3 and n-6 fatty acids which appear to be transferred from mother to fetus during pregnancy( Reference Schwarz, Cox and Sharma 62 ). However, in women in their last trimester, who have smoked during pregnancy, it has been reported that breath ethane was higher than for a control group of non-smokers, and inversely related to serum vitamin C( Reference Schwarz, Cox and Sharma 63 ). Dietary n-3 fatty acid supplementation also appeared to increase lipid peroxidation as assessed by breath alkane output, and this was not prevented by co-administration of vitamin E( Reference Allard, Kurian and Aghdassi 64 ).
Dietary studies
The effects of dietary constituents on breath composition are complex, as alluded to in Fig. 2. The acute effects of diet on breath have been described briefly above. Medium- and longer-term effects may be mediated by changes of flora in the gastrointestinal tract( Reference Fooks and Gibson 65, Reference Steer, Carpenter and Tuohy 66 ) and direct or indirect effects on gastro-caecal transit time( Reference Musso, Gambino and Cassader 67, Reference Van Citters and Lin 68 ), together with effects on metabolism, systemic inflammation( Reference Kontogiorgis, Bompou and Ntella 69– Reference Garcia-Lafuente, Guillamon and Villares 71 ) and redox state( Reference Kowaltowski 72, Reference Bouayed and Bohn 73 ).

Fig. 2. Dietary and metabolic sources of the major metabolites in human breath. GI, gastrointestinal; F1P, fructose 1-phosphate; G6P, glucose 6-phosphate; G1P, glucose 1-phosphate; LCFA, long-chain fatty acids; BCFA, branched-chain fatty acids; HMG, hydroxy methyl glutaryl; carbomyl P, carbomyl phosphate. The grey boxes represent compounds that have been identified in breath.
Effects of macronutrients and dietary energy restriction
High- and low-fat diets
Rosenkranz et al. ( Reference Rosenkranz, Townsend and Steffens 74 ) have investigated the acute effects of a high-fat meal on pulmonary function and expiratory NO. They found that a high-fat meal was associated with increased expiratory NO, but had no effect on a systemic marker of inflammation, or pulmonary function in normal individuals, and the authors concluded that a high-fat diet may contribute to inflammation within the airway. Studies in patients with asthma have found that a diet containing a high n-6:n-3 fatty acid ratio was associated with worsening of asthma control and higher concentrations of NO in exhaled breath( Reference Barros, Moreira and Fonseca 75 ).
Ketogenic diets are high in fat, low in carbohydrate and contain adequate levels of protein. Under these conditions fat is metabolised in preference to carbohydrates, and ketone bodies (acetone, acetoacetate and β-hydroxybutyrate) are generated in the liver, leading to ketosis( Reference Kalapos 76 ). In these circumstances, expiratory breath acetone concentrations are increased substantially. Even in healthy subjects, breath acetone has been reported to rise more than five-fold following a ketogenic diet( Reference Musa-Veloso, Likhodii and Cunnane 77, Reference Španěl, Dryahina and Rejskova 78 ). Breath acetone appears to be indicative of systemic ketosis associated with a ketogenic diet( Reference Musa-Veloso, Likhodii and Cunnane 77, Reference Musa-Veloso, Rarama and Comeau 79 ). Under certain circumstances acetone is reduced to isopropanol by hepatic alcohol dehydrogenase and this then also appears in the breath( Reference Jones and Rossner 80 ).
Simple carbohydrates and alcohol
H2 breath tests have been used for the assessment of carbohydrate malabsorption and abnormal bacterial colonisation of the gut for many years( Reference Levitt 81 ). Basal breath H2 is dependent on dietary carbohydrate( Reference Avallone, De Carolis and Loizos 82 ). H2 production in man is primarily dependent upon the delivery of ingested, fermentable substrates to an abundant intestinal flora that is normally present only in the colon. In the normal intestine, more than 99 % of H2 production appears to be of colonic origin, but small-bowel production may be increased in a patient with excessive numbers of small-bowel bacteria. H2 breath tests are based on the fact that there is no source for H2 gas in humans other than bacterial metabolism of carbohydrates. Respiratory H2 excretion can therefore be used as an indicator of intestinal H2 production. In carbohydrate tolerance tests, different carbohydrates are administered orally and the concentration of H2 is measured in expired air. When defective sugar absorption is present, unabsorbed sugars are available in the colon for bacterial fermentation( Reference Rumessen, Hamberg and Gudmandhoyer 83, Reference Rumessen, Hamberg and Gudmandhoyer 84 ). Approximately 14 % of the total H2 production is excreted by the lungs, and rates of breath H2 excretion and production correlates well( Reference Simren and Stotzer 85 ). Smoking raises and exercise lowers H2 concentrations and is therefore not allowed during these tests( Reference Simren and Stotzer 85 ).
Orocaecal transit time is increased in subjects with alcoholism, but it also appears to be increased among individuals who drink moderate amounts of alcohol as assessed by the H2 breath test( Reference Addolorato, Montalto and Capristo 86, Reference Wegener, Schaffstein and Dilger 87 ). Clearly, this has the potential to alter the occurrence of specific breath constituents and the overall postprandial breath profile. Alcohol is largely metabolised to acetaldehyde by dehydrogenase enzymes, leading to the appearance of high concentrations of acetaldehyde in the breath after alcohol consumption( Reference Smith, Wang and Španěl 88, Reference Mitsubayashi, Matsunaga and Nishio 89 ). Somatic cells and microbes representing normal human gut flora are also able to produce acetaldehyde from ethanol( Reference Salaspuro 90 ). After the ingestion of alcoholic beverages, there are high local acetaldehyde concentrations in the saliva, gastric juice and the contents of the large intestine. In addition, microbes may produce acetaldehyde endogenously in the absence of exogenous alcohol administration( Reference Salaspuro 90 ).
Complex carbohydrates and fibre
Complex carbohydrate and fibre increase gut transit time and therefore increase the quantity of fermentable, non-absorbed carbohydrate reaching the distal intestine, and hence increase the production of gut-derived H2 and CH4 ( Reference Fritz, Siebert and Kasper 91– Reference Madsen, Linnet and Rumessen 93 ). In some groups of subjects, there appears to be an adaptation to high intakes of resistant starch over time and an apparent relationship with insulin sensitivity( Reference Behall and Howe 94 ). Using breath H2 analysis, Strocchi & Levitt( Reference Strocchi and Levitt 95 ) found that 5–10 % of starch in wheat, potatoes and maize is not absorbed by healthy subjects, while rice starch is nearly completely absorbed. The physiological effects of dietary fibre are not always predictable from their physicochemical properties( Reference Cherbut, Aube and Mekki 96 ). For example, maize fibre has been reported to resist fermentation better than potato fibre and to have a lower digestibility( Reference Cherbut, Aube and Mekki 96 ). However, both dietary fibres increased faecal output of DM, neutral sugars and water. Orocaecal transit time is increased by potato fibre, and it is reported to reduce postprandial plasma levels of total and esterified cholesterol. In contrast, maize fibre has been reported to lower fasting blood cholesterol concentrations and increase the non-esterified cholesterol ratio. A class of non-digestible but fermentable oligosaccharides, trans-galacto-oligosaccharides, was found to increase the concentration of breath H2 and the N density of the faeces( Reference Alles, Hartemink and Meyboom 97 ), whilst dietary fibre from maize, cassava and amaranth all increased faecal energy loss. Expired breath H2 was highest for those individuals consuming maize or cassava( Reference Hamaker, Rivera and Morales 98 ). In critically ill patients receiving jejunal feeding with a semi-elemental diet, fibre supplementation appeared to improve microbiota mass and function, being associated with increased carbohydrate fermentation, measured as breath H2 and CH4 ( Reference O'Keefe, Ou and Delany 99 ).
Protein
The ingestion of a high protein-energy meal is associated with some complex changes in breath compounds; the changes in exhaled acetone, NH3 and ethanol concentrations( Reference Smith, Španěl and Davies 26 ) have been discussed above.
Effects of entire diets
A diet that is chronically energy restricted is associated with longevity, which is probably related to a reduction in oxidant stress( Reference Beckman and Ames 100, Reference Trepanowski, Canale and Marshall 101 ); such a diet is also associated with low breath ethane concentrations, that again may relate to reduced oxidant stress( Reference Habib, Dickerson and Mooradian 102 ).
Kundu et al. have found that the amount of breath acetone in exhaled breath was correlated with the rate of fat loss( Reference Kundu, Bruzek and Nair 103 ) in subjects on a restricted-energy weight-loss programme.
Using a randomised controlled design of the effects of a diet rich in fruit and vegetables, with or without low-fat dairy products for 8 weeks' duration, Miller et al. ( Reference Miller, Appel and Risby 104 ) found that breath ethane was significantly reduced in patients on both fruit- and vegetable-rich diets, but particularly in subjects on a low-fat dairy diet. The endogenous production of methanol is increased after the consumption of fruit( Reference Lindinger, Taucher and Jordan 105 ), its concentrations increasing by as much as an order of magnitude. This is thought to be due to the degradation of natural pectin (which is esterified with methyl alcohol) in the colon. In vivo studies showed that pectin in either a pure form (10 to 15 g) or a natural form (in 1 kg of apples) induces a significant increase of methanol in the breath (and by inference in the blood) of humans( Reference Lindinger, Taucher and Jordan 105 ) to a level similar to that seen following the consumption of alcohol spirits( Reference Taucher, Lagg and Hansel 106 ).
Effects of pre- and probiotics
In vitro studies of isolated bacterial cultures have demonstrated that the VOC profile that they produce is distinctive, and may be used to differentiate bacterial species( Reference Zhu, Bean and Kuo 107 ). Whilst some of these molecules, for example ethanol and acetone, are produced by their human host, other trace gases, for example H2 and indole, are only produced in detectable quantities by bacteria( Reference Zhu, Bean and Kuo 107 ). Furthermore, the gases emitted by bacteria also appear to be dependent on the strain of the bacterial isolate( Reference Shestivska, Španěl and Dryahina 108 ) and culture conditions( Reference Chippendale, Španěl and Smith 109 ). Bartram et al. ( Reference Bartram, Scheppach and Gerlach 110 ) have reported that daily yogurt enriched with Bifidobacterium longum and 5 g lactulose/l increased breath H2 exhalation and mouth-to-caecum transit time.
SCFA are produced by bacterial fermentation of carbohydrates in the colon, influence gastrointestinal motility( Reference Cherbut, Aube and Blottiere 111 ), and can affect motility at a distance from their site of production. The mechanisms of action of SCFA on gastrointestinal motility have not been completely elucidated. They may involve systemic humoral and neural pathways as well as local reflexes and myogenic responses.
Cellobiose has a β-1,4 linkage, so it is resistant to hydrolysis by human small-intestinal disaccharidase and, hence, reaches the colon undigested. The excretion of breath H2 gas after cellobiose ingestion was found to be significantly greater than after glucose ingestion( Reference Nakamura, Oku and Ichinose 112 ). In another study, prebiotic treatment increased breath H2 excretion by 3-fold and reduced hunger( Reference Cani, Lecourt and Dewulf 113 ). The AUC for plasma glucagon-like peptide 1 and the volatile release curve for breath-H2 excretion measured after the meal were significantly correlated with each other( Reference Cani, Lecourt and Dewulf 113 ).
Dietary micronutrients
Micronutrients have the potential to affect redox status and prevailing inflammation, or they may have direct effects on constituents within breath. Furthermore, lung function (forced expiratory volume) appears to be related to dietary vitamin C( Reference Britton, Pavord and Richards 114 ) and fruit intake( Reference Cook, Carey and Whincup 115 ), although the latter study was in children and so the findings may not be the same for adults. Increased concentrations of breath alkanes are associated with reduced antioxidant micronutrient status( Reference Aghdassi and Allard 45 ). Supplementation with a cocktail of antioxidant vitamins (vitamin C, vitamin E and β-carotene) has been reported to be associated with reduced breath pentane in smokers( Reference Steinberg and Chait 116 ). In contrast, Fe supplements have been found to increase breath ethane concentrations in young women( Reference Mertz, Woodhouse and Donangelo 117 ). The amount of breath dimethyl selenide has been reported to increase after the ingestion of Se supplements( Reference Kremer, Ilgen and Feldmann 118 ) and substantial amounts are found in the breath of individuals with Se toxicity( Reference Barceloux 119 ), and this may account for the characteristic breath odour in individuals with this condition( Reference Barceloux 119 ).
Principles of measurement
The ability to accurately measure concentrations of trace gases in humid breath has only been possible in the last 20–30 years. GC-MS has been used widely used for breath analysis and continues to be vigorously exploited to great effect for this purpose. In GC-MS, breath samples are collected and volatile compounds extracted and pre-concentrated before offline analysis. Whilst GC-MS has allowed the identification of compounds in breath it is not possible to use this technique in real time( Reference Phillips 3, Reference Phillips, Herrera and Krishnan 4 ). It is disturbed by the large amount of water vapour present in humid exhaled breath.
More recent analytical advances include SIFT-MS, proton transfer reaction (PTR)-MS and various optical spectroscopic or electronic ‘nose’ devices; these are techniques that have allowed real-time analysis of breath( Reference Smith and Španěl 120– Reference Smith and Španěl 123 ). Spectroscopic detection methods have been designed to detect specific simple molecules of permanent gases, such as NO and ethane( Reference Wang and Sahay 121 ) rather than a profile of VOC in breath, but are amenable to real-time applications. The physicochemical principle of electronic nose devices is that exposure of the detector to specific compounds is associated with a change in surface conductivity of the sensor; however, interpretation may be complicated for humid samples( Reference Smith and Španěl 123 ) and they generally lack positive identification.
Methods commonly used for breath analysis
Those methods used for breath analysis mentioned above are briefly described below and summarised in Table 2. The most widely reported breath analytes are shown in Table 3, together with the methods used to detect them, their concentrations, sources and potential confounding factors.
Table 2. Summary of methods used for breath analysis

PTR, proton transfer reaction; TOF, time of flight; VOC, volatile organic compounds; SIFT, selected ion flow tube.
Table 3. Summary of breath analytes with reported ranges and sources
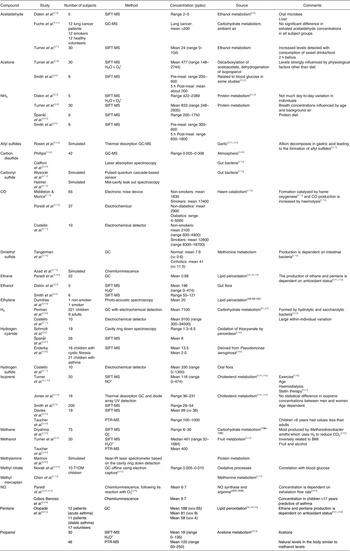
ppbv, Parts per billion by volume; SIFT, selected ion flow tube; PTR, proton transfer reaction; T1DM, type 1 diabetes mellitus.
Ion mobility spectrometry
The aim of ion mobility spectrometry is to identify trace gases by the mobility of their characteristic gas-phase ions or their derivatives in a buffer/carrier gas( Reference Ruzsanyi, Baumbach and Sielemann 124 ). These ions are produced by exposing the carrier gas/trace gas to a radioactive source or electrical discharge when chemical ionisation reactions result in the analytical drifting ions. The movement of these ions is dependent on their mass and molecular geometry, and their dwell times are used to characterise the original mixture of trace gases. Whilst this approach is not recommended for the identification of unknown compounds, it has been used to determine differences in breath metabolite profiles associated with specific diseases( Reference Smith and Španěl 123 ).
Proton transfer reaction-MS and proton transfer reaction-time of flight
In these techniques, precursor hydronium ions (H3O+) are injected into the buffer gas, which is usually the gas sample to be analysed, and react with the trace gas present in the sample. The precursor ions react with the trace gas species, producing characteristic ion products that are detected and quantified using a down-stream analytical MS. PTR-MS is sensitive down to and below ppbv( Reference Moser, Bodrogi and Eibl 125– Reference Jordan, Haidacher and Hanel 127 ). The precursor molecules react with most trace gas molecules to produce a protonated molecule (MH+). However, the latter nascent ion may be unstable for some compounds, for example, when M is an alcohol. Furthermore, when the carrier gas in PTR-MS is humid breath, this leads to the formation of cluster ions, for example H3O+·(H2O)1,2,3 that may make quantitative analysis more complex, although this cluster ion formation is inhibited by the presence of the axial electric field along the flow tube( Reference Blake, Monks and Ellis 128 ). In PTR-time-of-flight analysis, ions are accelerated to uniform energy by an electric field, and subsequently traverse a defined distance. The time of flight of the ion is directly related to the ion's mass:charge ratio, and this allows a mass resolution that is substantially better than for conventional PTR-MS( Reference Herbig, Muller and Schallhart 129 ). Whilst the original instruments relied on long integration times to attain sufficient sensitivity, recently developed PTR-time of flight instrumentation has improved sensitivity( Reference Jordan, Haidacher and Hanel 130 ), with integration times of 1 s and a corresponding limit of detection approaching 100 parts per trillion for most compounds, allowing online breath analysis( Reference Herbig, Muller and Schallhart 129 ).
Selected ion flow tube-MS
SIFT-MS combines the fast flow tube technique, chemical ionisation using selected precursor ions, either H3O+, NO+ or O2 +, and quantitative MS that allows online, real-time quantitative analysis of the trace gases (such as ethanol, acetaldehyde, NH3, acetone and isoprene, etc.) in single breath exhalations down to concentrations in the ppbv range in a timescale of seconds( Reference Wang 131 ). SIFT-MS relies on chemical ionisation by the chosen precursor ions of the trace gas molecules in air/breath samples introduced into He carrier gas. These reactions proceed for an accurately defined time, the precursor and product ions being detected and counted by a downstream quadrupole mass spectrometer, thus effecting quantification. Because the absolute concentrations of trace gases in single breath exhalation can be determined by SIFT-MS down to ppbv concentrations, this obviates the need for offline sample collection for the most common breath trace gases. A numerical algorithm allows the calculation, in real time, of absolute concentrations of trace gases, including VOC and water vapour( Reference Španěl, Dryahina and Smith 132 ).
Optical and laser spectroscopic detection
Laser spectroscopic detection techniques have high sensitivity and high selectivity, but also have the advantageous features of near real-time response and low instrument cost. Of approximately thirty-five biomarkers quantified using this method, fourteen species have been analysed in exhaled human breath by high-sensitivity laser spectroscopic techniques, for example acetone, NH3, CO2, ethane, CH4 and NO. The spectral fingerprints of these potentially useful biomarkers span from the UV to the mid-IR spectral regions and the detection limits achieved by the laser techniques range from parts per million by volume to ppbv. Sensors using the laser spectroscopic techniques are already commercially available for a few breath biomarkers, for example CO2 and NO( Reference Wang and Sahay 121 ).
Electronic nose detection
Electronic noses, or artificial sensors of volatiles including odorants, have been developed over the last 10 years to perform a variety of identification tasks in various industries. Electronic noses produce a chemical fingerprint of the sample, and this is matched to a reference database( Reference Di Francesco, Fuoco and Trivella 133 ). This powerful technology is only beginning to be introduced in the field of medicine, but is promising in its potential to assist in diagnosis( Reference Thaler, Kennedy and Hanson 134 ).
Chemiluminescence
Chemiluminescence (CL) is a powerful analytical tool in trace gas analysis. CL monitoring has been used as universal nitrogen and sulfur detectors for GC and capillary electrophoresis( Reference Sye and Cheng 135 ). CL detection can be used as the basis of compact and sensitive analysers for real-sample analysis. Isoprene and sulfur compounds in expired breath and atmospheric samples have been successfully measured by coupling to a small collection system. Short-term adsorbent collection enhances the sensitivity and considerably reduces interference. The organosulfur compounds methyl mercaptan and dimethyl sulfide can be separated on the same column that is used for collection( Reference Toda and Dasgupta 136 ).
Conclusions
Breath analysis is becoming more accessible for clinical and physiological applications. Expired breath is a complex mixture of low-molecular-weight volatile compounds that are derived from diet and endogenous metabolism, or from micro-organisms in the gastrointestinal and respiratory tracts. Metabolic, inflammatory and neoplastic conditions are reported to be associated with characteristic breath profiles, and breath analysis has been promoted as a potentially simple, non-invasive method for screening and monitoring conditions such as asthma, diabetes mellitus and lung cancer. However, there are a number of factors that affect the concentrations of compounds in breath, including diet, physical activity and smoking habit, and it will be important to better understand how these factors influence breath composition as the applications of breath analysis broaden in scope. In order to apply breath analysis to investigations of human nutrition, it would be important to consider any concomitant co-morbidity, including renal and liver dysfunction, neoplastic disease, infection and inflammation. Breath sampling should probably take place under standardised conditions, for example after an overnight fast, and involve diurnal and longitudinal monitoring. A method should be used that is less sensitive to the local release of compounds from the oral cavity. Whichever methods are used should probably also have defined age-related reference ranges.
Acknowledgements
The North Staffordshire Medical Institute provided a grant to G. A. A. F., P. Š. and D. S., but had no role in the writing of the review.
All the authors were involved in the drafting and revision of the manuscript.
P. Š. and D. S. are shareholders and directors of Trans Spectra Limited, UK.







