Anterior ischemic optic neuropathy (AION) causes acute, painless vision loss due to hypoperfusion or nonperfusion of the optic nerve head. Reference Hayreh1 An uncommon cause of AION is papilledema. Papilledema is defined by optic disc edema secondary to raised intracranial pressure (ICP). While mild papilledema often presents with little to no vision loss, chronically elevated ICP can progress to AION with irreversible vision deterioration. Reference Hayreh1 Identifying papilledema cases at risk for AION remains a difficult task. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) values have been investigated in the diagnosis of AION due to their applications in acute brain ischemia. Reference Adesina, Scott McNally and Salzman2,Reference Al Othman, Naser, Kini and Lee3 We present a case of a 48-year-old man with papilledema that worsened and infarcted both optic nerve heads, revealing a unique MRI finding of restricted diffusion of both optic nerve heads.
A 48-year-old man was referred to neuro-ophthalmology for bilateral optic disc edema. He had a past medical history of hepatocellular carcinoma with cardiac metastasis, Alagille syndrome, liver transplant, chronic kidney disease, and spinal stenosis. His medications included aspirin, tacrolimus, mycophenolic acid, and vitamin D. One month prior to presentation, he noticed a new onset of blurry vision in both eyes and was referred to the emergency room by an optometrist that detected bilateral optic disc edema. He underwent a CT/CTV that showed a calvarial mass invading the superior sagittal sinus. He was suspected of having idiopathic intracranial hypertension (IIH) and underwent a lumbar puncture that showed an opening pressure of 36 cm of water with normal cerebrospinal fluid (CSF) contents. He was started on acetazolamide and referred to neuro-ophthalmology.
His initial examination revealed a visual acuity of 20/60 OD and 20/50 OS, full confrontation visual fields (Humphrey visual fields were not possible due to mobility issues), and no relative afferent pupillary defect. Dilated fundus examination revealed severe hemorrhagic optic disc edema in the right eye with blot hemorrhages in the macula and moderate optic disc edema in the left eye with a few blot hemorrhages OS (Figure 1). A diagnosis of papilledema related to venous hypertension due to external compression of the superior sagittal sinus by a likely metastasis was made and an MRI/MRV was performed. This revealed a calvarial lesion overlying the middle third of the superior sagittal sinus and compressing the superior sagittal sinus (Figure 2). Since he could not tolerate acetazolamide, he was switched to topiramate and a neurosurgical consultation was obtained. General anesthesia was deemed high risk due to his cardiac ventricular metastasis and he was unable to undergo CSF diversion. He was treated with radiation therapy for the calvarial metastasis.
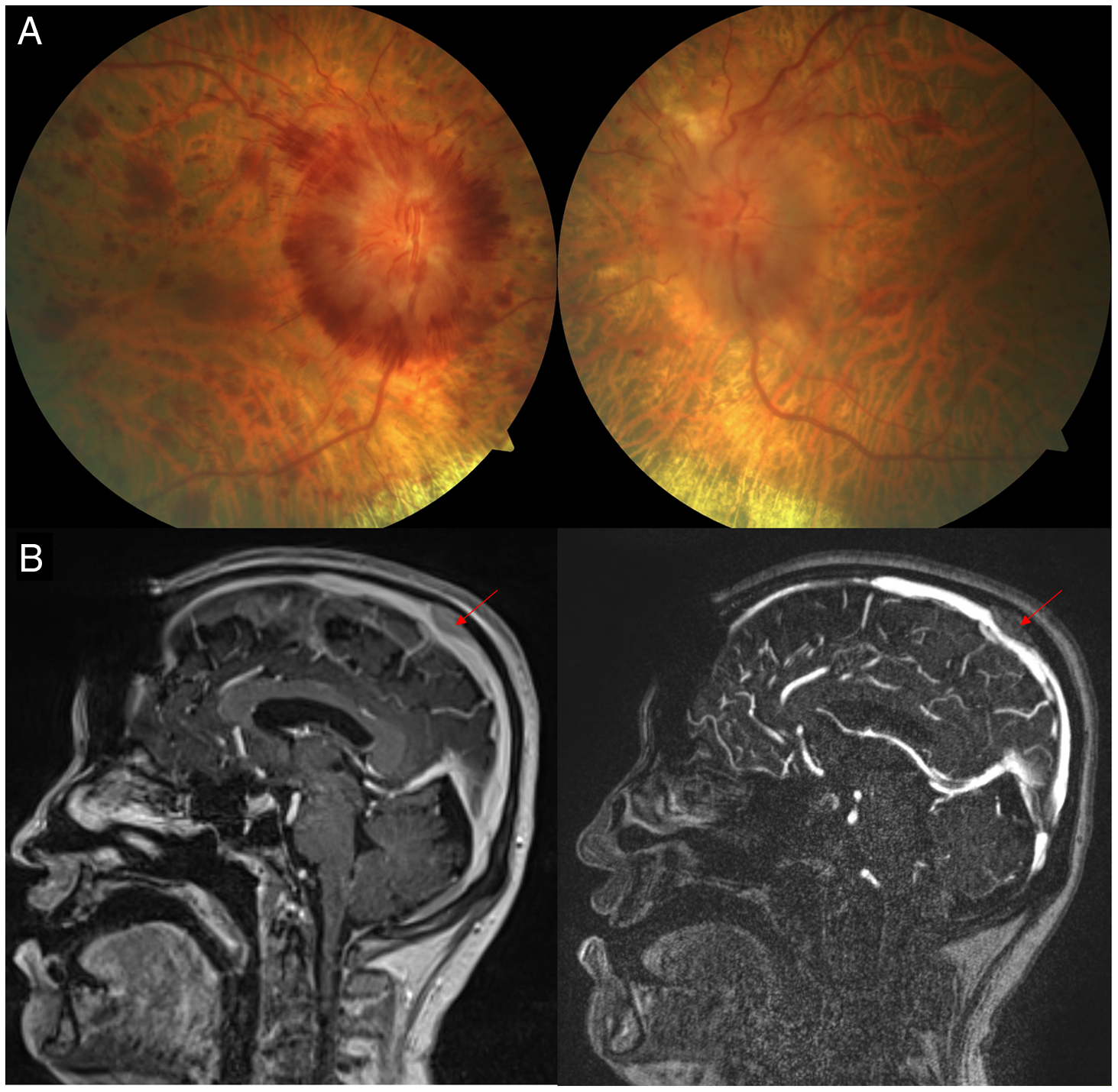
Figure 1: (A) Fundus photographs demonstrating severe bilateral optic disc edema. (B) Sagittal magnetic resonance imaging of the brain with contrast (A) and magnetic resonance venography (B) demonstrating extrinsic compression of the superior sagittal sinus by a presumed metastasis (red arrow).
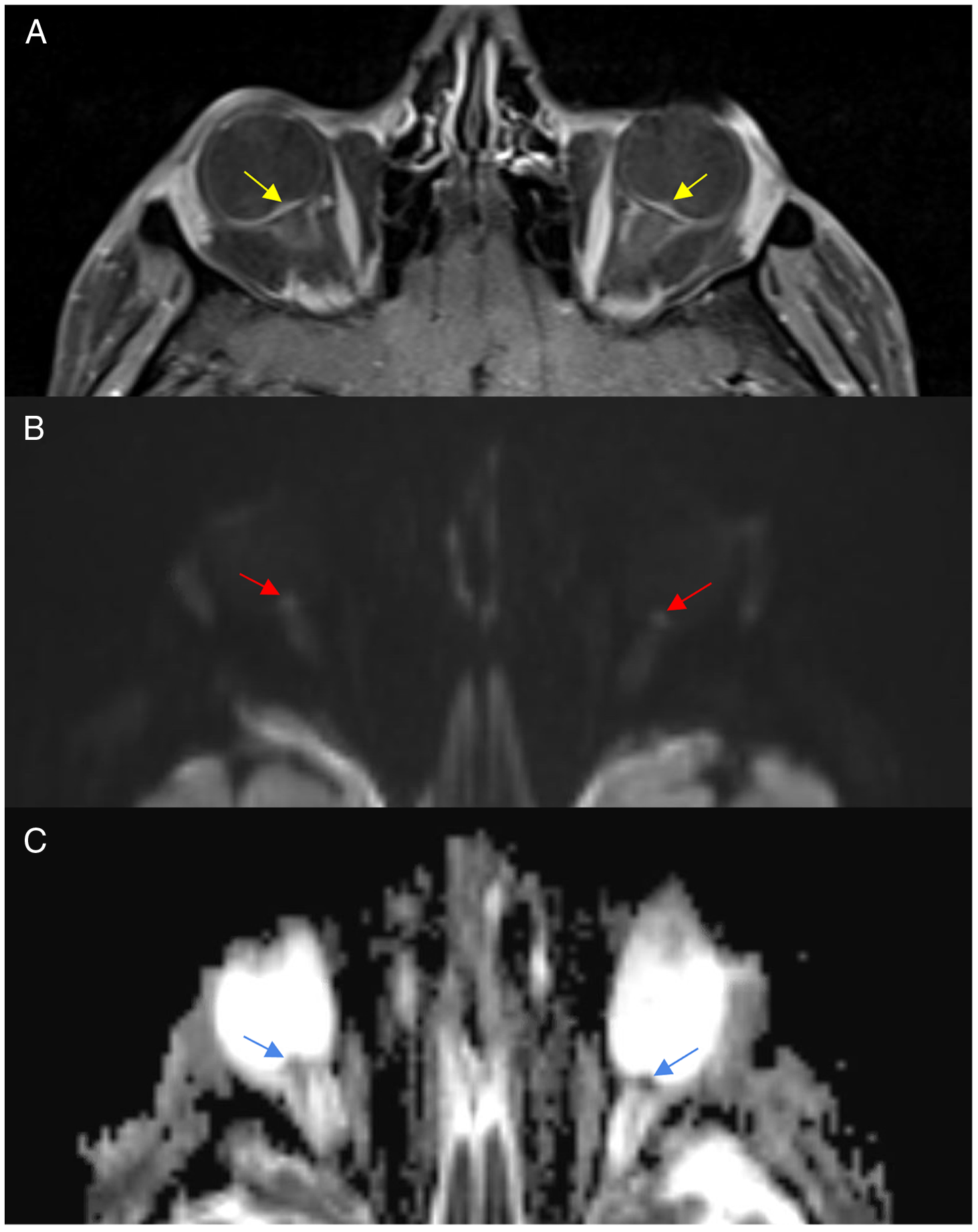
Figure 2: Magnetic resonance imaging T1-post-contrast with fat suppression (A), diffusion-weighted imaging (B) and apparent diffusion coefficient demonstrating flattening of the posterior sclera and optic nerve head enhancement (A), increase signal on DWI (B) and low signal on ADC (C).
This patient’s visual function continued to gradually decline in both eyes despite increased topiramate dosing, the addition of methazolamide, and dexamethasone. Three months after initial presentation, his visual acuity declined to no light perception OD and 20/500 OS. He had interval atrophy of his optic nerves and increasing hemorrhages in the retina. He underwent a repeat MRI orbits and brain that showed severe flattening of the posterior sclera and enhancement of the optic nerve head. On DWI sequences, there was restricted diffusion within the head of the optic nerve with associated low ADC signal. His vision declined to no light perception in both eyes 2 weeks later and he died 6 months later.
This was a rare and unfortunate case of a patient who had papilledema refractory to medical treatment. He could not undergo general anesthesia due to his cardiac issues and no surgical intervention could be performed. This allowed for the natural history of papilledema to be observed, which progressed to AION as confirmed with MRI.
Our literature review revealed two cases of papilledema progressing to AION and two cases of AION superimposed on papilledema. Reference Jiraskova, Studnicka and Rozsival4,Reference Ma and Micieli5,Reference Green, Lessell and Loewenstein6,Reference Lamirel, Bruce, Newman and Biousse7 All four cases had papilledema due to IIH. These were clinical diagnoses and restricted diffusion was not reported on MRI in these cases. Our patient did not have IIH and this report appears to be the first to investigate DWI and ADC findings in papilledema evolving to AION. Our literature review revealed three studies that have reported on DWI and ADC signs in AION. Two case reports support our findings of DWI hyperintensity at the optic nerve head and corresponding low ADC signal in the acute stages of vision decline in AION. Reference Adesina, Scott McNally and Salzman2,Reference Al Othman, Naser, Kini and Lee3 A larger study comparing 72 nonarteritic AION (NAION) eyes and 32 optic neuritis eyes found that, after adjusting for age and sex, the two conditions could not be differentiated by positive DWI or post-contrast enhancement at the optic disc alone. Reference Adesina, Scott McNally and Salzman2 However, NAION eyes had a longer average time between symptom onset and imaging. In addition, positive DWI eyes in both groups were imaged an average of 6 days earlier than eyes with negative DWI findings. Reference Adesina, Scott McNally and Salzman2
The progression of papilledema to AION depends on blood flow to the anterior optic nerve, which receives segmental blood supply from the posterior ciliary arteries (PCAs). This segmental network creates watershed zones, pockets of relatively poor vascularity at the margins of end-arterial territories. Reference Hayreh1 Between-individual differences in PCA branch number and distribution result in various watershed zone patterns. AION progression is thought to be influenced by the degree of overlap between watershed zones and the optic disc. Reference Hayreh1 Susceptibility of the optic disc to ischemia is also influenced by compressive forces from papilledema. Optic disc swelling compresses veins in the prelaminar optic disc with less impact on higher pressure arterial vessels. Chronic and elevated compression can cause prelaminar vascular resistance, venous stasis, and delayed capillary filling, which is well-documented in AION. Reference Hayreh1,Reference Arnold8 A reduction in perfusion pressure to an already susceptible optic disc can cause ischemia and AION. Reference Hayreh1 Our patient had a crowded optic disc, similar to a “disc at risk”, which is a risk factor for NAION. In this case, papilledema could have progressed to NAION due to a combination of the following: risk factors, watershed zone distribution, papilledema compressive forces, and hypoperfusive events.
In conclusion, NAION may develop from papilledema, especially in untreated and refractory cases. Restricted diffusion of the optic nerve head may be seen on MRI when performed in the acute stage.
Funding
The authors have no financial support to disclose.
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
Statement of Authorship
JM prepared the case summary, figures, and figure captions. NP prepared the introduction and discussion.




