Infants with complex CHD who require surgical intervention experience significant disruptions in their first few months after birth which impact their ability to feed orally. Twenty-two to 50% of children with complex CHD experience feeding challenges. Reference Maurer, Latal, Geissmann, Knirsch, Bauersfeld and Balmer1–Reference Yi, Kim, Huh, Jun, Cheon and Kwon3 Structural and physiologic cardiopulmonary differences in the infant with complex CHD impact neurodevelopment, as well as the respiratory and gastrointestinal systems, all of which are instrumental to promoting safe and efficient oral feeding. Understanding the medical complications and physiology of children with complex CHD is important in the assessment and treatment of feeding difficulties in order to change long-term trajectories with early intervention. Medical complications impacting the neurological, gastrointestinal, and respiratory systems after surgery and its disruptions to feeding development have been discussed in great detail. Reference Jones, Desai and Fogel4 The current manuscript reviews assessment and treatment of oral feeding skills in the infant with complex CHD, taking into consideration the unique medical consequences which impact feeding development.
Respiratory
Infants born with complex CHD often require respiratory support both before and after surgical intervention. The need for respiratory support interferes with opportunities for natural oral stimulation and oral feeding due to the placement of the endotracheal tube or non-invasive respiratory support equipment and the physiological status of the infant.
Assessment
Currently, standardised protocols to assess oral feeding readiness and swallowing safety while an infant is receiving higher levels of respiratory support are not available. Reference Canning, Fairhurst, Chauhan and Weir5 Literature looking at safety of oral feeding for preterm infants on nasal continuous positive airway pressure and high-flow nasal cannula reveal inconsistent results, with some indicating little to no impact on swallowing safety while others revealing an increased risk for aspiration. Reference Leder, Siner, Bizzarro, McGinley and Lefton-Greif6–Reference Ferrara, Bidiwala and Sher8 The flow rate of high-flow nasal cannula or continuous positive airway pressure at which suck/swallow/breathe coordination is disrupted is currently unknown, as contributing factors such as weight, air leak, and individual patient physiology result in variable positive pressure generation. Reference Dysart, Miller, Wolfson and Shaffer9 Infants on high-flow nasal cannula with a flow rate as low as 2.5 L/min can generate levels of positive pressure that increase the risk for swallowing dysfunction. Reference Sreenan, Lemke, Hudson-Mason and Osiovich10 Institution specific protocols should take into consideration respiratory status, oral feeding readiness cues, oral motor and swallowing coordination, and endurance to determine safe level for small volume of therapeutic feeding trials for infants with acute respiratory illness on high-flow nasal cannula. Reference Raminick and Desai11
Recommendations
Infants born with complex CHD often have delayed experiences with oral sensory stimulation, which are important for the development of neuromotor and sensory pathways responsible for the establishment of oral sensory motor and feeding skills. Reference Dalgleish, Kostecky and Blachly7 Early intervention by skilled feeding therapists can help promote improved oral motor strength, coordination, and sensory processing. Reference Coker-Bolt, Jarrard, Woodard and Merrill12
Non-nutritive oral motor stimulation has been proven to shorten time to nutritive oral feeding in infants and decrease length of hospital stay in infants with single ventricle anatomy in the cardiac ICU compared to infants who did not receive the intervention. Reference Coker-Bolt, Jarrard, Woodard and Merrill12 Providing oral stimulation with a small volume (e.g., 0.2 ml) of colostrum or human milk facilitates sensory stimulation. The anti-microbial and anti-bacterial properties of colostrum and human milk may help with decreasing permeability in the gut lining Reference Jakaitis and Denning13,Reference Davis, Baumgartel, Morowitz, Giangrasso and Demirci14, thus reducing risk for necrotising enterocolitis, ventilator-associated pneumonia, and sepsis. Reference Medoff-Cooper, Naim, Torowicz and Mott15–Reference Patel, Kim and Saunders17 If the infant tolerates a small presentation of milk and does not exhibit stress signs while intubated, additional stimulation can be provided to the body of the tongue with the swab or a tiny pacifier under the intubation tube to facilitate lingual cupping in a gentle/respectful approach. When the infant tolerates non-invasive respiratory support (Non-invasive Neurally Adjusted Ventilatory Assist (NIV-NAVA), high-flow nasal cannula, or nasal continuous positive airway pressure), a pacifier or finger dipped in human milk can be offered to encourage lingual/palatal proprioceptive awareness, improve oral seal, increase lingual movement, and improve strength for later bolus control once bottle or breastfeeding begins. Reading infant cues while providing oral stimulation is critical to ensure positive oral experiences. Signs of stress might include colour changes, frowning, finger splaying, stretching out arms and legs, decreasing or increasing heart rate, and gagging.
There can be oral sensory deprivation or overload when learning to eat which needs to be in careful balance for success. Reference Dalgleish, Kostecky and Blachly7 While initiating early oral feeding is important, a slow transition is often necessary toward bottle or breastfeeding. Encouraging an infant to suck on their own hand with milk promotes hand to mouth movement and improved proprioceptive awareness for pre-feeding skills. When beginning with breast or bottle feeding, the infant may be able to start with a small volume (e.g., 3–5 ml twice a day) if showing feeding readiness such as an awake state, rooting, or spontaneously bringing hands to mouth while still on high-flow respiratory support. Use of the slowest flow nipple to control the bolus and elevated side-lying position (Fig 1) to decrease the impact of gravity and improve breathing are strategies that can be implemented as an infant is weaning from respiratory support.
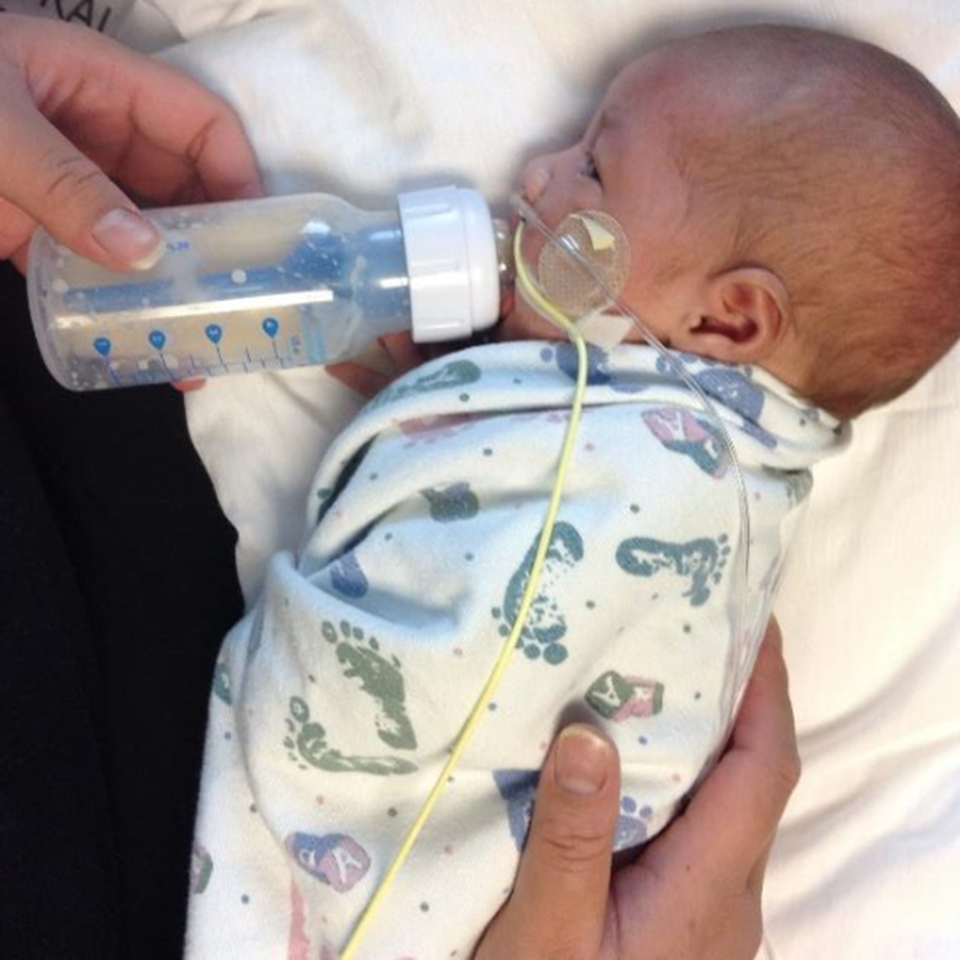
Figure 1 Side-lying position: head, shoulder, and hips supported midline, exposed ear pointing towards the ceiling, hands swaddled near the infant’s face.
Skilled therapists can initiate therapeutic trials and advance volume or increase duration of time or frequency of feeding based on the infant’s behavioural responses to stimulation, progression in weaning from respiratory support, improved cardiorespiratory endurance, and tolerance of enteral feedings. Clinical pathways such as The Safe Individualized Nipple Feeding Competence can be effective if done consistently and according to the protocol. Reference Dalgleish, Kostecky and Blachly7 Safe Individualized Nipple Feeding Competence is a systemic approach to safe and slow progressive oral feeding experiences based upon age, behavioural cues, respiratory support, and per cent of total volume the patient needs for nutrition. Reference Dalgleish, Kostecky and Blachly7
Gastrointestinal
Infants with complex CHD often experience gastrointestinal difficulties after cardiac surgery due to multiple causes, with vagal nerve dysfunction being the most highly cited in the research literature. Reference Pados and Davitt18 Twenty-five per cent of infants with complex CHD experience gastroesophageal reflux disease compared to 10–20% of infants without complex CHD, though this may be a low estimate due to difficulty diagnosing in the infant population. Reference Indramohan, Pedigo, Rostoker, Cambare, Grogan and Federman19–Reference Esposito, Roberti and Turrà22 Gastroesophageal reflux disease may cause discomfort and/or respiratory compromise, resulting in poor oral intake, and difficulties with weight gain. Most infants are diagnosed with gastroesophageal reflux disease based on clinical symptoms (i.e., coughing, irritability, apnoea, feeding difficulties). Reference Hasenstab and Jadcherla23 Pharyngeal and oesophageal motility may also be compromised after cardiac surgery due to transesophageal echocardiography and sedation/medication resulting in difficulties with oral feeding progression. Furthermore, inflammation, surgical/visceral trauma, circulatory changes, and chronic ventilation can alter sensorimotor reflexes of the pharynx and oesophagus.
Assessment
Collaboration with a gastroenterologist is beneficial to identify appropriate diagnostic testing to diagnose and manage gastrointestinal symptoms. An upper gastrointestinal tract series, oesophageal pH probe monitoring or multichannel intraluminal impedance with pH (pH-MII) can be performed to detect acid reflux and determine correlation between symptoms and acidic reflux episodes. High-resolution manometry is a diagnostic tool to assess motility difficulties when an infant presents with persistent oral feeding progression challenges in light of typical oral motor and pharyngeal swallowing skills. Diagnostic testing is beneficial and has been researched in infants with complex CHD to guide medical management, especially if symptoms are atypical and not responsive to treatment strategies. Reference Malkar and Jadcherla24
Recommendations
Management of gastroesophageal reflux disease is critical in promoting positive oral feeding experiences and feeding progression, as studies have shown increased feeding difficulties in children with gastroesophageal reflux disease, impacting their growth and development. Reference Sdravou, Emmanouilidou-Fotoulaki, Mitakidou, Printza, Evangeliou and Fotoulaki25 Non-pharmacologic management has been recommended as the initial treatment for infants with gastroesophageal reflux disease, followed by a limited trial of medication. Reference Rosen, Vandenplas and Singendonk26 Thickening formula or expressed breastmilk, with careful oversight, and using extensively hydrolysed protein formula are strategies used to decrease vomiting and reflux episodes. Reference Malkar and Jadcherla24,Reference Corvaglia, Martini, Aceti, Arcuri, Rossini and Faldella27 Positioning in the left lateral position is noted to decrease the severity of vomiting on pH impedance testing in symptomatic infants with gastroesophageal reflux disease. Reference Loots, Kritas and van Wijk28 Decreasing infant stress via skin-to-skin and supporting breastfeeding to improve gut microbiome and reduce discomfort are also interventions used to potentially improve symptoms of gastroesophageal reflux disease. Reference Pados and Davitt18,Reference Malkar and Jadcherla24 Anti-reflux medications have been widely used to treat reflux in infants. However, there is evidence suggesting that these medications do not have an effect on reducing gastroesophageal reflux disease symptoms in infants and may increase risk of abnormal bacterial colonisation and infections. Reference Pados and Davitt18 The literature, along with expert clinical consensus, recommends use of proton-pump inhibitors for the treatment of symptoms of reflux (e.g., erosive oesophagitis) in infants, but not for clinical signs (distress, fussiness, visible regurgitation) in otherwise healthy infants. Reference Rosen, Vandenplas and Singendonk26 Erythromycin is the only promotility agent with proven effectiveness in improving gastric motility and feeding tolerance in preterm infants. Reference Martinez, Douglas, Nurko and Mehta29 In addition, abdominal massage has also been found to increase vagal nerve activity and improve gastric motility in preterm infants. Reference Diego, Field, Hernandez-Reif, Deeds, Ascencio and Begert30
Nutrition
Infants with complex CHD present with challenges with weight gain due to hypermetabolism, increased demand on respiratory muscles resulting in tachypnoea, and poor endurance to consume sufficient volumes to meet caloric needs. Reference Jones, Desai and Fogel4
Assessment
The World Health Organization provides guidelines for nutritional assessment standards and integrated anthropometric classification which include weight, length and head circumference measurements, nutritional lab results, dietary intake, medical complexity, illness severity, energy requirements, and clinical observations of fat stores, mucous membrane,s and skin colour changes. Reference Green Corkins31–Reference de Onis and Habicht33 With a complete nutritional assessment, interventions can be determined to promote growth and development, providing safe perioperative nutrition. Reference Kalra, Vohra and Negi34–Reference Lisanti, Savoca and Gaynor38 The dietitian is also a crucial team member in decision and timing of gastrostomy tube placement.
Recommendations
Dietitians provide recommendations for individualised nutrition and hydration for critically ill infants. Reference Chinawa, Chinawa, Duru, Chukwu and Obumneme-Anyim39 Management of proper nutrition may include fortified human milk or formula to meet the energy needs of the infant, which are increased for infants with complex CHD especially when in recovery. Reference Kelleher, Laussen, Teixeira-Pinto and Duggan40,Reference McHoney, Eaton and Pierro41 Fat-free formula or fat-free human milk and dairy-free alternative may be used to support recovery from chylothorax and medical necrotising enterocolitis, respectively, after a period of gut rest or nothing by mouth. Reference Neumann, Springer, Nieschke, Kostelka and Dähnert42 Feeding advancement algorithms to methodically increase volume can improve intestinal comfort and decrease time to full enteral feeds. Reference Furlong-Dillard, Neary and Marietta35
The way an infant’s nutrition is advanced post-operatively can affect intestinal comfort and tolerance. As tube feeding is advanced and the amount of food is increased over time, the infant’s response to the nutrition impacts oral feeding readiness. If the infant has poor tolerance for tube feeding this can negatively impact the infants’ oral feeding cues and delay eating by mouth. Signs that infants are not tolerating tube feeding include gagging/retching, increased fussiness, frequent emesis, bilious emesis, increased abdominal girth, and poor tolerance to oral stimulation such as taking pacifier or touch near the mouth, bloody stool, high stool output, and overall discomfort. The medical team, including the dietitian and feeding therapist, should work together to promote adequate nutrition, intestinal comfort with tube feedings, positive oral motor experiences, and creation of a plan to support oral feeding. The dietitian’s role perioperatively is crucial to ensure the infant receives adequate nutrition to recover and grow Reference McHoney, Eaton and Pierro41,Reference Tsintoni, Dimitriou and Karatza43 in order to demonstrate sufficient endurance for oral feeding trials.
Bottle feeding
Delayed oral motor skill and coordination contributes to oral feeding challenges in infants with complex CHD due to respiratory, neurological and gastrointestinal comorbidities. Reference Jones, Desai and Fogel4
Assessment
There is currently no standardised oral feeding assessment to evaluate the bottle-feeding abilities of infants with complex CHD. As a result, clinicians must rely on other evaluation tools which have been primarily based on data collected in the preterm population. The Early Feeding Skills Assessment Reference Thoyre, Shaker and Pridham44 , the Neonatal Oral Motor Assessment Scale Reference Palmer, Crawley and Blanco45 , the Neonatal Eating Assessment Tool – Bottle Feeding Reference Pados, Thoyre, Estrem, Park and McComish46, and the Neonatal Eating Outcome Assessment Reference Pineda47 have all been developed for clinical use in the preterm population. The Early Feeding Skills takes a holistic approach to feeding, assessing behavioural state, feeding readiness, muscle tone, energy level, behavioural stress signs, swallowing, physiologic stability, and oral motor function. Reference Pados, Park, Estrem and Awotwi48 It has acceptable internal consistency reliability and construct validity and is available for clinicians from the first author. Reference Pados, Estrem, Thoyre, Park and McComish49 The Neonatal Oral Motor Assessment Scale focuses primarily on oral motor skills for sucking, with two test questions regarding fatigue and suck/swallow/breathe incoordination. Reference Pados, Park, Estrem and Awotwi48 It can be used for either bottle or breastfeeding and has been used in many other research studies to quantify an infant’s oral motor skills. Reference Howe, Lin, Fu, Su and Hsieh50,Reference Bickell, Barton, Dow and Fucile51 The Neonatal Eating Assessment Tool – Bottle Feeding is a valid and reliable parent report measure to assess infant bottle-feeding skills based on scores on five subscales looking at gastrointestinal tract function, infant regulation, energy and physiological stability, sensory responsiveness, and symptoms of problematic feeding. Reference Pados, Thoyre, Estrem, Park and McComish46 The Neonatal Eating Outcome assessment is also a standardised assessment of feeding skills for premature infants which can be used to identify feeding difficulties and provide appropriate interventions. Bickell et al. 2018 reviewed the Oral Feeding Scale Reference Lau and Smith52 , which provides objective measure of skill and endurance, but does not assist in identifying oral motor dysfunction which may impact oral feeding. Reference Bickell, Barton, Dow and Fucile51,Reference Lau and Smith52 Ehrmann et al. 2017 described a standardised feeding readiness assessment, adapted from a previously validated guideline, systematically measured feeding readiness in eight stages ranging from pre-feeding skills to nutritive sucking to help predict the need for enteral tube feeds. Reference Ehrmann, Mulvahill and Harendt53 While these assessment tools will allow a clinician to look at feeding skills holistically, they do not take into consideration the complexity of post-operative recovery of the infant with complex CHD.
Recommendations
When facilitating feeding skills in children with complex CHD, maintaining cardiorespiratory stability and safe oropharyngeal swallowing function should be considered. Positioning an infant in side-lying has been used to support less variation of oxygen saturations during feedings, increased saturations in the middle of feedings, and respiratory rates closer to baseline compared to infants held in a supine or semi-upright position. Reference Park, Pados and Thoyre54 The elevated side-lying position may improve oral transit control by optimising slower bolus flow through reduction of hydrostatic pressure in the bottle. This position can also promote improved chest wall movement and airway patency by decreasing the gravitational effects on rib cage expansion. The use of side-lying position and co-regulated pacing also attempts to mimic the physiologic norm of breastfeeding. Reference Park, Pados and Thoyre54,Reference Thoyre, Park, Pados and Hubbard55
Another common feeding technique to promote physiologic stability is co-regulated paced bottle feeding Reference Thoyre, Park, Pados and Hubbard55 which includes attention to the number of suck/swallow combinations before a breath and physiologic stability. Implemented rest breaks periodically during a feeding may improve energy level and breathing rate, but this has not been directly researched in children with complex CHD. In addition, changing the bottle nipple to alter the rate of milk flow is a strategy that has been shown to improve physiologic stability during bottle feeding in term and preterm infants. Reference Daley and Kennedy56,Reference Pados, Park, Thoyre, Estrem and Nix57 Faster flow rate nipples lead to larger bolus size, requiring the infant to compensate by increasing frequency of swallowing or holding their breath for a longer duration (in order to swallow a larger bolus), which can result in decreased ventilation. Reference al-Sayed, Schrank and Thach58 While typically developing infants maintain adequate oxygenation by altering their sucking pattern in response to milk flow, it is unclear if infants with complex CHD are as capable. Reference Pados, Park and Dodrill59 The use of slower flow nipples may allow the infant to have more opportunities to breathe, maintain physiologic stability, and feed more efficiently.
Breastfeeding
Breastfeeding is often challenging for infants with complex CHD due to initial separation of infant from mother for surgery, lack of privacy in the ICU, inability to measure volumes and belief of breastfeeding being more difficult than bottle feeds. Reference Jones, Desai and Fogel4 Additionally, there is poor consensus regarding clinical practice to support breastfeeding in this population. Reference Elgersma, McKechnie, Gallagher, Trebilcock, Pridham and Spatz60
Assessment
Assessing and supporting breastfeeding perioperatively should be standard for infants with complex CHD if possible. Unfortunately, there are no formal breastfeeding assessments specific to infants with complex CHD. The Bristol breastfeeding assessment tool was developed for healthy full-term infants to increase efficacy and maternal self-confidence. Reference Ingram, Johnson, Copeland, Churchill and Taylor61 The LATCH is a breastfeeding charting system consisting of a five-item assessment of the following: latch, audible swallowing, type of nipple, comfort of breast and holding. Reference Jensen, Wallace and Kelsay62 It was developed by experts but does not have a target population, formal content validity, or consistency between raters. Reference Pados, Park, Estrem and Awotwi48 Studies comparing healthy newborns and predicting long-term feeding success have revealed that the higher the LATCH score, the more likely the infant will be breastfeeding at 6 weeks postpartum. Reference Sowjanya and Venugopalan63 The Neonatal Eating Assessment Tool -Mixed Feeding is a parent report of 68 measures of breast and bottle-feeding behaviour for infants less than 7 months and demonstrates validity and reliability to identify infants with problematic feeding in order to intervene appropriately. Reference Pados, Thoyre and Galer64 Informal assessments of breastfeeding can be completed by lactation specialists and feeding therapists with a focus on transitioning the infant to the breast as soon as possible. Providing supports such as best positioning, assessing, and assisting with the infant’s latch, monitoring swallowing safety, pacing at the breast or pre-pumping through the mother’s let down and offering devices such as nipple shields are beneficial to assist with breastfeeding goals.
Recommendations
Interventions to support the breastfeeding infant with complex CHD vary across the nation. Breastfeeding is an established safe practice for infants with complex CHD, with the benefits of human milk and supporting the mother-child dyad well documented. Reference Davis and Spatz65 The literature supports the potential for improved physiologic stability and oxygenation in breastfeeding compared to bottle feeding. Reference Combs and Marino66 Contributing factors include the opportunity for natural positioning (e.g., cross cradle hold), independent control of flow rate, benefits of skin-to-skin, and natural rest breaks between letdowns which could improve minute ventilation. Reference Goldfield, Richardson, Lee and Margetts67
Supporting early and consistent development of this unique motor planning and programming at the breast is essential for establishment of the least restrictive feeding plan for children with complex CHD. Reference Goldfield, Richardson, Lee and Margetts67,Reference Medoff-Cooper, Shults and Kaplan68 Studies have demonstrated improved timing to full breastfeeding with the simple introduction of skin-to-skin in typically developing infants and in infants with complex CHD. Reference Sharma69–Reference Lisanti, Demianczyk and Costarino71 Skin-to-skin can safely be offered perioperatively with the adoption of ICU holding protocols. Reference Lisanti, Demianczyk and Costarino71 Additionally, supporting the breastfeeding mother during the tumultuous perioperative period with easy access to hospital-grade breast pumps, preferably in the patient’s room to facilitate skin-to-skin or non-nutritive breastfeeding after pumping. Other supports include the use of pasteurised donor milk to support transitions to the mother’s full human milk supply, and hospital supported milk centres to ensure safe management of human milk. Recommendations include early and frequent lactation support during the time breastmilk supply is established, ensuring the mother is receiving adequate nutrition and hydration (some centres provided a meal daily for breastfeeding mothers) and assuring mothers have a hospital-grade pump at discharge. Reference Davis and Spatz65
Coordinated sucking on a pacifier and holding along with pacifier use during tube feeding has demonstrated faster transition to breastfeeding in preterm infants Reference Kaya and Aytekin72 and a reduction in the duration of hospital stay. Reference Say, Simsek, Canpolat and Oguz73
Dysphagia
Perinatal events and medical or surgical interventions may impact neurosensory and neuromotor pathways, leading to maladaptive oral feeding patterns and a diagnosis of oropharyngeal dysphagia or swallow dysfunction. Reference Jadcherla, Khot, Moore, Malkar, Gulati and Slaughter74 Infants receiving a stage 1 palliation and aortic arch reconstruction are at high risk for recurrent laryngeal nerve injury, with 48–59% resulting in vocal cord paresis/paralysis and subsequent dysphagia. Reference Pham, Connelly, Wei, Sykes and O’Brien75–Reference Benjamin, Smith, Cotten, Jaggers, Goldstein and Malcolm79 Though the presence of vocal fold paresis increases risk of dysphagia, studies describe higher incidence of dysphagia in children born with complex CHD even without vocal fold paresis. Reference Pham, Connelly, Wei, Sykes and O’Brien80,Reference McGrattan, McGhee and DeToma81 For note, the majority of infants with complex CHD and aspiration have normal vocal fold function and are asymptomatic. Reference Raulston, Smood and Moellinger82 Prematurity is an added risk factor for dysphagia for infants with complex CHD. Reference Karsch, Irving, Aylward and Mahle83
Assessment
The Videofluoroscopic Swallow Study [also referred to as the Modified Barium Swallow Study] and Fiberoptic Endoscopic Evaluation of Swallow are considered the gold standards in the evaluation of the swallow mechanism, with known benefits and limitations to assist with clinical decision-making. Both are instrumental evaluations which assess the anatomical and physiologic characteristics of the oropharyngeal swallow to determine pathology that contributes towards swallowing disorders and ultimately airway threat from a bolus. The Videofluoroscopic Swallow Study allows for observation of the oral, pharyngeal, and cervical oesophageal phases of the swallow. Reference Arvedson84 It is performed by a feeding therapist and radiologist to allow for implementation of feeding strategies such as nipple flow modification, external pacing, thickening, and positional change that may be useful in improving swallowing function and decreasing risk for aspiration. Fiberoptic Endoscopic Evaluation of Swallow is usually performed by a speech pathologist in collaboration with an otolaryngologist to directly visualise the nasal, pharyngeal and laryngeal structures during swallowing. Fiberoptic Endoscopic Evaluation of Swallow may be performed on an infant while breastfeeding and can be completed at bedside for infants in the intensive care unit. Reference Schroeder, Willette and Molinaro85
Recommendations
Alterations to liquid viscosity is a treatment method in the management of infant pharyngeal phase dysphagia. Reference Duncan, Larson and Rosen86,Reference Dion, Duivestein, St Pierre and Harris87 Although not completely understood, it is thought that the sensory input from the thickened liquids impacts timing of oral and pharyngeal transfer of the bolus, duration of upper oesophageal sphincter opening as well as magnitude of hyoid and laryngeal vestibule movements. Reference Steele, Alsanei and Ayanikalath88–Reference Newman, Vilardell, Clavé and Speyer90 Thickening infant formula and breastmilk does not come without controversial clinical application and should not be the first intervention used for treatment of pharyngeal phase dysphagia. McGratten et al 2017 studied dysphagia in infants following stage 1 surgical palliation revealing that nectar thick barium, compared to thin barium contrast, allowed significantly more infants to swallow without aspiration. Reference McGrattan, McGhee and DeToma81 In this same study, an increase in viscosity to nectar thick resulted in significant extraction challenges such as increased number of sucks to form a bolus. In addition, increased viscosity may not be a viable solution for infants with complex CHD who aspirate due to energy expenditure and continued aspiration risk. Reference McGrattan, McGhee and DeToma81 Large quantities of starch-based thickeners may negatively impact the immature and maladaptive infant gut resulting in malabsorption, necrotising enterocolitis, shift of macronutrient composition and constipation. Concerns also include arsenic exposure with use of rice cereal to thicken and reduction or discontinuation of human milk due to viscosity maintenance challenges. Reference Almeida, Almeida, Moreira and Novak91–Reference McCallum93 Extended use of thickened liquids may contribute to atypical motor planning of swallow, lending to challenges with weaning to thin liquids when indicated. Reference Wolter, Hernandez and Irace94 Unfortunately, without direction, many clinicians caring for infants with complex CHD have little to no guidance of thresholds for introduction of thickened liquids as a dysphagia treatment for this vulnerable population with variability of clinical practices. Reference Madhoun, Siler-Wurst, Sitaram and Jadcherla95 Support from a skilled feeding therapist is recommended in determination of thickening liquids to manage dysphagia.
The infants’ safe swallowing impacts oral acceptance and protects positive oral stimulation for long-term oral feeding. Timing of long-term supplemental nutrition via gastrostomy tube and the influence on promoting or hindering oral feeding development should be considered individually and addressed globally for our infants with complex CHD.
Neurodevelopment
Neurodevelopmental abnormalities are common and documented in greater than 50% of newborns with complex CHD before surgery with abnormality persisting after surgery. Reference Limperopoulos, Majnemer, Shevell, Rosenblatt, Rohlicek and Tchervenkov96–Reference Butler, Sadhwani and Rofeberg100 These challenges can affect the infant’s ability to develop higher level motor and cognitive skills for safe and efficient oral feeding. Neurodevelopmental testing on newborns has demonstrated poor state regulation and decreased motor skill development, including oral motor skills. Reference Donofrio and Massaro98,Reference Butler, Sadhwani and Stopp99 Stability in autonomic state, sensorimotor system, and behavioural state are critical for the complex process of coordinating sucking, swallowing, and breathing. Unfortunately, decreased attention is often a concern for infants with complex CHD and is associated with poorer oral feeding outcomes. Reference Gakenheimer-Smith, Glotzbach and Ou101 In addition, behavioural state regulation to transition from sleep to alert state is necessary to interact with the environment and take adequate nutrition for growth. Reference Desai and Lim102
Assessment
Several infant measurements are helpful to review infant neurodevelopmental strength and weakness. The developmental assessment measures which are based on the Brazelton Neonatal Behavioral Assessment Scale Reference Brazelton, Nugent, Lester and Osofsky103 such as the Newborn Behavioral Observations, Reference Nugent, Keefer, Minear, Johnson and Blanchard104 Assessment of Preterm Infants’ Behavior, Reference Als, Lester, Tronick and Brazelton105 and NICU Network Neurobehavioral Scale, Reference Lester, Tronick and Brazelton106 all measure infant state, motor skills, autonomic stability, self-regulation, and social interaction. The NICU Network Neurobehavioral Scale-II has been used most often on research of infants with complex CHD and examines both neurologic integrity as well as behavioural functioning and scores a full range of infant neurodevelopmental performance that was intended to have broad applicability for detecting at-risk infants and has been correlated with infant feeding. Reference Butler, Sadhwani and Stopp99,Reference Gakenheimer-Smith, Glotzbach and Ou101 The Newborn Behavioral Observations system has also been used in children with complex CHD and is an infant-focused, family centred, relationship-based tool, designed to sensitise parents to their infant’s competencies and individuality to foster positive parent-infant interactions and provide early support to the relationship. Reference Butler, Sadhwani and Stopp99 The Newborn Behavioral Observations itself is not an assessment but is a set of shared observations designed to help the clinician and parent to collaborate, observe the infant’s behavioural capacities and identify the best support for successful growth, oral feeding and overall development. Reference Nugent, Keefer, Minear, Johnson and Blanchard104
Recommendations
The Supporting of Oral Feeding in Fragile Infants method is an evidence-based strategy which has been used with preterm infants to assess and reassess infant cues and use neurodevelopmental strategies to respond to the cues. Reference Ross and Philbin107 Auditory, Tactile, Visual, and Vestibular is a multisensory intervention that has been found to improve sucking organisation and pressure in preterm infants by facilitating behavioural organisation and alert state. Reference Medoff-Cooper, Rankin, Li, Liu and White-Traut108,Reference White-Traut, Nelson and Silvestri109 Sensory strategies are utilised to decrease infant stress signs and promote behavioural subsystem stability to achieve oral feeding readiness. Recognition of infant stress signs and use of neurodevelopmental strategies such as decreasing the challenge, providing external postural support via swaddling, reducing environmental stimulation, and providing gentle touch can facilitate a more organised response to the feeding intervention. Reference Desai and Lim102,Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler110
In addition, the Newborn Individualized Developmental Care and Assessment Program is an intervention which is theory-based and supported by scientific evidence. Reference Als111 The model focuses on detailed reading of each individual infant’s behavioural cues. These cues dictate the environmental and care adaptations that are required to support and enhance each infant’s strengths and self-regulation capacities. Newborn Individualized Developmental Care and Assessment Program has been shown to improve outcomes for infants including decreased hospital stay, decreased days on tube feeding and increased weight gain in preterm infants and is recommended in infants with CHD. Reference Medoff-Cooper, Rankin, Li, Liu and White-Traut108,Reference Als, Duffy and McAnulty112–Reference Butler, Huyler, Kaza and Rachwal113
Multidisciplinary team
It is important to foster and value a team approach to feeding safety and skill development. Collaboration among feeding therapists, bedside nurses, developmental therapists, families, and physicians/advanced practice providers is necessary for successful feeding of newborns and infants with complex CHD. Each team member has unique and critical insight regarding how best to optimise the feeding approach. This begins with formal and informal discussions of patient-specific goals for growth and nutrition based on cardiopulmonary status, which then guide establishment of a feeding plan post-clinical assessment. The bedside nurse plays a key role in gathering relevant information that guides the daily medical plan and implementation of feeding practice. However, advanced paediatric feeding assessment, pre-feeding interventions, neurodevelopmental support, and modulation of therapeutic techniques are not taught in undergraduate nursing programmes. A structured support system for bedside nurses to advance their knowledge of common feeding strategies is recommended to carry over targeted feeding behaviours and skills throughout the day. Educational strategies can be implemented via in-services, hands-on skills labs, shadowing of feeding therapists, and most importantly demonstration and discussion when feeding plans are being established and implemented at bedside. Feeding therapists and bedside nurses can also educate and train parents to implement strategies to be able to feed their child safely and successfully during hospital admission. Automatic order sets are helpful to allow for immediate pre-feeding and feeding support with referrals to feeding therapist along with supports for overall neurodevelopment through physical therapy, occupational therapy, child life, and neurodevelopmental specialists.
Family support
Lisanti and colleagues (2017) examined maternal stress and anxiety in mothers of infants in the paediatric cardiac intensive care unit and found the greatest maternal stressors to be infants’ appearance and behaviour, followed by parental role alteration. Reference Lisanti, Allen, Kelly and Medoff-Cooper114 Supporting families to re-establish their roles as a primary caretaker and decision-makers can be implemented as early as the first day of life for a baby who is intubated through education and information sharing. Reference Gramszlo, Karpyn and Demianczyk115
Options for outpatient therapy after discharge
Infants with complex CHD discharged with a feeding tube or with other feeding challenges require skilled feeding therapy after discharge. The American Heart Association recommends early intervention for all infants with complex CHD upon discharge from primary interventions, which could include feeding therapy. Reference Marino, Lipkin and Newburger116 Utilisation and timing of services varies by location in the United States. Often infants can receive feeding therapy through their local Early Intervention programme, through private feeding therapists in the community or through a hospital-based feeding programme. Coordinating feeding therapy can be difficult to navigate, especially when an infant’s surgical intervention is in a different state than their residence. The local paediatrician, cardiologist, and hospital case managers can help support referral to feeding therapy close to home. Recently, cardiac-specific feeding clinics have been developed to support the transition from discharging with a feeding tube and tube weaning. Cardiac feeding clinics can be provided in conjunction with follow-up cardiac neurodevelopmental programmes or cardiology medical visits.
Weaning from the feeding tube typically happens post-discharge home and can be especially challenging for children with complex CHD. Often providers of children with complex CHD are concerned about weight gain and growth and less likely to agree to decreasing calorie intake through tube usage while working on increasing hunger and encouraging oral feeding. A systematic review and meta-analysis of weaning programmes for toddlers ages 15–48 months (27% with cardio/pulmonary medical concerns), including intensive day treatment and/or inpatient hospital programmes, reported that dependence on tube feeding was eliminated in 71% of children at discharge from the feeding programme. When follow-up data was provided at an average of 9 months post-treatment, 80% of these patients were able to maintain independence from tube feedings after the formal intervention. Reference Sharp, Volkert, Scahill, McCracken and McElhanon117 Key components for successful tube weaning in the toddler population included utilisation of a multidisciplinary team, hunger provocation, behavioural interventions, positive mealtimes and oral experiences, and caregiver involvement. Reference Slater, Spader, Fridgen, Horsley, Davis and Griffin118 Tube weaning duration is variable depending on the treatment approach and intensity, taking between three weeks up to four months. Reference Taylor, Purdy, Jackson, Phillips and Virues-Ortega119
Conclusions
Treatment and management of oral feeding challenges in the pre- and post-operative period in the infant with complex CHD requires attention to multisystem factors which all need to interact together to develop and maintain safe and efficient oral feeding and swallowing skills. Clinicians need to be skilled at evaluating and analysing the systems which impact oral feeding such as respiratory, neurological, and gastrointestinal. Use of appropriate feeding strategies can facilitate improved respiration, endurance and swallow safety during bottle or breastfeeding. In addition to aiding the infant during the hospitalisation, the family also should be supported to reduce stress and re-establish their role as a parent in the care of their child. The family and the infant benefit from continued support after discharging from the hospital through outpatient therapy services and nutrition guidance to achieve oral feeding goals (Table 1).
Table 1. Summary of feeding assessments and management recommendations

Despite the considerable evidence for feeding challenges in infants with CHD, specific assessment tools and intervention strategies which consider their unique post-surgical sequelae have not yet been established. Current clinical practice to improve oral feeding difficulties in this population applies knowledge based on neonatal literature. However, we are uncertain if these practices are effective for infants with CHD, as there are significant gaps in the research examining assessment and intervention strategies. Additional studies exploring oral feeding with non-invasive respiratory support, gastrointestinal complications and effect on oral feeding development and feeding outcomes with use of common therapeutic feeding strategies in infants with CHD are some examples of future research that is necessary to facilitate treatment and management of the complex feeding difficulties in this population.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.





