Introduction
Paediatric cholesteatoma is thought to be more aggressive than adult cholesteatoma because of rapid growth and expansion.Reference Palva, Karma and Kärjä1 It is associated with higher rates of ossicular erosion and is more likely to present at a higher stage of disease than adult cholesteatoma.Reference Fontes Lima, Carvalho Moreira, Sousa Menezes, Esteves Costa, Azevedo and Sá Breda2 The rate of recidivism has been found to be more than twice as high in children as in adults.Reference Møller, Pedersen, Grosfjeld, Faber and Djurhuus3
The optimal surgical approach to paediatric cholesteatoma is a topic of debate.Reference Dornhoffer, Friedman and Gluth4 In an effort to reduce the morbidity associated with an unstable mastoid cavity, canal wall up tympanomastoidectomy has gained popularity.Reference Dornhoffer, Friedman and Gluth4 However, planned second-look surgical procedures are often necessitated by this technique because of difficulty accessing disease within the sinus tympani and epitympanum during primary surgery.Reference Schraff and Strasnick5 Because of the risk of recidivism with paediatric cholesteatoma, planned second-look procedures can also be recommended for the canal wall down approach, particularly where there is concern regarding residual disease at primary surgery.Reference Dornhoffer, Friedman and Gluth4
Diffusion-weighted magnetic resonance imaging (MRI) has been utilised since the early 2000s to demonstrate cholesteatoma of the temporal bone, as it has greater specificity than conventional MRI.Reference Fitzek, Mewes, Fitzek, Mentzel, Hunsche and Stoeter6 Cholesteatoma displays a bright signal on diffusion-weighted MRI, corresponding to restricted water diffusion.Reference Fitzek, Mewes, Fitzek, Mentzel, Hunsche and Stoeter6 Non-echoplanar diffusion-weighted MRI has been reported to be superior to the older echoplanar diffusion-weighted MRI for detecting cholesteatoma because of higher sensitivity and specificity, as a function of reduced magnetic susceptibility artefacts and better spatial resolution.Reference Sharifian, Taheri, Borghei, Shakiba, Jalali and Roshanfekr7
Based on recent evidence, our local practice has adapted to include non-echoplanar diffusion-weighted MRI, both for the diagnosis of residual and recurrent disease post-operatively from canal wall up and canal wall down procedures and as a decision-making tool for planning further surgery. We report our experience with this method.
Materials and methods
Patients
A retrospective review was carried out for all non-echoplanar diffusion-weighted MRI scans performed to detect residual disease or recurrence after surgery for cholesteatoma in children. Diffusion-weighted MRI scans were conducted at our tertiary care paediatric centre, over a 5-year and 11-month period, from 1 January 2012 to 30 November 2017. Follow-up MRI scans were retrieved to 16 August 2019. Children were defined as those aged 16 years or younger. Children had undergone primary surgery for congenital or acquired cholesteatoma.
A clinical records review was carried out. The following data were extracted: date and type of initial surgery (transcanal tympanoplasty, canal wall up or canal wall down tympanomastoidectomy); symptoms at time of scanning; date of second-look surgery if conducted; and presence or absence of recurrence at surgery. Site and size of recurrence were recorded if detailed at second-look surgery. At out-patient follow up, audiogram (stability of hearing level) results, examination findings, symptoms and follow-up duration were also recorded if patients did not undergo second-look surgery. All revision surgery was conducted by, or performed under the direct supervision of, a consultant surgeon with subspecialisation in otology.
Imaging
Within our department, non-echoplanar diffusion-weighted MRI is routinely conducted at around 18 months after surgery, to check for the presence or absence of recurrence, and to aid decision-making for second-look surgery. Non-echoplanar diffusion-weighted MRI is also utilised to aid decision-making should there be clinical suspicion of recurrence at other time points. The MRI scanner used was an Avanto 1.5 T (Siemens, Erlangen, Germany). The protocols used during this time period are shown in Table 1.
Table 1. MRI parameters*

* Magnetic resonance imaging (MRI) parameters for diagnosis of post-operative cholesteatoma in our institution between 1 January 2012 and 30 November 2017. The MRI scanner used was an Avanto 1.5 T (Siemens, Erlangen, Germany). N/A = not applicable; CISS = constructive interference in steady state; HASTE = half-Fourier acquisition single-shot turbo spin-echo imaging; EPI = echoplanar imaging
Image review
All images were reviewed by a consultant radiologist experienced in paediatric ENT radiology. This radiologist was blinded as to the clinical details and operative outcome of second-look surgery. Diffusion-weighted MRI scans were classified into a qualitative scale regarding the presence of cholesteatoma, as previously described.Reference Lingam, Khatri, Hughes and Singh8 This classification system is: (1) definite absence of cholesteatoma; (2) probable absence of cholesteatoma; (3) possible cholesteatoma; (4) probable cholesteatoma; and (5) definite cholesteatoma. For the purposes of sensitivity and specificity analysis, ‘1’ and ‘2’ were defined as negative report findings, and ‘3’, ‘4’ and ‘5’ were defined as positive report findings.
Statistical review
Sensitivity, specificity, and positive and negative predictive values were calculated for the presence of cholesteatoma on diffusion-weighted MRI. As a secondary analysis, the sensitivity of the use of symptoms alone as a predictor of cholesteatoma recurrence was calculated.
Results
Thirty-nine diffusion-weighted MRI scans were carried out (Figure 1). Four of these were echoplanar diffusion-weighted MRI and were therefore excluded from this study. One diffusion-weighted MRI scan was conducted and then repeated a year later on the same patient without any intervening surgery; the initial diffusion-weighted MRI was excluded. Thirty-four diffusion-weighted MRI scans were therefore included in this study, belonging to 29 patients. Five patients had two diffusion-weighted MRI scans each included within this study, both of the same ear. For these five patients, revision surgery was carried out between the initial and second diffusion-weighted MRI scans, with the second diffusion-weighted MRI being conducted to identify the presence of a recurrence after revision surgery.

Fig. 1. Flow chart of study inclusion, outcomes after radiology reporting, and surgical and clinical follow up. dwMRI = diffusion-weighted magnetic resonance imaging
Demographics
The mean age of patients at diffusion-weighted MRI was 12 years (standard deviation (SD) = 3) (range, 6–16 years). The mean time between initial surgery and diffusion-weighted MRI was 25 months (SD = 17) (range, 5–67 months). The mean time between diffusion-weighted MRI and revision surgery was nine months (SD = 10) (range = 1–35 months). Regarding the initial surgery before diffusion-weighted MRI, 13 cases were canal wall up and 21 were canal wall down tympanomastoidectomy. Nine of these initial surgical procedures were revisions. The overall rate of cholesteatoma at second-look surgery was 47 per cent (16 of 34 cases).
Positive imaging findings
Eighteen diffusion-weighted MRI scans were reported positive for cholesteatoma (Figure 1). In six cases, patients had diffusion-weighted MRI over three years after surgery because of clinical suspicion of cholesteatoma based on a history of chronic discharge. For the remaining 12 cases, diffusion-weighted MRI was carried out less than two years post-operatively as part of the post-operative follow-up protocol within our unit. Of these 18 diffusion-weighted MRI scans, 13 were true positives and 5 were false positives based on second-look surgery (Table 2, Figure 2).
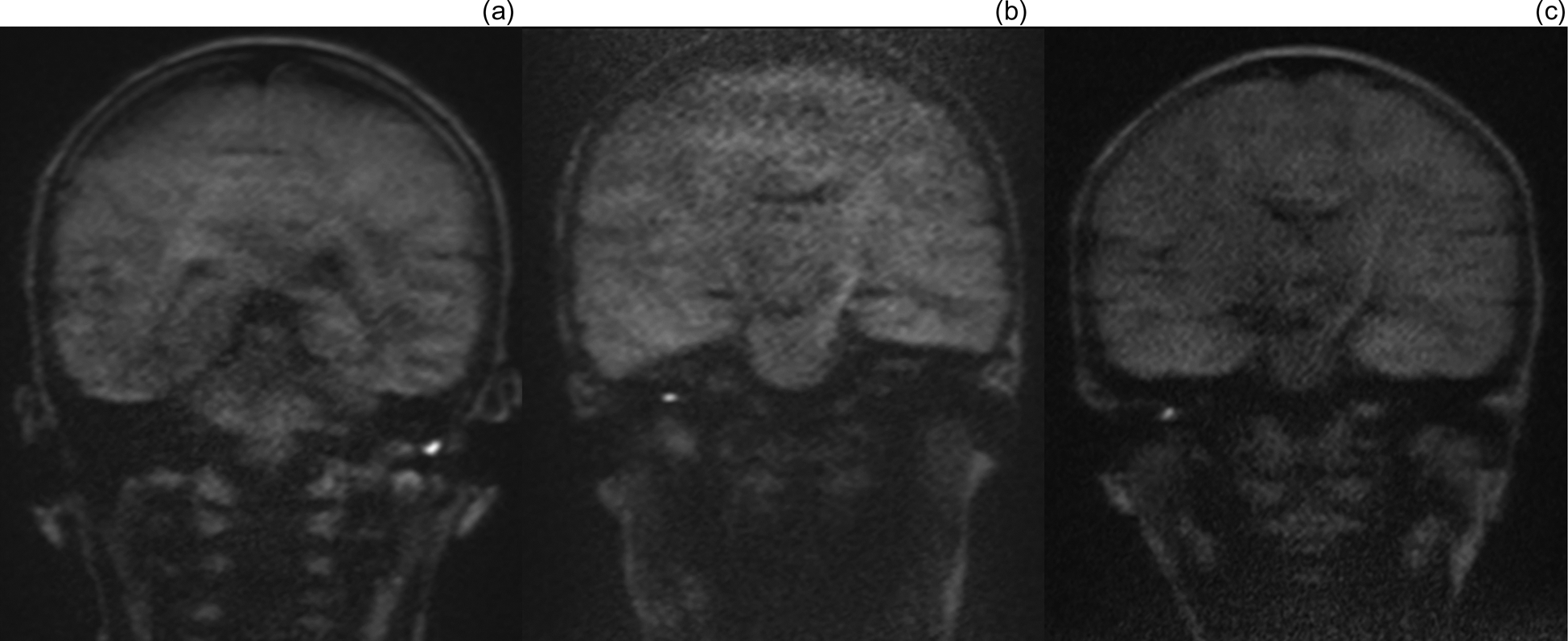
Fig. 2. (a–c) Non-echoplanar diffusion-weighted magnetic resonance imaging coronal scans of false positive cases, demonstrating small areas of avidity, scored on imaging review as: (a) 5 (definite cholesteatoma), (b) 4 (probable cholesteatoma) and (c) 4 (probable cholesteatoma). Operative findings at second-look surgery were of: (a) cartilage reconstruction and cerumen, (b) mucoid discharge and cartilage reconstruction, and (c) adhesions and retained debris in retraction pocket. All were negative for cholesteatoma at second-look surgery.
Table 2. False positives*

* False positives are defined as post-operative diffusion-weighted magnetic resonance imaging (dwMRI) findings reported as positive but with negative findings at second-look surgery. †1 = definite absence of cholesteatoma; 2 = probable absence of cholesteatoma; 3 = possible cholesteatoma; 4 = probable cholesteatoma; 5 = definite cholesteatoma. CWU = canal wall up; CWD = canal wall down
Negative imaging findings
Sixteen diffusion-weighted MRI scans were reported as negative for cholesteatoma (Figure 1). Four of these were surgically confirmed as negative. Despite negative diffusion-weighted MRI findings, three of these operations were carried out based on clinical suspicion of cholesteatoma secondary to chronic discharge, and the other was conducted based on the examination finding of a bulging tympanic membrane, suspicious for cholesteatoma. Nine patients were not operated upon, based on the negative diffusion-weighted MRI result, and were followed up in the out-patient setting without evidence of recurrence. Mean follow-up time was 48 months post surgery (SD = 11.6) (range, 27–65 months). Three patients were surgically confirmed as positive for recurrence despite a negative diffusion-weighted MRI (Figure 1, Table 3). Again, these patients were operated upon based on clinical suspicion of recurrence (Table 3).
Table 3. False negatives*

* False negatives are defined as post-operative diffusion-weighted magnetic resonance imaging (dwMRI) findings reported as negative but with positive findings at second-look surgery. †1 = definite absence of cholesteatoma; 2 = probable absence of cholesteatoma; 3 = possible cholesteatoma; 4 = possible cholesteatoma; 5 = definite cholesteatoma. CWU = canal wall up; CWD = canal wall down
Overall findings
Overall, the sensitivity and specificity values of diffusion-weighted MRI for detecting post-operative cholesteatoma were 81 per cent and 72 per cent, respectively. Positive predictive and negative predictive values were 72 per cent and 81 per cent, respectively.
Presence of symptoms
As a secondary analysis, the presence of symptoms raising the clinical suspicion of recurrence was also studied as an independent predictor. These symptoms were: otorrhoea (16 cases), unexplained otalgia (1 case) and otoscopy findings of possible cholesteatoma (3 cases). The sensitivity and specificity values of symptoms alone for detecting the presence of post-operative cholesteatoma were 69 per cent and 50 per cent, respectively; the positive predictive and negative predictive values were 55 per cent and 64 per cent, respectively.
Discussion
Overall results
This study aimed to evaluate the performance of non-echoplanar diffusion-weighted MRI for detecting cholesteatoma in our paediatric population. In this retrospective review, non-echoplanar diffusion-weighted MRI was found to have a sensitivity of 81 per cent, specificity of 72 per cent, positive predictive value of 72 per cent and negative predictive value of 81 per cent for predicting residual or recurrent cholesteatoma.
Comparison with literature
A recent meta-analysis of 8 studies with 117 patients reported that non-echoplanar diffusion-weighted MRI in a paediatric population had a pooled sensitivity of 89.4 per cent (95 per cent confidence interval (CI) = 51.9–98.5 per cent) and specificity of 92.9 per cent (95 per cent CI = 81.4–97.5 per cent).Reference Bazzi, Wong, Jufas and Patel9 Of these 8 studies, only 5 included over 10 paediatric patients within the series.Reference Nash, Wong, Kalan, Lingam and Singh10–Reference Lecler, Lenoir, Peron, Denoyelle, Garabedian and le Pointe14
The result of a UK study, and the largest series studying diffusion-weighted MRI in the paediatric population, demonstrated a sensitivity of 97 per cent and a specificity of 95 per cent for residual disease or recurrence for non-echoplanar diffusion-weighted MRI in their paediatric population of 54 patients undergoing second-look surgery.Reference Nash, Wong, Kalan, Lingam and Singh10 Both the sensitivity and the specificity values are greater than those reported in our centre. The recurrence rate of cholesteatoma was 61 per cent within the group who underwent second-look procedures in this population.Reference Nash, Wong, Kalan, Lingam and Singh10 The authors took the decision to omit 36 patients who did not undergo second-look surgery from their analysis of sensitivity and specificity, opening their results to verification bias, particularly as selection criteria for patients undergoing second-look surgery were not described.Reference Nash, Wong, Kalan, Lingam and Singh10,Reference Weinstein, Obuchowski and Lieber15 We took the decision to include patients who did not undergo second-look surgery in order to reduce this bias, perhaps accounting for our diffusion-weighted MRI not performing as well diagnostically in our population.Reference Weinstein, Obuchowski and Lieber15
Excellent results were also found by Rajan et al.; however, the prevalence of disease at second-look surgery was 13 per cent (2 of 15 cases), which may be a function of conducting this surgery within six months of primary surgery.Reference Rajan, Ambett, Wun, Dhepnorrarat, Kuthubutheen and Chow11 In comparison, our recurrence or residual disease rate was 47 per cent (16 of 34 cases). This aspect makes comparisons difficult.
Our results agree more closely with those of Geoffray et al.,Reference Geoffray, Guesmi, Nebbia, Leloutre, Bailleux and Maschi12 who reported a sensitivity of 87 per cent and specificity of 71 per cent for diffusion-weighted MRI. Like our group, they did not routinely provide revision surgery for all patients, based on diffusion-weighted MRI results; they used follow up as an alternative, and included all patients in their analysis. Their mean time from diffusion-weighted MRI to surgery was 27 months, similar to our study.Reference Geoffray, Guesmi, Nebbia, Leloutre, Bailleux and Maschi12
Clinical implications: false positives
Our false positives were related to: use of cartilage as graft material, cerumen, fluid, adhesions and a debris-filled retraction pocket (Table 2). Additional studies in the paediatric population have demonstrated artefacts from dental braces,Reference Plouin-Gaudon, Bossard, Fuchsmann, Ayari-Khalfallah and Froehlich13 cholesterol granuloma,Reference Nash, Wong, Kalan, Lingam and Singh10 calcified cartilageReference Nash, Wong, Kalan, Lingam and Singh10 and scarring around a Silastic® elastomer prosthesis as causes for false positives.Reference Geoffray, Guesmi, Nebbia, Leloutre, Bailleux and Maschi12
A previous study of the paediatric population revealed a trend towards smaller areas of avidity where there were false positives.Reference Hervochon, Elmaleh-Berges, Francois, Marhic, Bahakim and Teissier16 In our study, small areas of avidity on diffusion-weighted MRI were also seen in our false positive group (Figure 2). One study, specifically focusing on false positives in the adult population, reported that in three of the four false positive cases, areas of high avidity disappeared on subsequent imaging.Reference Muhonen, Mahboubi, Moshtaghi, Sahyouni, Ghavami and Maducdoc17 They reported false positives to be related to cartilage grafts.Reference Muhonen, Mahboubi, Moshtaghi, Sahyouni, Ghavami and Maducdoc17
Within our study, one of the false positives was reported as ‘possible cholesteatoma’ and did not have symptoms at the time of diffusion-weighted MRI; this patient could possibly have been followed up with subsequent imaging. However, the other four patients were reported as ‘probable cholesteatoma’ or ‘definite cholesteatoma’, and three of the four had symptoms, making any decision not to operate challenging.
Clinical implications: false negatives
We had three cases of false negatives in our audit (Table 3). Of these, one was found to have a small pearl of cholesteatoma identified at surgery. It is known that small-volume cholesteatoma of less than 3 mm in size or small pearls of cholesteatoma are not well identified on diffusion-weighted MRI.Reference Lecler, Lenoir, Peron, Denoyelle, Garabedian and le Pointe14 The mean duration between operation and diffusion-weighted MRI was 25 months (SD = 17) (range, 5–67 months), perhaps accounting for our reasonably low proportion of false negatives and higher sensitivity than reported elsewhere, compared with studies where the time between second-look surgery and initial surgery was much shorter.Reference Plouin-Gaudon, Bossard, Fuchsmann, Ayari-Khalfallah and Froehlich13,Reference Lecler, Lenoir, Peron, Denoyelle, Garabedian and le Pointe14 Indeed, Lecler et al. found the median size of cholesteatoma to be 2 mm where second-look surgery was carried out within 12 months of initial surgery.Reference Lecler, Lenoir, Peron, Denoyelle, Garabedian and le Pointe14
• High rates of recidivism are reported after paediatric cholesteatoma surgery
• We use non-echoplanar diffusion-weighted magnetic resonance imaging (MRl) to diagnose residual or recurrent cholesteatoma; it is rapid and non-invasive
• There is no intravenous contrast or radiation exposure, and no risk of the operative morbidity associated with second-look surgery
• Non-echoplanar diffusion-weighted MRI had respective sensitivity, specificity and positive predictive values of 81, 72 and 72 per cent for predicting residual or recurrent cholesteatoma
• Diffusion-weighted MRI is a recommended replacement for routine second-look surgery in paediatrics
• However, given the negative predictive value of 81 per cent and the aggressive nature of paediatric cholesteatoma, all patients require close follow up
Interestingly, one of our false negative cases had extensive cholesteatoma discovered at second look surgery, 29 months after negative diffusion-weighted MRI; surgery had not been carried out initially, in light of these negative diffusion-weighted MRI findings. Rather than representing a true negative, it is feasible that cholesteatoma could have developed de novo post MRI. This underlines the aggressive nature of paediatric cholesteatoma, and demonstrates the importance of close follow up over time, even when diffusion-weighted MRI findings are negative.
Limitations
Our study has several limitations. Firstly, it was a retrospective case review; further studies would benefit from a prospective design. Secondly, patients for whom there was low clinical and radiological suspicion of cholesteatoma did not undergo second-look surgery (26 per cent; 9 of 34 cases), which is the ‘gold standard’ for identifying cholesteatoma. However, omitting those patients who were not undergoing surgery from the analysis would have opened the study to verification bias,Reference Weinstein, Obuchowski and Lieber15 and operating on all patients regardless of diffusion-weighted MRI result would have had ethical implications. Thirdly, our case numbers were limited. Our study included 34 non-echoplanar diffusion-weighted MRI scans from 29 patients. Although this is the second largest case series in the paediatric population of non-echoplanar diffusion-weighted MRI, there is a requirement for larger, prospectively designed studies. Fourthly, the time between diffusion-weighted MRI and surgery was often prolonged (mean of 9 months; SD = 10.0) (range, 1–35 months), meaning there was an opportunity for cholesteatoma to develop after a diffusion-weighted MRI scan with negative findings.
Conclusion
Diffusion-weighted MRI is a rapid and non-invasive investigation, without the requirement for intravenous contrast or radiation exposure, and does not carry the risk of operative morbidity. Based on the results of our study, we would recommend the use of diffusion-weighted MRI as a replacement for routine second-look procedures in the paediatric population. It has a higher sensitivity and specificity compared with symptoms alone. However, it must be noted that the negative predictive value is reported at 81 per cent; we therefore advise that all patients with negative diffusion-weighted MRI findings have close clinical follow up and that there is a low threshold for surgical intervention if there is clinical suspicion of recidivism. We report a greater number of false positives than previously reported in other series. However, it is difficult to justify a non-operative approach for these patients in our paediatric population when there is a high recidivism rate.
Acknowledgement
We would like to thank Mr Hitesh Tailor for his help with formatting the figures.
Competing interests
None declared







