Many epidemiological studies(Reference Williams1–Reference Pal, Khossousi and Binns3) have reported that consumption of fruit and vegetables may play a role in the prevention of chronic diseases and in decreasing the risk of mortality from cancer and CVD. This protective action of plant-based food has been primarily attributed to secondary metabolites known as phytochemicals, which include polyphenolic products of the phenylpropanoid biosynthetic pathway. Polyphenols are widely distributed in plants, and are generally considered beneficial, as it has been documented that they can affect a number of processes in mammalian cells, suggesting an anti-carcinogenic and anti-atherogenic role(Reference Duthie4). However, some polyphenols and plant secondary metabolites, especially at higher concentrations, have proved to be genotoxic in various cell systems(Reference Brusick5–Reference Stopper, Schmitt and Kobras7).
Plant secondary metabolites can be constitutively expressed, but they can also be induced by a range of factors, including attack by fungal and bacterial pathogens and herbivores(Reference Wink8). Recent investigations have shown that the plant secondary metabolic pathway is influenced by beneficial soil micro-organisms – arbuscular mycorrhizal (AM) fungi (AMF) – which establish mutualistic symbioses with the root systems of about 80 % of land plant species, including the most important agricultural crops. AMF absorb and translocate soil mineral nutrients – mainly P, N, S, K, Ca, Fe, Cu and Zn – to host roots, enhance plant growth and biomass production in many crops, and positively affect crop tolerance of biotic and abiotic stresses(Reference Smith and Read9). In addition, AMF symbioses have been shown to modify terpenoid metabolism in species belonging to the Poaceae family(Reference Peipp, Maier and Schmidt10), increase the activity of several antioxidant enzymes in some shrubs(Reference Alguacil, Hernández and Caravaca11), increase total superoxide dismutase activity in bean roots(Reference Lambais, Rios-Ruiz and Andrade12) and induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with apocarotenoid accumulation(Reference Walter, Fester and Strack13, Reference Fester, Schmidt and Lohse14). Although most of these data were obtained by analysing the roots of mycorrhizal plants, recent studies have reported that mycorrhizal symbioses increase phenolic content and antioxidant properties of artichoke leaves and flower heads in the field(Reference Ceccarelli, Curadi and Martelloni15) and enhance tomato fruit ascorbic acid content(Reference Subramanian, Santhanakrishnan and Balasubramanian16).
Some isoflavones and other flavonoids, as well as lignans, coumestans and stilbenes, display oestrogenic (and anti-oestrogenic) activity, and are commonly referred to as phyto-oestrogens. Phyto-oestrogens, which do not necessarily show structural similarities to naturally occurring oestrogen(Reference Dornstauder, Jisa and Unterrieder17), have been investigated for their putative preventive role in osteoporosis, menopausal symptoms, arteriosclerosis, heart disease and cancer(Reference Ososki and Kennelly18, Reference Marinangeli and Jones19). They have a complex mode of action via interaction with the nuclear oestrogen receptor isoforms ERα and ERβ, exhibiting either oestrogen-agonist or oestrogen-antagonist effects(Reference Moutsatsou20). Interestingly, the levels of isoflavones with oestrogenic activity (e.g. of biochanin A, formononetin, genistein or daidzein) were impaired in some mycorrhizal plant species(Reference Khaosaad, Krenn and Medjakovic21).
Tomato fruits, extensively cultivated in Europe and throughout the world, have assumed the status of ‘functional food’, due to epidemiological evidence of reduced risk of certain types of cancers and CVD(Reference Willcox, Catignani and Lazarus22–Reference Palozza, Bellovino and Simone25). Tomato fruits are a reservoir of a range of antioxidant molecules, such as lycopene, ascorbic acid, vitamin E, carotenoids, flavonoids and phenolic compounds and, due to their high consumption rates, can provide a substantial part of the total intake of these beneficial nutraceutical molecules(Reference Raffo, Leonardi and Fogliano26). Among carotenoids, lycopene has been shown to have strong antioxidant activity and exhibits the highest physical quenching rate constant with singlet oxygen(Reference Di Mascio, Kaiser and Sies27). In addition to these properties, lycopene has also been shown to induce cell-to-cell communications and modulate hormones, immune systems and other metabolic pathways(Reference Rao and Agarwal28).
In the present study, we developed a multidisciplinary approach to acquire knowledge on the nutraceutical value and safety of mycorrhizal tomato fruits produced by plants inoculated with the beneficial AM fungus Glomus intraradices and grown in the greenhouse (harvested at the ripening stage – full-red skin). Thus, we assessed, on inoculated and control plants, (1) the establishment of mycorrhizal colonisation, (2) plant growth parameters and nutrient uptake, (3) lycopene and main antioxidant content of raw tomato fruit extracts, (4) genotoxic activity in vitro of raw tomato fruit extracts and (5) oestrogenic/anti-oestrogenic activity of raw tomato fruit extracts.
Materials and methods
Plant and fungal material and growth conditions
Seeds of Solanum lycopersicum L., var. Money maker, were germinated in sterile moist quartz grit. After 8 d growth, plantlets were transferred to 100 ml plastic pots (one plant/pot) containing a mixture (1:1, by vol.) of soil and Terragreen (calcinated clay; OILDRI, Chicago, IL, USA), which was steam-sterilised (121°C for 40 min) to kill naturally occurring AMF. The soil was a sandy loam collected near S. Piero (Pisa, Italy). Chemical and physical characteristics of the soil were as follows: pH (water) 8·1; clay, 4·9 %; silt, 6·1 %; sand, 88·9 %; total N, 0·9 g/kg; available P (NaHCO3 soluble P, Olsen analysis), 4·15 mg/kg; available K, 127 mg/kg. In mycorrhizal treatments, each plant was inoculated with 30 g of the whole inoculum (mycorrhizal roots and soil containing spores and extraradical mycelium) of the AM fungal species G. intraradices Schenck & Smith (isolate IMA6 from France, collector V. Gianinazzi-Pearson), obtained from Helianthus annuus L. pot cultures maintained in the collection of the Soil Microbiology Laboratory of the Department of Crop Plant Biology, University of Pisa, Italy. All pots received 50 ml of a filtrate, obtained by sieving the mycorrhizal inoculum through a 50 μm pore diameter sieve and a Whatman® paper no. 1 (Whatman International Ltd, Maidstone, Kent, UK), to ensure common microflora for all treatments. A total of thirteen control and thirteen mycorrhizal pots were set up. Plants were grown in a growth chamber, supplied with tap water as needed and with weekly fertilisation of half-strength Hoagland's solution (10 ml per pot). After 1 month of growth, three pots per treatment were harvested and roots were observed after clearing and staining with 0·05 % Trypan blue in lactic acid(Reference Phillips and Hayman29). Mycorrhizal root length was assessed by using the gridline intersect method(Reference Giovannetti and Mosse30). Both mycorrhizal and control plants were transferred with their soil to 7 litre plastic pots containing a mixture of steam-sterilised soil and Terragreen. Plants were grown in a greenhouse for 4 months. Each pot was watered and fertilised by a timer-controlled drop irrigation system. Fertigation was applied to both treatments, using the nutrient solution with the following macronutrient composition: N, 6·0 mm; P, 0·1 mm; K, 3·9 mm, dissolved in tap water. Rate and frequency of fertigation varied during the tomato growth cycle. For the first 2 weeks after the inoculation step, 20 ml of the solution were manually added to the pots every third day. Thereupon, an automated drip irrigation device was utilised, and nutrients were dissolved in a 266 litre tank with well water. Frequency of solution delivery was increased throughout the experiment to compensate for the increased nutrient demand by plants. The solution in the tank was replaced every week.
The experiment was a completely randomised design with two inoculum treatments (inoculated plants and uninoculated control) and ten replicates.
Plant growth and mycorrhizal colonisation
Tomato fruits from control and mycorrhizal plants at the full-red stage were harvested from the first three trusses. Fully red ripe fruits with bright red colour and marketable appearance were selected. Fresh weight of fruits produced by each plant was recorded and DM (%) was obtained by drying the fruit pieces in a thermo-ventilated oven at 70°C up to constant weight. The pH of tomato fruit was measured using a pH meter. Soluble solid concentration was measured with an optic refractometer (Model RL-3, Polskie Zaklady Optyczne, Warsaw, Poland) and expressed as °Brix at 20°C. Acidity was determined by titration with 0·1 m-NaOH to reach pH 8·1 and expressed as g citric acid/100 g of fresh fruit weight. Shoot dry weight (DW) was determined after drying at 70°C in a thermo-ventilated oven for 3 d. Host benefit was calculated for each fungal species as ((DW mycorrhizal plant − DW non-mycorrhizal plant)/DW non-mycorrhizal plant) × 100. Each plant root system was extracted from soil and both mycorrhizal and control roots were carefully washed with tap water, cut into 5 cm pieces, cleared and stained as described above. Percentage of AMF colonisation was assessed on each root sample by using the gridline intersect method. Colonised roots were mounted on microscope slides and observed under a Reichert-Jung (Wien, Austria) Polyvar light microscope to detect intraradical fungal structures.
Mineral content of tomato fruits
Fruit samples from control and mycorrhizal plants were oven dried for a week at 60°C and ground to powder with a mortar and pestle; 0·1 g of each sample were then transferred into 25 ml beakers. Concentrated nitric–perchloric acid solution (ratio 5:2) was added to each beaker and allowed to digest for 12 h at 120°C on a heating plate. The solution was transferred to 50 ml volumetric flasks and double-distilled water was added. Fruit C and N were determined with a Finnigan Flash E1112 CHN-S elemental analyser (Thermo Fisher Scientific, Waltham, MA, USA), whereas Ca, Fe, K, Mg, Mn, Na, P and Zn analysis was performed with Optima 2000 OES DV ICP-OES (Perkin-Elmer, Waltham, MA, USA).
Fruit sample preparation for activity assays
A total of twenty-five fruits from each treatment were washed, cut into four pieces and seeds discarded. Fruit pieces were then pooled and stored at − 70°C until analyses. For biochemical analyses, the material was extracted by homogenising 1 g of frozen tomatoes in 10 ml of 50 mm-pottasium-phosphate buffer (pH 7·5). The homogenate was then centrifuged for 15 min at 6000 g at 4°C. Supernatants were collected and referred to as ‘hydrophilic extract’ (HE). The pellet was resuspended in 10 ml of ethyl acetate, stirred for 15 min at 4°C and then centrifuged for 10 min at 6000 g at 4°C, and the supernatant was collected. This procedure was performed twice. The organic phase was evaporated with a rotary evaporator, resuspended in 2 ml of ethyl acetate and referred to as ‘lipophilic extract’ (LE).
Biochemical analyses
Analysis of lycopene
Lycopene content was determined according to the reduced volumes of organic solvents(Reference Fish, Perkins-Veaziea and Collins31). About 0·5 g of homogenised tomato were weighed, and combined with 5 ml of acetone with 0·05 % butylated hydroxytoluene, 5 ml of ethanol and 10 ml of hexane. The mixture was put on an orbital shaker for 15 min. Water (3 ml) was then added, before an additional 5 min on the shaker. Samples were then left in an upright position at room temperature for 5 min to allow for phase separation. Absorbance of the upper phase (hexane) was measured in a 1 cm path length quartz cuvette at 503 nm, blanked with hexane.
Analysis of ascorbate
Total ascorbate, reduced ascorbate (ASC) and oxidised ascorbate were measured as reported(Reference Gillespie and Ainsworth32), with some modifications. The assay is based on the reduction of Fe3+ to Fe2+ by ascorbate and the spectrophotometric detection of Fe2+ complexed with 2,2′-dipyridyl. Oxidised ascorbate was reduced to ASC by pre-incubation of the sample with dithiothreitol (DTT). The excess of DTT was removed with N-ethylmaleimide, and total ascorbate was determined by the 2,2′-dipyridyl method. Oxidised ascorbate concentration was estimated from the difference between total ascorbate and ASC (without pre-treatment with DTT). Frozen tomato slurry (1 g) from control and mycorrhizal plants was ground in a prechilled mortar with 0·8 ml TCA (6 %, w/v). The mixture was homogenised and then allowed to stand for several minutes. The homogenates were transferred to 2 ml tubes and adjusted to a volume of 2 ml with 6 % TCA, and subsequently centrifuged for 5 min at 14 000 g. Total ascorbate was measured in a 2·0 ml reaction mixture containing 0·1 ml aliquot of the supernatant, 0·2 ml of 200 mm-phosphate buffer (pH 7·5) and 0·1 ml of 10 mm-DTT. After incubation for 20 min at 42°C, 0·1 ml of 0·5 % N-ethylmaleimide were added. ASC was determined in the same reaction mixture, except that 0·3 ml of 200 mm-phosphate buffer (pH 7·5) and 0·1 ml water were added instead of DTT and N-ethylmaleimide. Colour was developed in the reaction mixture after addition of the following reagents: 0·5 ml of 10 % TCA, 0·4 ml of 42 % ortho-phosphoric acid, 0·4 ml of 4 % 2,2′-dipyridyl dissolved in 70 % (v/v) ethanol and 3 % (w/v) FeCl3. After mixing, the mixture was incubated at 42°C for 40 min and absorbance of α,α′-bipyridyl complexed with reduced ferric iron was read at 525 nm using a UV-1204 spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD, USA). A standard curve in the range 0–200 μg/ml of ASC was prepared.
Analysis of glutathione
Total, reduced (GSH) and oxidised (GSSG) glutathione content were determined with an enzymatic-recycling assay based on glutathione reductase(Reference Anderson33) with minor modifications. Frozen tomato slurry (0.5 g) from control and mycorrhizal plants were added to a chilled solution of 6 % m-phosphoric acid and 1 mm-EDTA-K2 and transferred into 2 ml tubes. Tubes were centrifuged at 20 000 g for 15 min at 4°C. The supernatant was collected and used for analysis. Then, 0·1 ml of the supernatant were used for the assay of total glutathione (GSH+GSSG). Another 0·1 ml of the aliquot were first neutralised by adding 5 μl of triethanolamine and subsequently derivatised with 4 μl of 2-vinylpyridine to mask GSH. The tube was mixed until an emulsion was formed and incubated for 60 min at room temperature. This sample was used for the GSSG assay. GSH was estimated as the difference between total glutathione and GSSG. Glutathione content was measured in 1·2 ml of the reaction mixture containing 0·3 ml of 5 % K2HPO4.3H2O, 0·4 ml of reagent 1 (150 mm-phosphate buffer, 15 mm-EDTA, 0·3 mm-5,5′-dithiobis-2-nitrobenzoic acid and 0·04 % bovine serum albumin), 0·32 ml reagent 2 (1 mm-EDTA, 50 mm-imidazole solution, 0·02 % bovine serum albumin and 25·05 nkat/ml of glutathione reductase) and 0·1 ml of the sample. The reaction was started by the addition of 0·08 ml of 9 mm-NADPH. The reaction was allowed to proceed for 2 min at room temperature and then immediately stopped by dipping tubes in boiling water for few seconds. A standard GSH curve in the range of 0–30 μg/ml was prepared.
Total phenolic compounds
Total soluble phenolics were extracted from frozen tomato slurry with 70 % methanol, determined using Folin–Ciocalteu reagent, and expressed as quercetin equivalents(Reference Ainsworth and Gillespie34). The reaction mixtures included 0·125 ml methanol extract, 0·5 ml distilled water, 0·125 ml Folin–Ciocalteu reagent, 1·25 ml of 7 % sodium carbonate and 1 ml water. Absorbance was read at 760 nm after 90 min of incubation at room temperature. A standard curve for quercetin in the range of 0–50 μg/ml was prepared.
Total antioxidant activity
Antioxidant activity was measured using a method previously described(Reference Cano, Acosta and Arnao35), with some modifications. The assay is based on the capacity of different antioxidant components to scavenge the 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical cation (ABTS∙+) compared with a standard antioxidant (Trolox; Sigma Aldrich). ABTS∙+ was generated by mixing 2·45 mm-potassium persulfate with 7 mm-ABTS, which was then allowed to react in the dark at room temperature for 12–16 h. For analysis of HE and LE antioxidant capacity, 110 μl of the ABTS∙+ solution were diluted with 6 mm-pottasium-phosphate buffer or 10 ml ethanol, respectively, in order to obtain an absorbance of 0·70 ± 0·02 at 734 nm. Then, 5, 10, 15 μl of HE or LE were added to 1 ml of the ABTS∙+ solution. The decrease in absorbance was determined at 734 nm 15 min after initial mixing and every 15 min until the reaction reached a plateau (after 1 h). Absorbance was determined as the difference between the A 734 nm value before and after the addition of the sample (after 1 h). Antioxidant activity is expressed as Trolox equivalents/fresh weight (mm/g fresh weight).
Determination of genotoxic activity: in vitro mutagenicity assay
Ames test
The mutagenicity Ames test is based on the ability of histidine-dependent mutated (his − ) bacterial strains to revert to the wild-type phenotype (his+) when allowed to grow on agar plates containing minimal medium without histidine. The assay was performed on TA98 and TA100 tester strains of S. typhimurium, in the presence and absence of metabolic activation (S-9 mix) as described(Reference Maron and Ames36). Using a semi-log scale, LE and HE were tested from 0·03 to 2·625 mg/plate and from 0·04 to 25 mg/plate, respectively. 2-Aminofluorene was used as a positive control at 1 μg/plate to check for functioning of both cell and S-9 mix. Negative controls were also set up, according to the solvent used to prepare each extract. For each experimental point, three independent plates per strain in the presence and absence of S-9 mix were set up.
Micronucleus assay
The analysis of micronucleus (MN) formation in phytohaemagglutinin-stimulated and cytochalasin B-blocked human peripheral lymphocytes, which detects chromosome breakage and/or malsegregation, is commonly used as a marker of genome damage(Reference Fenech37). Heparinised peripheral blood samples were obtained from three healthy, young, non-smoking male donors. After adding 0·3 ml of the whole blood to 4·7 ml of Roswell Park Memorial Institute (RPMI)-1640 (Invitrogen, Milano, Italy) supplemented with 20 % fetal bovine serum (Invitrogen), 1·5 % phytohaemagglutinin (Invitrogen) and 1·0 % penicillin/streptomycin (Invitrogen), stimulated lymphocytes were incubated and cultured at 37°C for 72 h. Using a semi-log scale, tomato extracts were assayed in the final concentration range of 0·0024–1·5 and 0·016–10 mg/ml for LE and HE, respectively. Bleomycin (Sanofi-aventis, Milano, Italy) was used as a positive control. Negative controls were also set up, according to the solvent used to prepare each extract. Cytochalasin B (6 μg/ml; Sigma-Aldrich, Milano, Italy) was added at 44 h to all culture tubes to block cell cytokinesis. As described in detail elsewhere(Reference Scarpato, Paganucci and Bertoli38), lymphocytes were harvested at the end of cell culturing by 4 min centrifugation at 2400 rotations/min, treated with 10 ml of 0·075 mm-KCl for a few minutes to lyse erythrocytes, prefixed in methanol–acetic acid (3:5), fixed in 100 % methanol, washed twice in methanol–acetic acid (5:1) and dropped onto clean glass slides. The air-dried slides were then stained in 5 % Giemsa. For each experimental point, 2000 bi-nucleated cells (1000 cells from replicate cultures) were scored for the presence of MN. MN frequency is expressed as the number of micronucleated bi-nucleates per 1000 bi-nucleated scored cells. Toxicity and/or cell cycle delay is expressed as cell proliferation index, which was calculated according to the following formula: (M+2B+3P)/(M+B+P), where M (mononucleated), B (bi-nucleated) and P (plurinucleated) are the number of cells that have not yet entered the first mitosis (M), and cells that have divided once (B) and twice (P, comprising both tri- and tetranucleated), respectively. M+B+P represents a total of at least 1000 cells scored.
Determination of oestrogenic/anti-oestrogenic activities: the yeast oestrogen screen assay
Oestrogenic/anti-oestrogenic activity of tomato samples was assessed using the Saccharomyces cerevisiae yeast strain RMY326 containing the human oestrogen receptor α and a Xenopus laevis vitellogenin ERE sequence linked to a lacZ reporter gene encoding for the enzyme β-galactosidase(Reference Liu, Jeannin and Picard39). Induction of reporter gene transcription by the complex receptor–ligand was detected and quantified by spectrophotometry (ULTROSPEC®2100 pro; Amersham Pharmacia Biotech, Cologno Monzese, Italy). The yeast oestrogen screen (YES) assay was described previously(Reference Pinto, Picard and Reali40, Reference Pinto, Garritano and Reali41). Determination of oestrogenic and anti-oestrogenic activity was conducted on both LE and HE. The extracts were tested in the range 0·001–2·0 mg/ml. To test for oestrogenic activity, yeast cultures were incubated overnight in the presence of 10 nm-17-β-oestradiol (E2) as a positive control, vehicle (negative control), and increasing concentrations of tomato extracts alone. The enzymatic reaction was started by adding o-nitrophenyl β-d-galactopyranoside (Sigma-Aldrich) and incubating at 30°C for 5–10 min. The reaction was stopped by adding Na2CO3 and absorbance was measured at 420 nm. β-Galactosidase activity was normalised to the number of cells assayed (optical density at 600 nm). The amount of β-galactosidase is correlated with the potential oestrogenic activity of the sample and can be quantified by means of a logistic dose–response curve generated with E2 (GraphPad 4.0; GraphPad Software Inc., San Diego, CA, USA). Oestrogenic (agonistic) activity of samples is expressed as a percentage of β-galactosidase activity obtained with 10 nm-E2 (positive control). For anti-oestrogenic activity, yeast cells were co-treated overnight with 1 nm-E2. Samples that successfully inhibited the activity of the natural ligand E2 led to a dose-dependent decrease in β-galactosidase expression, which was associated with a concurrent decrease in the rate of change in colour of the medium. A yeast culture containing 1 nm-E2 alone was used as a positive control. Antagonistic activity, defined as ability of the extracts to antagonise the binding of the natural hormone to its receptor, is expressed as inhibition (%) of 1 nm-E2 activity by each sample. The drug 4-hydroxy tamoxifen (Sigma-Aldrich), which acts as an E2-antagonist in yeast(Reference Liu, Jeannin and Picard39, Reference Beresford, Routledge and Harris42), was used as the control for antagonistic activity.
Lycopene, one of the most representative constituents of tomato fruits, accounting for more than 80 % of total tomato carotenoids(Reference Ho43), was also tested in order to evaluate assay sensitivity in detecting the endocrine disrupting activity of this compound. Lycopene (L9879 Sigma-Aldrich) was tested from 10− 9 to 10− 4 m. E2, 4-hydroxy tamoxifen, lycopene and samples were dissolved in dimethyl sulfoxide (dimethyl sulfoxide–water 1:1, v/v for the HE) and added to the yeast culture so that solvent concentration did not exceed 2 % (v/v). The results are the mean of three independent experiments and standard deviations.
Statistical analysis
ANOVA of plant growth and mycorrhizal colonisation data were performed on SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA), after the necessary transformations, and differences between means were determined by the appropriate test. The Tukey B procedure was used for comparing means. Percentage colonisation data were arcsine-transformed before analysis. Results from the Ames test are expressed as the mean number of revertants per plate, and evaluation of statistical significance was obtained by linear regression analysis calculated on the dose–response curve. MN frequencies and cell proliferation index values were processed by the Dunnett test, which performs a multiple comparative calculation of treated v. control cultures. Oestrogenic/anti-oestrogenic activity was evaluated by ANOVA first-order regression analysis, (PROC GLM of SAS, version 8.2; SAS Institute Inc., Cary, NC, USA) and each activity was considered significant when it reached at least a 20 % increase compared with the control (agonistic activity) or a 40 % decrease (antagonistic activity).
Results
Mycorrhizal colonisation
At harvest, G. intraradices successfully established mycorrhizal symbioses with tomato plants, showing percentages of colonised root length ranging from 40 to 70 %. No colonisation was observed in uninoculated plants.
Plant biomass, mineral content and quality parameters
Shoot biomass production was significantly affected by mycorrhizal symbiosis (P = 0·001): host benefit was 18·3 (se 3·2) %, based on total shoot weights. Fruit Ca, K, P and Zn concentrations were higher in mycorrhizal plants than that of control plants. Fruit concentrations of all other elements were unaffected by AM fungal colonisation (Table 1). Fruit quality parameters of mycorrhizal and control tomatoes are shown in Table 2. Fruit DM, pH, soluble solids and acidity were not significantly affected by mycorrhizal colonisation.
Table 1 Mineral element concentrations in control and mycorrhizal tomato fruits
(Mean values with their standard errors of three replicates)
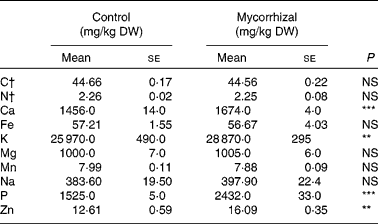
DW, dry weight.
Mean values were significantly different: **P ≤ 0·01 and ***P ≤ 0·001 (two-tailed paired t test).
† C and N are expressed in g/100 g DW.
Table 2 Quality parameters in control and mycorrhizal tomato fruits
(Mean values with their standard errors of three replicates)
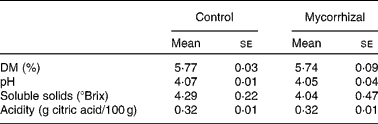
Mean values were not significantly different between control and mycorrhizal values (P>0·05; two-tailed paired t test).
Lycopene and main antioxidant compounds
Mean values of bioactive compounds and of antioxidant activity in the fruits of control and mycorrhizal plants are given in Table 3. Comparing the composition of tomatoes on a fresh matter basis is essential when the nutritional value of the fruit is considered. On a fresh matter basis, the concentrations of the bioactive compounds assayed, except lycopene, were similar in both treatments. Lycopene content of tomato fruits from mycorrhizal plants was 18·5 % higher than that of controls.
Table 3 Bioactive compounds in control and mycorrhizal tomato fruits
(Mean values with their standard errors of three replicates)
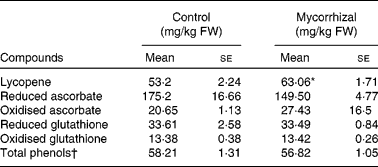
FW, fresh weight.
* Mean values were significantly different between control and mycorrhizal values (P ≤ 0·01; two-tailed paired t test).
† mg quercetin/kg FW.
The redox state of ascorbate expressed as the ratio of ASC to total ascorbate ranged from 0·85 to 1·0 in fruits from both treatments. The glutathione ratio (GSH:GSH+GSSG) was roughly 0·7 in fruits both of control and mycorrhizal tomato plants.
In the present study, the ABTS∙+ radical cation assay was performed to test antioxidant activity. This assay is widely used because it is easy to perform and helpful in analysing both hydrophilic and lipophilic compounds. Antioxidant activity values of water-soluble and water-insoluble fractions showed no significant differences between treatments.
In vitro mutagenicity assays
The presence of mutagenic compounds in raw plant extracts was assessed by the in vitro Ames test and the MN assay in human lymphocytes for evaluation of gene and chromosome mutation, respectively. In no case did HE or LE (from fruits of mycorrhizal and non-mycorrhizal tomato plants) increase the spontaneous reversion frequency of Salmonella strains with and without metabolic activation (data not shown). Table 4 lists the results of the MN test. Treatment of cultures with HE from fruits of mycorrhizal and control plants induced no significant variation in the baseline levels of MN or in cell proliferation up to the maximum concentration tested (10 mg/ml). Similar results were observed when lymphocytes were treated with LE of tomato fruits from mycorrhizal and non-mycorrhizal plants, although a slight, but non-significant, increase in cytotoxicity was observed at 1·5 mg/ml. As expected, the reference mutagen bleomycin induced highly significant (P < 0·001) increases (18·00 (se 8·48) and 68·50 (se 19·50) ‰) in the spontaneous MN frequencies (0·50 (se 0·50) and 11·50 (se 3·50) ‰) and a substantial reduction in cell division.
Table 4 Micronucleus (MN) assay in human lymphocytes treated by hydrophilic and lipophilic extracts of tomato fruits obtained from control and mycorrhizal plants
(Mean values with their standard errors of two independent experiments)

CPI, cell proliferation index; DMSO, dimethyl sulfoxide.
Mean values were significantly different from the control (0 mg/ml): **P < 0·01, ***P < 0·001.
Oestrogenic/anti-oestrogenic activity
LE exhibited weak oestrogenic activity in YES, depending on the concentration tested. At 0·1 mg/ml, this extract produced 26·6 % β-galactosidase activity. A significant difference (P < 0·0001) between extracts of fruits produced by mycorrhizal and control plants was observed. The antagonistic action of tomato extracts is shown in Fig. 1. Both LE (Fig. 1(a)) and HE (Fig. 1(b)) exerted a significant (P < 0·0001) dose-dependent inhibition of E2 activity. The activity expressed by LE was characterised by an exponential dose–response relationship and, overall, it was higher than the activity induced by HE (P < 0·05). In particular, at the highest concentration tested, LE of tomato fruits produced by mycorrhizal plants exhibited a 93·3 % inhibition of the activity induced by 1 nm-E2, while HE of the same tomatoes showed a 70·7 % decrease in E2 activity. For both LE and HE, the inhibitory action was significantly affected by the mycorrhizal treatment (P < 0·0001). Moreover, HE of fruits from both mycorrhizal and control plants affected yeast cell proliferation (185·5 and 173·5 %, respectively).

Fig. 1 Anti-oestrogenic effect induced by (a) the lipophilic and (b) hydrophilic extracts of control (□) and mycorrhizal (■) tomato fruits. To test for anti-oestrogenic activity, the yeast strain was incubated with 17-β-oestradiol (E2) in the presence or absence of samples. Activity of the extracts is expressed as inhibition (%) of the activity induced by 1 nm-E2 (100 %). Values are means of three independent experiments, with standard deviations represented by vertical bars. β-gal, β-Galactosidase.
In the concentration range tested, lycopene was not oestrogenic, but showed a dose-dependent inhibition of E2 activity (20–50 % inhibition, depending on the dose).
Discussion
To the best of our knowledge, this is the first multidisciplinary study on the nutraceutical value and safety of mycorrhizal tomato fruits. The present data show that inoculation with G. intraradices positively affected the growth and mineral nutrient content of tomato plants, and enhanced the nutritional and nutraceutical value of tomato fruits through modifications of plant secondary metabolism, without production of mutagenic compounds. Moreover, fruits produced by mycorrhizal tomato plants showed significantly higher anti-oestrogenic activity.
Mycorrhizal colonisation by G. intraradices exerted a positive effect on plant growth and nutrition, with enhanced uptake of soil mineral nutrients, in particular P, K, Ca and Zn. This is not unexpected, since a considerable body of evidence has shown improved nutritional status of mycorrhizal plants compared with controls(9). Tomato is not considered a highly mycotrophic plant and both mycorrhizal establishment and host benefit may depend on host varieties(Reference Bryla and Koide44) and fungal species(Reference Gao, Delp and Smith45).
AMF inoculation enhanced the nutritional status of tomatoes. Ca, K, P and Zn content of fruits of mycorrhizal plants was higher than in control plants. Interestingly, fruit P and Zn content of mycorrhizal plants was 60 and 28 % higher than that of controls, respectively. Similar results were obtained in a field experiment using a mycorrhizal defective tomato mutant (rmc) and its mycorrhizal wild type (76R MYC+plants)(Reference Cavagnaro, Jackson and Six46). The present data show that AMF colonisation can improve the nutritional value of tomatoes, which is an important finding since Zn has been identified as a key human mineral nutrient(Reference Welch and Graham47).
A particularly interesting result of the present study is the enhanced level of lycopene found in tomato fruits produced by mycorrhizal plants compared with controls, while the levels of other bioactive compounds were similar to control values.
In this context, we demonstrated that extracts of tomato fruits produced by mycorrhizal plants induced no in vitro genotoxic effect. In the present study, two tests were used for genotoxicity assessment: the Ames Salmonella/microsome mutagenicity assay(Reference Mortelmans and Zeiger48), which represents the ‘first line’ for detection of gene mutation, and the human lymphocyte MN test(Reference Fenech49), a ‘first line’ test for detecting chromosome aberrations. In the present findings, we detected no mutagenic polyphenol with DNA-damaging activity(Reference Brusick5, Reference Stopper, Schmitt and Kobras7). Although several studies have shown the chemoprotective/chemopreventive effects of phytochemicals(Reference Scarpato, Paganucci and Bertoli38, Reference Mersch-Sundermann, Kassie and Böhmer50–Reference Siddique and Afzal52), other investigations have reported that plant extracts can be genotoxic due to the presence of high concentrations of particular secondary metabolites(Reference Rietjens, Boersma and van der Woude53, Reference Noel, Kasinathan and Rath54). On the basis of the present results, and since lycopene has been found to possess anti-genotoxic/anti-carcinogenic properties(Reference Velmurugan, Santhiya and Nagini55, Reference Scolastici, Alves de Lima and Barbisan56), we hypothesised that the higher lycopene content detected in mycorrhizal tomato plants succeeded in neutralising the DNA-damaging activity of any possible mutagenic compound occurring in tomato fruits.
Tomato extracts from control and mycorrhizal plants exhibited weak oestrogenic action, as maximum β-galactosidase activity, observed for LE, was 26·6 %. The present results indicated that tomato extracts, above all the lipophilic fraction, strongly inhibited E2–human oestrogen receptor binding – and that mycorrhizal inoculation significantly increased the anti-oestrogenic power of tomato fruits. The higher inhibitory effect induced by LE could bear a relationship to the distribution of compounds capable of binding oestrogen receptor, such as flavonoids and lycopene, in tomato fruits. More specifically, the major flavonoids of tomato, naringenin-calchone and rutin(Reference Slimestad, Fossen and Verheul57), are mainly contained (98 %) in LE(Reference Lavelli, Hippeli and Dornisch58), as well as other compounds of a lipophilic nature (e.g. carotenoids). The inhibitory effect observed for lycopene in the YES test is in agreement with data concerning the E2-antagonistic effect of this compound on different in vitro systems(Reference Fornelli, Leone and Verdesca59, Reference Hirsch, Atzmon and Danilenko60).
Recently, polyphenols and lycopene have been proposed as promising pharmacological agents in cancer prevention on account of their antiproliferative effects and their inhibitory action on the human oestrogen receptors(Reference D'Incalci, Steward and Gescher61–Reference Pinto, Bertoli and Noccioli63). In particular, anti-oestrogenic compounds can antagonise oestrogen-dependent processes in their target tissues, counteracting the growth of oestrogen-related cancers. The present results suggest that intake of functional foods such as tomato fruits produced by mycorrhizal plants could antagonise in vivo the oestrogen-like activity known to be elicited by several environmental–industrial xenobiotics to which humans are exposed through the food chain(Reference Pinto, Garritano and Cristofani64, Reference Pinto and Reali65).
In conclusion, AMF are ecologically and economically important micro-organisms playing a major role in sustainable food production systems, by reducing the input of chemical fertilisers and pesticides(Reference Giovannetti, Avio, Khachatourians and Arora66). Thus, they not only ensure lesser environmental damage but also allow safe production of high-quality food, which is an important societal issue, strongly in accordance with the demand expressed both by consumers and producers.
Acknowledgements
The present study was supported by the University of Pisa (Progetti di Ateneo 2006), by Cassa di Risparmio di Lucca Pisa e Livorno and by CNR. The authors declare no conflicts of interest. M. G., R. B. and D. R conceived and designed the experiments. L. A., N. C., R. C., A. I., F. M., P. P., B. P., C. S. and R. S. performed the experiments and analysed the data. M. G., R. B. and D. R. contributed reagents/materials/analysis tools. M. G., N. C., P. P., B. P., D. R., C. S. and R. S. wrote the manuscript.







