Antipsychotic medication has been the mainstay of schizophrenia treatment for the past 50 years. The first-generation antipsychotics, introduced in the mid-20th century, were unevenly effective in relieving the symptoms of schizophrenia, often at the expense of extrapyramidal side-effects (EPS) such as acute dystonia, akathisia, Parkinsonism and tardive dyskinesia. The development of second-generation antipsychotic drugs and their promotion by the pharmaceutical industry were predicated on indications that these medications would have a milder EPS profile and therefore greater tolerability than the earlier drugs. Reference Arvanitis and Miller1–Reference Kane11 The results of recent large trials and meta-analyses have shown that there is no effectiveness or tolerability advantage. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12–Reference Rochon, Stukel, Sykora, Gill, Garfinkel and Anderson15 There is a view that the two generations of antipsychotics should preferably be seen as lying on a multidimensional continuum rather than as distinct classes, and that the heterogeneity and complexity of side-effects are masked by a spurious dichotomy. Reference Fleischhacker16,Reference Barnes17 Nevertheless, there has been a dramatic increase in the prescription of second-generation drugs. Reference Verdoux, Tournier and Bégaud18
The Cost Utility of the Latest Antipsychotics in Schizophrenia Study Band 1 (CUtLASS-1) was a pragmatic randomised controlled trial (RCT) that tested the hypothesis that the clinical and cost-effectiveness of second-generation antipsychotics would be superior in individuals whose antipsychotic treatment was being changed owing to an inadequate response or side-effects. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12 The primary analysis of quality of life, symptoms and costs over 1 year refuted this hypothesis. In fact, the older drugs were associated with a trend towards better outcomes and lower costs, although the more conservative conclusion is one of broad equivalence between the two classes when prescribed in the context of a multicentre pragmatic trial. Second-generation drugs were not superior, even on measures of patient preference. One possible explanation for the relative lack of distinction between drug classes seen in CUtLASS-1 is that the second-generation antipsychotics were not associated with the expected relief from EPS. In the USA, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated, similarly, that there was no significant difference between second-generation anti-psychotics when compared with perphenazine in terms of the emergence of EPS. Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins19,Reference Miller, Caroff, Davis, Rosenheck, McEvoy and Saltz20 It has been suggested in the analysis of CATIE and elsewhere that the differences between first- and second-generation drugs in early studies could have resulted from the use of haloperidol – often in relatively high dose – as the comparator. Reference Miller, Caroff, Davis, Rosenheck, McEvoy and Saltz20,Reference Hugenholtz, Heerdink, Stolker, Meijer, Egberts and Nolen21 In fact, a systematic review published early in the second-generation drug epoch demonstrated no evidence that these drugs were more effective or better tolerated than the first generation, and attributed any perceived benefit to the dosage of the comparator drug. Reference Geddes, Freemantle, Harrison and Bebbington22 Furthermore, the doses of several second-generation antipsychotics used in clinical practice are higher than those used in the benchmarking studies sponsored by their manufacturers.
We report the results of a secondary analysis of data from the CUtLASS-1 study focusing on emergent and resolved EPS, together with the mediating effect of anticholinergic drug treatment. Based on the results of the CATIE study, we predicted that the two drug classes (when prescribed with care in the context of a pragmatic trial) would not have markedly different EPS profiles, particularly when combined with judicious use of anticholinergic medication.
Method
The CUtLASS-1 study was a pragmatic, multicentre, rater-masked RCT, conducted between July 1999 and January 2002 within 14 community psychiatry services affiliated with five medical schools in the English National Health Service. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12,Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23 It was designed to test the effectiveness of antipsychotic medications in routine clinical practice. The 227 participants were randomised by means of a remote telephone service to receive either a first- or a second-generation antipsychotic (other than clozapine). Randomisation to a class of drugs allowed the managing physician to select a drug from the choices available locally within that class, approximating to real-life clinical practice. Clinicians and participants knew the identity of the prescribed drug but clinical raters did not. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23 Clinicians were asked to try as much as possible within good practice to keep participants on the randomised medication for at least the first 12 weeks and, if it was necessary to switch drugs, to select a second drug within the same class. This was supported by a good-practice manual produced for clinicians in the trial based on contemporary guidelines that covered antipsychotic and anticholinergic prescribing. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23 Masked clinical assessments were conducted at baseline and at 12 weeks, 26 weeks and 52 weeks.
Participants
A research ethics committee at each site approved the study. Participants were 18–65 years of age and were receiving care from a clinician who was considering changing their prescribed drug because of poor clinical response or side-effects impairing global functioning. Each patient had a DSM-IV diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder or delusional disorder. One month was required to have passed since the first onset of positive symptoms. Patients for whom the clinician considered substance misuse to be the primary cause of the psychotic illness and those with a history of neuroleptic malignant syndrome were excluded.
Outcome measures
The main outcome measure for the primary analysis was the Quality of Life Scale (QLS) score, as previously reported. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12,Reference Henrichs, Hanlon and Carpenter24 Secondary measures relevant to this analysis were the Barnes Akathisia Rating Scale (BARS) for akathisia, the Simpson–Angus Scale (SAS) for Parkinsonism and the Abnormal Involuntary Movement Scale (AIMS) for tardive dyskinesia. Reference Barnes25–Reference Guy27 Side-effects were considered to be present at each time point according to the following operational criteria: for akathisia, when participants scored 2 or more on the global akathisia item of the BARS; for Parkinsonism, when participants had a total score of 3 or more on the SAS; and for tardive dyskinesia, when participants had one score of 3 or two scores of 2 on AIMS items 1–7 covering observed movements. An ‘emergent’ side-effect was defined as one that was not present at baseline but was noted at follow-up; a ‘relieved’ side-effect was one present at baseline but absent at follow-up.
Statistical analysis
Intention-to-treat (ITT) analysis was performed. Individuals were grouped depending on the treatment to which they were allocated at randomisation and data were recoded according to the operational criteria for EPS at baseline, 12 weeks and 52 weeks. To test whether emergent and relieved EPS differed between the two treatment groups, data were transformed into binary categories and presented in contingency tables from which chisquared statistics and odds ratios were calculated; P values and 95% confidence limits were used to determine statistical significance.
It is common practice for clinicians to prescribe anticholinergic adjuncts to patients in response to EPS, particularly Parkinsonism, and sometimes in anticipation of such problems. To distinguish between patients receiving anticholinergic adjuncts in each study arm, the sample was stratified according to whether adjunctive medication was prescribed, effectively creating four treatment groups:
-
(a) first-generation antipsychotic alone;
-
(b) second-generation antipsychotic alone;
-
(c) first-generation antipsychotic with anticholinergic adjunct;
-
(d) second-generation antipsychotic with anticholinergic adjunct.
Procyclidine or trihexyphenidyl hydrochloride were the only anticholinergic drugs prescribed in this sample. After stratification by adjunct, the above analyses were repeated for the Parkinsonism outcome at 12 weeks and 52 weeks, as this is the condition that the adjuncts are most commonly used to prevent or treat. Comparisons were made between subgroups (a), (b) and (c). Statistical power was constrained in this, as in any secondary analysis where the risks of type 1 and 2 errors need attention because the main trial design is predicated on the primary outcome. Thus, we defined clinically relevant effects as double or half the prevalence of EPS between the two study groups, Reference Last28 measured as an odds ratio of ⩾2.0 or ⩽0.5 respectively; the first-generation antipsychotic only subgroup was used as the baseline in all analyses. This allowed us to make statements about clinically meaningful differences and define their precision in terms of conventional statistical parameters. Post hoc analysis of statistical power for these comparisons assumed a 15% prevalence of EPS in the first-generation group and α = 0.05. For an odds ratio of 2.0 the analysis had 78% power to reject the null hypothesis. All subsequent analyses were carried out using SPSS for Windows XP Release 15.0.
Results
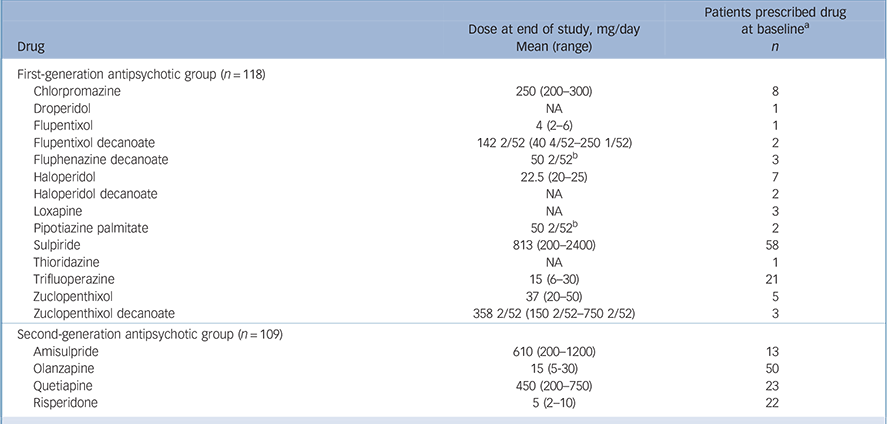
Table 1 lists the antipsychotic drugs prescribed to patients randomised into first- or second-generation treatment groups and the doses at the end of the study, all of which are within conventional limits. The most common first-generation drugs chosen were sulpiride and trifluoperazine; haloperidol was a relatively uncommon choice. The most commonly prescribed second-generation drugs were olanzapine, quetiapine and risperidone.
Emergent side-effects
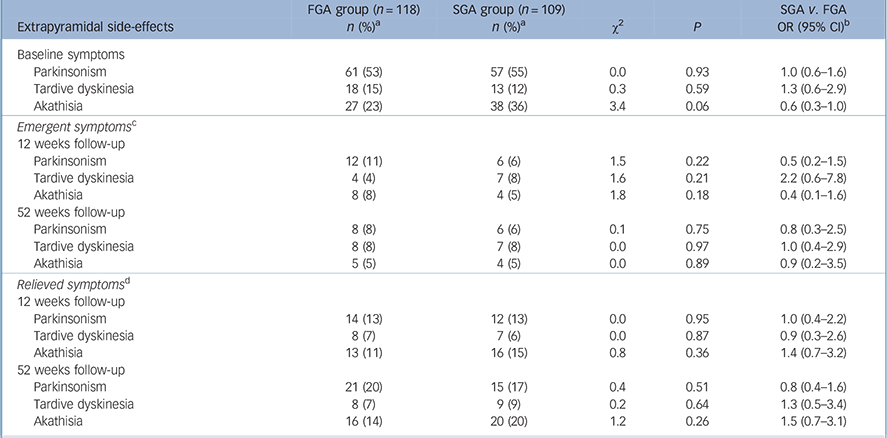
Table 2 describes the two treatment groups according to EPS at 12 weeks and 52 weeks, stratified into EPS that were emergent or resolved. There was no statistically significant difference between the groups in terms of emergent Parkinsonism, akathisia or tardive dyskinesia at either assessment point. Potentially clinically relevant differences in akathisia and Parkinsonism at 12 weeks in the second-generation group, both with odds ratios of 0.5 or less, did not reach statistical significance and were no longer present at the 52-week follow-up. Indication of a clinically significant increase in the development of tardive dyskinesia in the same group at 12 weeks (OR = 2.2, 95% CI 0.6–7.8) was similarly unconfirmed at conventional levels of statistical significance, and had disappeared by 52 weeks (OR = 1.0, 95% CI 0.4–2.9). These results suggest, overall, a null effect at 1-year follow-up.
TABLE 1 Antipsychotic drugs prescribed at baseline in the two treatment arms
| Drug | Dose
at end of study, mg/day Mean (range) |
Patients prescribed
drug at baselineFootnote a n |
|---|---|---|
| First-generation antipsychotic group (n = 118) | ||
| Chlorpromazine | 250 (200–300) | 8 |
| Droperidol | NA | 1 |
| Flupentixol | 4 (2–6) | 1 |
| Flupentixol decanoate | 142 2/52 (40 4/52–250 1/52) | 2 |
| Fluphenazine decanoate | 50 2/52Footnote b | 3 |
| Haloperidol | 22.5 (20–25) | 7 |
| Haloperidol decanoate | NA | 2 |
| Loxapine | NA | 3 |
| Pipotiazine palmitate | 50 2/52Footnote b | 2 |
| Sulpiride | 813 (200–2400) | 58 |
| Thioridazine | NA | 1 |
| Trifluoperazine | 15 (6–30) | 21 |
| Zuclopenthixol | 37 (20–50) | 5 |
| Zuclopenthixol decanoate | 358 2/52 (150 2/52–750 2/52) | 3 |
| Second-generation antipsychotic group (n = 109) | ||
| Amisulpride | 610 (200–1200) | 13 |
| Olanzapine | 15 (5-30) | 50 |
| Quetiapine | 450 (200–750) | 23 |
| Risperidone | 5 (2–10) | 22 |
NA, no end dose available owing to drug switching; 1/52, weekly; 2/52, fortnightly; 4/52, monthly.
a. Two data points are missing.
b. Equivalent dosing across all participants.
TABLE 2 Extrapyramidal side-effects in the first- and second-generation antipsychotic groups at baseline and at 12 weeks and 52 weeks follow-up, stratified by emergent and relieved symptoms at the two follow-up points
| Extrapyramidal side-effects | FGA group
(n = 118) n (%)Footnote a |
SGA group
(n = 109) n (%)Footnote a |
σ2 | P | SGA
v. FGA OR (95% CI)Footnote b |
|---|---|---|---|---|---|
| Baseline symptoms | |||||
| Parkinsonism | 61 (53) | 57 (55) | 0.0 | 0.93 | 1.0 (0.6–1.6) |
| Tardive dyskinesia | 18 (15) | 13 (12) | 0.3 | 0.59 | 1.3 (0.6–2.9) |
| Akathisia | 27 (23) | 38 (36) | 3.4 | 0.06 | 0.6 (0.3–1.0) |
| Emergent symptoms Footnote c | |||||
| 12 weeks follow-up | |||||
| Parkinsonism | 12 (11) | 6 (6) | 1.5 | 0.22 | 0.5 (0.2–1.5) |
| Tardive dyskinesia | 4 (4) | 7 (8) | 1.6 | 0.21 | 2.2 (0.6–7.8) |
| Akathisia | 8 (8) | 4 (5) | 1.8 | 0.18 | 0.4 (0.1–1.6) |
| 52 weeks follow-up | |||||
| Parkinsonism | 8 (8) | 6 (6) | 0.1 | 0.75 | 0.8 (0.3–2.5) |
| Tardive dyskinesia | 8 (8) | 7 (8) | 0.0 | 0.97 | 1.0 (0.4–2.9) |
| Akathisia | 5 (5) | 4 (5) | 0.0 | 0.89 | 0.9 (0.2–3.5) |
| Relieved symptoms Footnote d | |||||
| 12 weeks follow-up | |||||
| Parkinsonism | 14 (13) | 12 (13) | 0.0 | 0.95 | 1.0 (0.4–2.2) |
| Tardive dyskinesia | 8 (7) | 7 (6) | 0.0 | 0.87 | 0.9 (0.3–2.6) |
| Akathisia | 13 (11) | 16 (15) | 0.8 | 0.36 | 1.4 (0.7–3.2) |
| 52 weeks follow-up | |||||
| Parkinsonism | 21 (20) | 15 (17) | 0.4 | 0.51 | 0.8 (0.4–1.6) |
| Tardive dyskinesia | 8 (7) | 9 (9) | 0.2 | 0.64 | 1.3 (0.5–3.4) |
| Akathisia | 16 (14) | 20 (20) | 1.2 | 0.26 | 1.5 (0.7–3.1) |
FGA, first-generation antipsychotic; SGA, second-generation antipsychotic.
a. Minor discrepancies in column percentages due to missing data; these percentages could theoretically exceed 100% if multiple extrapyramidal side-effects in some participants.
b. Odds ratios >1.0 indicate SGA worse; OR <1.0 indicate FGA worse. Clinically meaningful effects defined as OR >2.0 or <0.5.
c. Symptoms occurring in those free from symptom at baseline.
d. Symptoms present at baseline but absent at follow-up.
There was no statistically significant difference between the treatment groups in terms of emergent Parkinsonism, akathisia or tardive dyskinesia at either follow-up point (Table 2). None of these effects achieved the a priori criteria for a clinically relevant effect in terms of symptom relief, suggesting that there was no clinically meaningful difference between the groups that was hidden by type 2 statistical error.
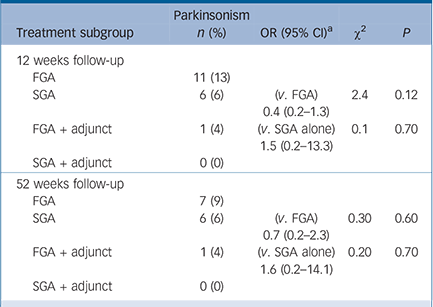
TABLE 3 Emergent Parkinsonism at 12 weeks and 52 weeks follow-up stratified by treatment arm and prescription of anticholinergic adjunct
| Treatment subgroup | Parkinsonism n (%) |
OR (95% CI)Footnote a | σ2 | P |
|---|---|---|---|---|
| 12 weeks follow-up | ||||
| FGA | 11 (13) | |||
| SGA | 6 (6) | (v. FGA) 0.4 (0.2–1.3) | 2.4 | 0.12 |
| FGA + adjunct | 1 (4) | (v. SGA alone) 1.5 (0.2–13.3) | 0.1 | 0.70 |
| SGA + adjunct | 0 (0) | |||
| 52 weeks follow-up | ||||
| FGA | 7 (9) | |||
| SGA | 6 (6) | (v. FGA) 0.7 (0.2–2.3) | 0.30 | 0.60 |
| FGA + adjunct | 1 (4) | (v. SGA alone) 1.6 (0.2–14.1) | 0.20 | 0.70 |
| SGA + adjunct | 0 (0) |
FGA, first-generation antipsychotic; SGA, second-generation antipsychotic.
a. All comparisons SGA v. FGA with or without anticholinergic adjunct such that OR <1.0 represents advantage for SGA, OR >1.0 represents disadvantage for SGA.
Use of anticholinergic adjuncts
In the first-generation antipsychotic group, 26 patients (22%) were prescribed an anticholinergic adjunct at baseline v. a single patient (1%) in the second-generation group; this 30-fold increase in odds was highly statistically significant (P<0.001), despite the equivalence of EPS at this time point. Table 3 shows the results of an analysis of emergent Parkinsonism stratified by prescription of anticholinergic adjunct in the study population. No effect reached statistical significance but the trends were as follows. At 12 weeks, individuals receiving a second-generation drug alone, were less likely to experience emergent Parkinsonism when compared with those taking a first-generation drug alone, according to the a priori criteria for a clinically relevant effect. At 52 weeks, there was no longer a clinically relevant effect size according to the pre-specified odds ratio criteria. In the subgroup comparison of patients prescribed a second-generation antipsychotic with those receiving a first-generation antipsychotic plus anticholinergic medication, there was no clinically or statistically significant difference at either time point.
Discussion
We report the results of a secondary analysis of EPS in the CUtLASS-1 trial. Owing to the statistical power constraints in such an analysis, we framed our results in the context of clinically important effects, defined as an odds ratio of 2.0 or 0.5, a double or half risk of EPS between the two study groups. Statistical power was limited but this approach allows us to conclude that there were few clinically relevant differences missed due to type 2 errors. The results were essentially null. The more frequent prescription of anticholinergic agents in the first-generation drug group despite equivalent EPS at baseline almost certainly represents clinical expectation of greater EPS in this arm. Overall, these results are in accord with those from other studies. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12–Reference Rochon, Stukel, Sykora, Gill, Garfinkel and Anderson15,Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins19,Reference Miller, Caroff, Davis, Rosenheck, McEvoy and Saltz20
Drug choice
Many RCTs showing second-generation antipsychotics to have a lower risk of EPS than a first-generation drug used haloperidol as the comparator. Haloperidol has a relatively high EPS liability and is often prescribed in a high dose that may be above the optimum dose for the study sample. The findings from the CUtLASS-1 study indicate that, in this pragmatic trial designed to emulate real physician prescribing behaviour, haloperidol was an uncommon choice for first-generation antipsychotic treatment (Table 1). This could contribute to the results suggesting that there are, generally, few clinically significant differences in the EPS profiles of first- and second-generation antipsychotics when those drugs are prescribed with a flexible approach and due care.
Emergent side-effects
The results from the analysis of emergent EPS showed that at 1-year follow-up there was no clinically significant difference between the two drug classes. Although there was a clinically significant decrease in Parkinsonism and akathisia for the second-generation antipsychotic group at 12 weeks (ORs 0.5 and 0.4 respectively), this effect was diminished at the 52-week follow-up point, suggesting that the benefit was not long-lasting. It does, however, remain possible that the clinically significant decrease in EPS at 12 weeks was real and that the null effect at 52 weeks was due to increased class switching that is not reflected in the ITT analysis but has been previously reported. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23
Despite the decreases in Parkinsonism and akathisia at 12 weeks, tardive dyskinesia was twice as common at this point in the second-generation group (OR = 2.2) but was not statistically significant and this potentially clinically relevant effect also disappeared by 52 weeks. One possible interpretation of this result is that tardive dyskinesia is temporarily exacerbated by withdrawal of dopamine-2 receptor blockade, reflecting a change in the neurotransmitter milieu resulting from the switch in drug class. In addition, the degree of tardive dyskinesia has been shown to worsen with adjunctive anticholinergic medication, Reference Klawans and Rubovits29 and to improve with its discontinuation. Reference Greil, Haag, Rossnagl and Rüther30 Therefore, the high intrinsic anticholinergic activity of some second-generation drugs such as olanzapine may have contributed to this effect. Reference Moore, Tye, Axton and Risius31
Relieved side-effects
There was no clinically significant difference between first- and second-generation antipsychotic drugs in terms of relief from baseline EPS at either 12-week or 52-week follow-up. Second-generation drugs appeared to be no more successful than the older ones in providing relief from these side-effects. This is surprising in the context of the common belief that first-generation anti-psychotics exacerbate such problems, but nonetheless is in line with the CATIE results.
Use of anticholinergic adjuncts
Anticholinergic adjuncts were more typically prescribed to prevent or mitigate EPS such as Parkinsonism in those receiving first-generation antipsychotics. However, the justification for the overwhelming difference in adjunct prescription between the two treatment arms is unclear, given the fact that there was no sustained, clinically relevant difference in EPS between the two groups and no difference at baseline. An anticholinergic adjunct was prescribed for just one patient taking a second-generation drug, despite the equivalence in EPS profiles between the classes in this study. One possible explanation for this finding is that clinicians are more likely to prescribe anticholinergic adjuncts on the basis of their expectations regarding the side-effect profile of the drug, especially given their likely assumption at the time that second-generation antipsychotics would have markedly lower liability to EPS. Reference Lloyd, Markwick, Page, Lewis and Barnes32 Clinicians prescribing a first-generation drug may have expected the development of EPS and/or had a lower threshold for the detection of these symptoms; thus, they would have been more likely to prescribe an anticholinergic drug as an adjunct in anticipation of or in response to EPS. Other studies have shown that adherence to second-generation antipsychotic treatment can be enhanced by the prescription of an anti-cholinergic along with the antipsychotic, Reference Ren, Huang, Lee, Miller, Qian and Kazis33 so it is notable that in this case they were so rarely used. The clinical threshold for detection of EPS may be higher than that applied when prescribing a first-generation antipsychotic where these symptoms are expected. This was true for psychotic symptoms, as clinicians switched patients randomised to first-generation antipsychotics at a lower score on the Positive and Negative Syndrome Scale than patients randomised to the newer drugs. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23
The analysis of emergent Parkinsonism stratified by adjunctive prescription showed a clinically significant difference when comparing second-generation antipsychotics with first-generation antipsychotics alone at 12-week follow-up. However, there was no clinically significant difference in the comparison between second-generation antipsychotics and first-generation antipsychotics plus anticholinergic at this time point. This suggests that potential EPS from first-generation antipsychotics can be effectively managed with adjunctive anticholinergic medication. At 52 weeks there was no clinically significant difference between any of the three groups. Anticholinergic prescription may have attendant problems, such as cognitive deficit and potential for misuse, but we note this was not reflected in the overall results of the CUtLASS-1 study. Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst12
Limitations
Secondary analyses of trial data often face limitations in terms of statistical power given that the original studies are designed around the primary outcome of the original trial. Estimates of power undertaken post hoc indicated that this was reasonable for the clinically relevant effects that we defined. By pre-defining these clinically significant odds ratios we were able to interpret our results in the context of patient experience with these medications; we do not think we have missed important effects due to type 2 error. An effort was also made to minimise the number of statistical tests carried out in this analysis to control for type 1 errors. For instance, the use of anticholinergic drugs by clinicians in response to Parkinsonism is based on the strongest evidence, so we restricted statistical tests to this side-effect. We did not stratify our analyses according to the reason for referral to the trial for similar reasons of limited statistical power.
Masking of raters to treatment allocation is an important source of potential bias. Considerable efforts were made to maintain the masking, including physical separation of raters from clinical teams, reminders to patients not to divulge their treatment, and technical aspects of the randomisation procedure and study database. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23 Known breaches were reported and affected four participants in the first-generation group and two in the second-generation group. Nevertheless, it is possible that subtle indications were apparent in more cases. If such bias was present, however, we believe that it would most probably have operated against the older drugs, for example EPS might be more likely to be rated as present in participants in whom signs of treatment with an anticholinergic agent were present. Thus, we consider this potential bias an unlikely cause of the null results.
Implications
This analysis illuminates the relative side-effect profiles of first- and second-generation antipsychotics in terms of EPS when used in the context of a clinical trial. It suggests some misconceptions prevalent among the participating clinicians at that time regarding their expectation of motor side-effects; Reference Lloyd, Markwick, Page, Lewis and Barnes32 they were, in fact, able to use the two classes of drugs with equivalence in EPS. This ITT comparison shows that there was weak evidence (not statistically significant) for few clinically significant differences in terms of emergent or relieved EPS between the two classes of antipsychotics at 12 weeks, and none at 52 weeks. One implication is that judicious prescription of adjunctive anticholinergic agents to manage EPS when prescribing first-generation antipsychotics can result in an EPS profile equivalent to second-generation drug treatment. Reference Rosenheck34 This analysis contributes to the existing literature on EPS profiles of schizophrenia medications by demonstrating the effects of these drugs in real clinical practice. However, further work is necessary to determine definitively the treatment regimens that will provide the greatest benefit while causing the fewest adverse effects for people with schizophrenia and similar disorders.
The results suggest that prescribers can rise to the challenge of using both first- and second-generation compounds at doses that result in levels in the domain between the dose–response curves for beneficial and extrapyramidal effects. This dose-dependent therapeutic domain differs not only between drugs but also between patients. Although the introduction of second-generation drugs may have improved prescribing through an increased emphasis on monotherapy and vigilance for EPS, these benefits can be obtained with first-generation drugs and may be better achieved with the latter in some cases. Indeed, many patients may not have been well served by the rapid restriction of the number of antipsychotic drugs in common use, as the second-generation drugs became the only antipsychotics used by most clinicians. Reference Cheng, Jones, Mortimer and McKenna35
The emergence of obesity and metabolic abnormalities leading to life-threatening increases in risk of cardiovascular disease is a serious complication of antipsychotic prescribing, with second-generation drugs in particular being implicated. Reference Lewis, Davies, Jones, Barnes, Murray and Kerwin23 In advance of new antipsychotic drugs with truly novel mechanisms of action that may not have these side-effects, patients urgently need us to reappraise the relative positions of the two generations of drugs on our therapeutic palette, Reference Mackin and Thomas36 ideally with further randomised trials to guide the use of a wider range of antipsychotic options. There are educational implications of a return to the careful use of first-generation drugs as a treatment option for some people, because a generation of psychiatrists is now unfamiliar with their use.






eLetters
No eLetters have been published for this article.