Introduction
Low back pain (LBP) is quite common in older adults and is often associated with impairments in both physical and psychosocial domains (Leopoldino et al., Reference Leopoldino, Diz, Martins, Henschke, Pereira and Dias2016; Wong, Karppinen, & Samartzis, Reference Wong, Karppinen and Samartzis2017). Some studies have suggested that factors such as advanced age, lower educational attainment, smoking habits, lower economic status, limited access to health services, and presence of multi-morbidities are associated with higher levels of pain and disability in older adults with LBP (Jarvik et al., Reference Jarvik, Comstock, Heagerty, Turner, Sullivan and Shi2014; Parreira et al., Reference Parreira, Maher, Ferreira, Machado, Blyth and Naganathan2017; Stewart Williams et al., Reference Stewart Williams, Ng, Peltzer, Yawson, Biritwum and Maximova2015); however, little is known about the influence of physical frailty on LBP.
Previous studies have shown an association between frailty and the presence of musculoskeletal conditions, such as osteoarthritis (Castell et al., Reference Castell, van der Pas, Otero, Siviero, Dennison and Denkinger2015; Misra et al., Reference Misra, Felson, Silliman, Nevitt, Lewis and Torner2015), chronic LBP (Coyle, Sions, Velasco, & Hicks, Reference Coyle, Sions, Velasco and Hicks2015), and chronic musculoskeletal pain (Megale et al., Reference Megale, Ferreira, Ferreira, Naganathan, Cumming and Hirani2018; Shega et al., Reference Shega, Dale, Andrew, Paice, Rockwood and Weiner2012; Wade et al., Reference Wade, Lee, McBeth, Ravindrarajah, Gielen and Pye2016). To the best of our knowledge, there is no study addressing the influence of frailty on health outcomes, such as pain intensity, disability, and health-related quality of life (HRQOL), following acute musculoskeletal condition.
Frailty represents a state of vulnerability caused by decreased physiological reserves. As it is known that frail older adults have decreased ability to deal with acute stressors (Chen, Mao, & Leng, Reference Chen, Mao and Leng2014), we hypothesized that such individuals might be at risk of experiencing higher levels of pain and more severe disability after an episode of acute non-specific LBP. Therefore, the aim of this study was to investigate whether frailty is independently associated with higher levels of pain and disability and lower HRQOL among older adults seeking care for acute non-specific LBP.
Methods
Study Design and Participants
A cross-sectional analysis was conducted using baseline data from the Back Complaints in the Elders (BACE-Brazil) study, a cohort study addressing the clinical course and prognostic factors related to acute non-specific LBP in older adults. Individuals 55 years of age and older who sought medical care for acute non-specific LBP were invited by physicians or allied health care professionals at primary care settings to contact the Brazilian BACE research team in charge of screening participants for eligibility. Non-specific LBP was defined as any pain without specific cause occurring between the last ribs and inferior gluteal folds, with or without leg pain (Dionne et al., Reference Dionne, Dunn, Croft, Nachemson, Buchbinder and Walker2008). A new acute episode was defined as one occurring within 6 weeks or less of the enrolment period, which was preceded by no less than a 6-month pain-free period. Participants were excluded if they had any cognitive impairment, severe medical disease, or motor, visual, or hearing loss that would prevent them from being assessed during data collection. A structured multidimensional questionnaire was used to obtain socio-demographic and clinical data. All participants signed an informed consent form. Details on the BACE study protocol have been published elsewhere (Scheele et al., Reference Scheele, Luijsterburg, Ferreira, Maher, Pereira and Peul2011). BACE-Brazil was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (ETIC 0100.0.203.00-11).
Independent Variable
Frailty assessment
The presence of frailty was assessed according to the Cardiovascular Health Study (CHS) frailty phenotype (Fried et al., Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener2001). Frailty was defined as the presence of three or more of the following criteria.
-
(1) Unintentional weight loss – defined as weight loss greater than or equal to 4.5 kg in the past year.
-
(2) Self-reported exhaustion – assessed using the questions: “Did you feel that you had to exert yourself to perform your daily tasks?” and/or “Were you not able to carry out your activities?” The answers "most often" or "always" to at least one of such questions indicated the presence of exhaustion.
-
(3) Low physical activity – defined as being below the lowest CHS quintile score for kilocalories expended per week adjusted for sex. In the BACE-Brazil, kilocalories expended per week were estimated based on physical activity level assessed with the Brazilian-Portuguese version of the Active Australia Questionnaire (Rocha et al., Reference Rocha, Soares, Leopoldino, Queiroz, Rosa and Lustosa2017).
-
(4) Weakness – defined as being below the lowest CHS quintile score for grip strength adjusted for sex and body mass index (BMI). Maximal grip strength in the dominant hand (average of three trials) was measured using the JAMAR® dynamometer.
-
(5) Slowness – defined as time to walk 4.6 m at a usual pace above the sex and height adjusted CHS cut-off points. This test was performed twice, with an interval of 1 minute between repetitions, and the average of the two trials was used for data analysis.
Participants who met one or two of these criteria were classified as pre-frail, and those who had not met any criteria were classified as robust.
Dependent Variables
Pain intensity assessment
LBP intensity was assessed using an 11-point numeric rating scale (NRS) ranging from 0 to 10, where 0 indicates no pain and 10 indicates extreme pain (Mawdsley, Moran, & Conniff, Reference Mawdsley, Moran and Conniff2002).
Disability assessment
Disability was assessed using the Roland Morris Disability Questionnaire (RMDQ), which consists of 24 questions that address functional limitations resulting from LBP (Costa et al., Reference Costa, Maher, Latimer, Ferreira, Pozzi and Ribeiro2007); the total RMDQ score is the sum of positive responses and ranges from 0 to 24, with higher scores indicating greater disability.
HRQOL assessment
The Medical Outcome Study (MOS) Short Form-36 (SF-36) – Physical Component Summary (PCS), and the MOS SF-36 – Mental Component Summary (MCS) were used to assess HRQOL (Ware & Sherbourne, Reference Ware and Sherbourne1992).
Covariates
Age, sex, marital status, education level, income, BMI, depressive symptoms, and the presence of co-morbidities were used as covariates in statistical models for assessing the association between frailty and pain intensity, disability, or HRQOL. Depressive symptoms were assessed by the Center for Epidemiologic Studies Depression (CES-D) scale, and their presence was defined as a score of 16 or greater on this scale (Lewinsohn, Seeley, Roberts, & Allen, Reference Lewinsohn, Seeley, Roberts and Allen1997). Co-morbidities were assessed by the Self-administered Comorbidity Questionnaire (SCQ), which addresses 12 self-reported medical conditions (Sangha, Stucki, Liang, Fossel, & Katz, Reference Sangha, Stucki, Liang, Fossel and Katz2003); scores on the SCQ range from 0 to 36, and greater scores indicate a greater co-morbid load.
Data Analysis
Descriptive data of the total sample and frailty subgroups were presented as mean and standard deviation (SD) for continuous variables and absolute (n) and relative (%) frequency for categorical variables. Differences in socio-demographic and clinical data among the three frailty subgroups (robust, pre-frail, and frail) were assessed using analysis of variance (ANOVA) for continuous variables and Fisher’s exact test for categorical variables. To assess pairwise differences in continuous variables, post-hoc analysis using a Bonferroni test was performed when statistical differences were detected in the ANOVA test. Simple linear regression models were used to determine unadjusted coefficients for the association between frailty (as an independent variable) and NRS, RMDQ, PCS, and MCS scores (as dependent variables). We conducted a multivariate analysis considering potential confounders to investigate whether the associations between frailty status and pain intensity, disability, or HRQOL were independent of socio-demographic and clinical factors. Three models of multivariate linear regression were used: (1) Model 1: adjusted for socio-demographic variables (age, sex, marital status, education level, and income); (2) Model 2: adjusted for clinical variables (BMI, depressive symptoms, and co-morbidities); and (3) Final model: adjusted for all independent variables that presented a p value < 0.20 in the previous statistical models. The level of significance was set at p < 0.20 to ensure that potential associated factors were not excluded. A significant level was considered for all other statistical analyses when the p value was < 0.05. Data were analyzed with the STATA software package, version 13 (StataCorp LP, College Station, TX, USA).
Results
Six hundred and two individuals (84.9% female) with a mean age of 67.6 (SD 7.0) years were enrolled in the BACE-Brazil study. However, 13 participants did not have information for frailty status and were excluded. Therefore, 589 participants composed our analytical sample. According to the CHS frailty criteria, 21.3 per cent of the sample were classified as robust, 59.2 per cent were classified as pre-frail, and 19.5 per cent were classified as frail. The descriptive data of the frailty subgroups at baseline and comparison across robust, pre-frail, and frail subgroups are presented in Table 1. There was a statistically significant difference among frailty subgroups in categorical variables education level, income, depressive symptoms, and obesity. For the continuous variables, the Bonferroni post-hoc test showed differences among all frailty subgroups for the SCQ score (robust vs. pre-frail: p = 0.001; robust vs. frail: p < 0.001; pre-frail vs. frail: p = 0.001) and for BMI there was a statistical difference only between the robust and pre-frail groups (p = 0.018).
Table 1: Baseline characteristics of the study participants and comparison among robust, pre-frail, and frail individuals (n = 589)

Note. Missing data: a0.2%; b0.3%; c1.7%; d13.8%.
e 1 minimum wage in 2014 was $302.80.
** Bonferroni test: robust versus pre-frail: p = 0.001; robust versus frail: p < 0.001; pre-frail versus frail: p = 0.001.
*** Bonferroni test: robust versus pre-frail: p = 0.018.
n = absolute number; SD = standard deviation; CES-D = Centre for Epidemiologic Studies Depression Scale; SCQ = Self-Administered Comorbidity Questionnaire; BMI = body mass index.
The NRS, RMDQ, PCS, and MCS scores stratified by frailty status, as well as the simple linear regression coefficients, are shown in Table 2. Pre-frail and frail participants had significantly higher pain intensity, higher disability levels, and lower scores in both the physical and mental domains of the SF-36 than the robust subgroup. According to our model, NRS scores are expected to be 0.65 (95% confidence interval [CI] 0.12–1.17; p = 0.016) and 1.15 (95% CI 0.50–1.80; p = 0.001) higher for pre-frail and frail individuals, respectively (robust as reference). Likewise, the RMDQ scores are expected to be 3.83 (95% CI 2.70–4.95; p < 0.001) and 7.24 (95% CI 5.84–8.63; p < 0.001) higher, the PCS scores are expected to be 3.64 (95% CI 2.08–5.19; p < 0.001) and 8.14 (95% CI 6.21–10.01; p < 0.001) lower, and the MCS scores are expected to be 6.73 (95% CI 4.07–9.38; p < 0.001) and 12.62 (95% CI 9.34–15.91; p < 0.001) lower in pre-frail and frail participants, respectively.
Table 2: Mean scores and unadjusted linear regression coefficients for the association between frailty status and numeric rating scale, Roland Morris Disability Questionnaire, and SF-36 Physical and Mental Component Summary scores in individuals with acute low back pain (n=589)

Note. SD = standard deviation; CI = confidence interval; NRS = numeric rating scale; RMDQ = Roland Morris Disability Questionnaire; PCS = physical component summary; MCS = mental component summary; NA = not applicable.
The inclusion of socio-demographic and clinical variables in the multivariate linear regression changed the regression coefficients and the statistical significance of the association between frailty status and NRS, RMDQ, PCS, and MCS scores. In model 1, adjusted only for socio-demographic variables, frailty status was still significantly associated with pain, disability, and HRQOL as measured by the PCS and MCS scores (Table 3). Model 2 was adjusted only by clinical variables (BMI, depressive symptoms, and co-morbidities). The inclusion of these variables, particularly depressive symptoms and co-morbidities, might be responsible for the association between frailty status and pain, and between frailty status and the MCS scores being no longer statistically significant (Table 3). The final model of the multivariate linear regression (including all variables that presented a p value < 0.20 in the previous statistical models) showed that frailty status was independently associated only with LBP-related disability and the physical component of HRQOL. The regression coefficients for the association between frailty status and RMDQ scores were 1.68 (95% CI 0.56–2.80; p = 0.003) and 3.69 (95% CI 2.19–5.19; p < 0.001), and those for the association between frailty status and PCS scores were -2.74 (95% CI -4.37 to -1.12; p = 0.001) and -6.50 (95% CI -8.63 to -4.38; p < 0.001) for pre-frail and frail participants, respectively (robust as reference). No association between frailty and pain intensity or t
Table 3: Multivariate linear regression coefficients for the association between frailty status and numeric rating scale, Roland Morris Disability Questionnaire, SF-36 Physical and Mental Component Summary scores in individuals with acute low back pain (n=589)
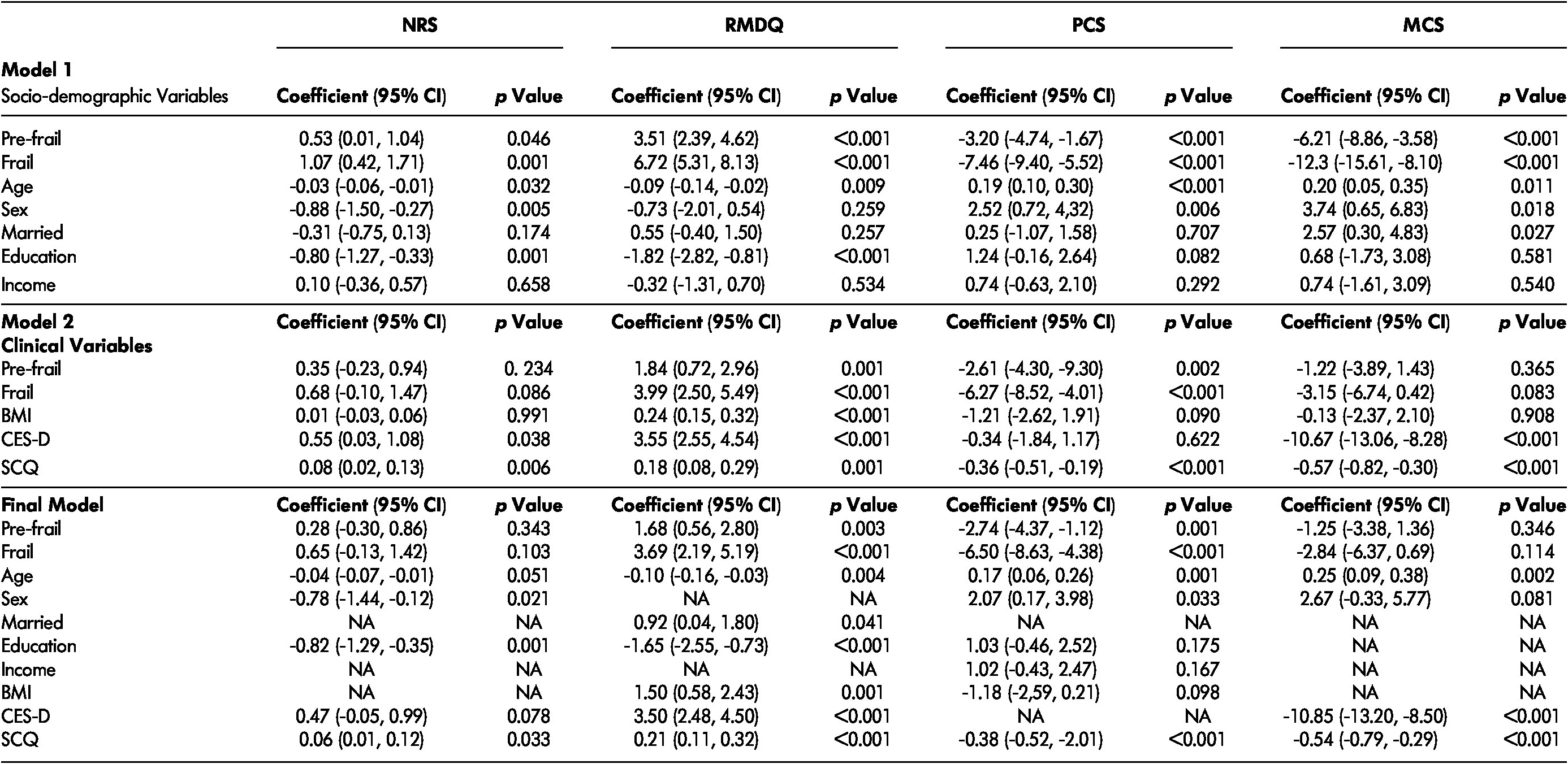
Note. NRS = numeric rating scale; RMDQ = Roland Morris Disability Questionnaire; PCS = physical component summary; MCS = mental component summary; CI = confidence interval; BMI = body mass index; CES-D = Centre for Epidemiologic Studies Depression Scale; SCQ = Self-administered Comorbidity Questionnaire; NA = not applicable.
he mental component of HRQOL was found (Table 3).
Discussion
This study has shown that, in older adults with acute non-specific LBP, frailty status is independently associated with LBP-related disability and the HRQOL physical component, but not with pain intensity or the HRQOL mental component. It was expected that frailty would influence both physical and psychosocial domains; nevertheless, our study shows that only physical domains are sensitive to frailty status.
Previous studies have shown an association between pain intensity and frailty (Nessighaoui et al., Reference Nessighaoui, Lilamand, Patel, Vellas, Laroche and Dantoine2015), which could be, in part, mediated by depressive symptoms (Chiou, Liu, Lee, Peng, & Chen, Reference Chiou, Liu, Lee, Peng and Chen2018; Sanders, Comijs, Bremmer, Deeg, & Beekman, Reference Sanders, Comijs, Bremmer, Deeg and Beekman2015; Tian et al., Reference Tian, Wang, Qiao, Liu, Dong and Butler2018). In the BACE study baseline, the adjustment for depressive symptoms was the main reason for not finding a significant association between frailty and pain intensity or SF-36 MCS scores in the multivariate analysis. Our results strengthen the hypothesis that depressive symptoms are important mediators in the association between pain and frailty. It is noteworthy to highlight that the BACE study included only individuals with acute pain, and so it is unlikely that pain had influence on the development of depressive symptoms or frailty in our sample. Conversely, depressive symptoms could have led frail older adults to report higher pain intensity after an episode of acute non-specific LBP.
There is evidence that frailty and late-life depression in older adults are overlapping syndromes with bidirectional association (Lohman, Dumenci, & Mezuk, Reference Lohman, Dumenci and Mezuk2016; Mezuk, Edwards, Lohman, Choi, & Lapane, Reference Mezuk, Edwards, Lohman, Choi and Lapane2012). Considering that late-life depression could be, in some patients, an intermediary step in the causal relationship between frailty and pain, the adjustment for depressive symptoms might be incorrect for part of our sample. Frailty and late-life depression have common risk factors, and it is still unclear if depression is a confounder variable in the frailty–pain relationship or if frailty and late-life depression are just different phenotypic expressions of the same underlying pathology.
Education is an important socio-demographic variable and, in our study, it was found to be significantly associated with pain intensity and disability, independent of the presence of frailty status. Low education reflects the deprivation of opportunities and inequality in the health status of older adults throughout their lives (Stewart Williams et al., Reference Stewart Williams, Ng, Peltzer, Yawson, Biritwum and Maximova2015). Poor socio-economic conditions, little formal education, and low income are characteristics present in more debilitated people, who are more susceptible to health problems, such as frailty (Casale-Martínez, Navarrete-Reyes, & Avila-Funes, Reference Castell, van der Pas, Otero, Siviero, Dennison and Denkinger2015). The CHS (n = 5,317) showed an association between frailty, and lower education and income, poorer health, and higher rates of co-morbidities and disability (Fried et al., Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener2001). In turn, the BACE-Brazil, with 602 older adults, compared groups with different levels of education and income and found that those with 4 years or less of education, and income equal to or less than two minimum wages, had worse scores on disability and pain catastrophizing (Jesus-Moraleida et al., Reference Jesus-Moraleida, Ferreira, Ferreira, Silva, Assis and Pereira2018).
The finding that frailty is associated with LBP-related disability and SF-36 PCS scores could be explained by the fact that frailty, by affecting multiple systems and diminishing physiological reserves, decreases the ability to maintain functional status after an acute episode of LBP (Fried, Ferrucci, Darer, Williamson, & Anderson, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004). Therefore, the odds of physical impairments would be increased in individuals who had both conditions simultaneously. The use of CHS frailty phenotype criteria to assess frailty may have played a role in our results. The CHS frailty phenotype criteria identify individuals with physical frailty, which represents a pre-disability condition (Xue, Reference Xue2011). We wonder if similar results would be found had a multidimensional concept of frailty been used instead.
Although the association between frailty and activities of daily life (ADL) disability has already been established in the literature (Chen et al., Reference Chen, Mao and Leng2014; Fried et al., Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener2001, Reference Fried, Ferrucci, Darer, Williamson and Anderson2004), our study specifically concerns disability caused by LBP, as assessed by the RMDQ. In addition, previous studies showing an association between frailty and poor HRQOL in older adults did not include only individuals with acute LBP (Chang et al., Reference Chang, Chen, Lin, Fang, Yen and Hsieh2012; Rizzoli et al., Reference Rizzoli, Reginster, Arnal, Bautmans, Beaudart and Bischoff-Ferrari2013). Not only the presence of acute LBP may worsen HRQOL, but poor HRQOL may have an influence on acute LBP.
This study represents the very first step towards understanding the influence of frailty on older adults with acute LBP. Although the influence of age in the course of LBP has been widely investigated in previous research (Stewart Williams et al., Reference Stewart Williams, Ng, Peltzer, Yawson, Biritwum and Maximova2015; Walker, Reference Walker2020), the influence of frailty has not been. Age itself is not a good predictor of physiological reserves, as older adults of the same age present with different levels of vulnerability. The follow-up of BACE participants will allow an investigation as to whether frailty is associated with a worse long-term prognosis in terms of recovery from disability and development of chronic LBP. Moreover, it will be possible to determine whether experiencing an episode of acute LBP is associated with future development of frailty in this population.
Strengths and Limitations
Our study has a number of strengths: (1) adequate sample size and power to address all study outcomes, which allowed adjustments for potential confounders and improved the estimates of the regression coefficients; (2) a standardized approach was used to define frailty and all study variables; and (3) this was the first study to investigate the association between frailty and pain intensity, disability, and HRQOL in individuals seeking care for acute LBP. The limitations of this study refer to: (1) the convenience sampling of the BACE-Brazil study, which caused men to be under-represented in our sample (< 20%); (2) the cross-sectional design that did not allow definitive inferences about the associations found; and (3) the fact that it was not possible to identify whether the same problem that was causing LBP was related to the development of frailty or not.
Conclusion
In older adults with acute non-specific LBP, frailty is independently associated with disability related to LBP and the physical component of HRQOL, but not with pain intensity or the mental component of HRQOL. Longitudinal studies are needed to investigate the influence of frailty status on recovery from LBP-related disability and development of chronic LBP.





