Introduction
In the summer of 2012, an exceptionally massive bloom of Karenia mikimotoi occurred off the coastal waters of Fujian province, with a high biomass of 107 cells L−1 and a long duration of 21 d (SOAC, 2013). During such outbreaks of K. mikimotoi, mass mortality of abalone, Haliotis discus hannai was observed in the farming regions and, consequently more than $330 million was lost (SOAC, 2013), which may be one of the most serious harmful algal bloom (HAB) economic losses recorded throughout the world. The large scale and huge economic impact made further research into the bloom essential.
In fact, K. mikimotoi has always been one of the most prevalent HAB species along Chinese coasts in recent decades (Zhou, Reference Zhou, Ishimatsu and Lie2010). Previously, the K. mikimotoi bloom was known as a famous ‘fish killer’ in China and frequently appeared in the South China Sea (Qi et al., Reference Qi, Chen, Wang, Xu, Wang, Shen, Lu and Hodgkiss2004; Ou, Reference Ou2006; SOAC, 2006; Li et al., Reference Li, Glibert, Zhou, Lu and Lu2009). Most seriously, it caused almost all of the cultured fish in cages to be killed in the adjacent coastal areas near Pearl estuary and was also associated with nearly $60 million in losses (Yang and Hodgkiss, Reference Yang and Hodgkiss2004). However, this outbreak in Fujian unexpectedly killed the abundant abalone instead of fish. It was thought possible that the lethal mechanisms of K. mikimotoi from Fujian differed from previous blooms, in particular the nature of the microalgal toxicity between them, given their various geographical biotope around China's coasts.
The cases of Karenia spp. blooms causing the death of shellfish in the wild have been long-known in Japan, South Africa, and other countries (Table 1). Except for the toxic effect of autologous algal toxicity, the dissolved oxygen (DO) depletion resulted from Karenia spp. blooms were also a major factor in the mortality of shellfish (Yasumoto et al., Reference Yasumoto, Underal, Aune and Graneli1990; Satake and Tanaka, Reference Satake and Tanaka2005; Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010; O'Boyle et al., Reference O'Boyle, McDermott, Silke and Cusack2016). In Fujian, abalones were cultured densely and abundantly in offshore rafts along the coast, and these also suffered from hypoxia in the presence of K. mikimotoi blooms. Therefore, it was necessary to further determine whether massive mortalities of abalones were associated with the microalgal toxicity of K. mikimotoi or DO depletion during the blooms.
Table 1. Records of shellfish species mortality due to Karenia species bloom

ND, No data.
A similar type of abalone mortality, when exposed to Karenia cristata and Karenia concordia has been recorded (Botes et al., Reference Botes, Smit and Cook2003; Chang et al., Reference Chang, Uddstrom, Pinkerton and Richardson2008), however, little is known about the reaction of abalone to Karenia hazards. During harmful algal blooms, the mortality of molluscs was usually due to damage to their main organs or immune system. Of these, gills are the most susceptible because of the direct contact with seawater for respiratory gas exchange. Harmful algae can cause some vacuolation, abnormality, and even shrinkage of mollusc gills (eg. Mercenaria mercenaria, Ruditapes philippinarum) (Basti and Segawa, Reference Basti and Segawa2010; Hégaret et al., Reference Hégaret, Smolowitz, Sunila, Shumway, Alix, Dixon and Wikfors2010). Additionally, haemocytes are crucial for the proper function of the immune system and are important health indicators. Exposure to HABs allows for the interaction with haemocytes found throughout the open circulatory system. The immune system is also easily destroyed by HABs through inducing haemocyte diapedesis (Haberkorn et al., Reference Haberkorn, Lambert, Le Goı̈c, Guéguen, Moal, Palacios, Lassus and Soudant2010; Medhioub et al., Reference Medhioub, Lassus, Truquet, Bardouil, Amzil, Sechet, Sibat and Soudant2012; Lassudrie et al., Reference Lassudrie, Soudant, Richard, Henry, Medhioub, Silva, Donval, Bunel, Goïc, Lambert, Montaudouin, Fabioux and Hégaret2014, Reference Lassudrie, Wikfors, Sunila, Alix, Dixon, Combot, Soudant, Fabioux and Hégaret2015). Therefore, K. mikimotoi blooms in Fujian probably damaged the gills and immune systems of abalones leading to mass mortality.
To test the hypotheses above, we evaluated the differential algal character and toxicity on abalones between K. mikimotoi strains isolated from the South China Sea (K.mikimotoi – SCS strain) and Fujian coastal areas (K.mikimotoi – FJ strain). The immunological and histopathological changes in abalones were also investigated by K. mikimotoi – FJ at low bloom concentrations to determine the specific mechanisms of killing abalones.
Materials and methods
Algal species and abalone cultures
Algal cultures and juvenile abalones were used to determine the effects of exposure to K. mikimotoi on abalones. The dinoflagellate K. mikimotoi – FJ strain was isolated during the 2012 bloom in the Fujian coastal area, China, provided by the Centre for Collections of Marine Algae, Xiamen University. K.mikimotoi – SCS strain was isolated from the South China Sea in 2005 and provided by two National Basic Research Priority Programmes of China, CEOHAB-I and -II. The two strains were cultured in modified f/2 medium in 5-L flasks, and prepared without the addition of silicate. The cultures were kept at 20 ± 1 °C on a 12:12 L:D cycle, with irradiance of 56 μE m−2 s−1.
Considering that a large amount of the juvenile abalone died during the algal bloom accident in Fujian, therefore, the juvenile abalone was used in this research. The juvenile abalone H. hannai was bought from Longwan Biotechnology Corporation, Qingdao, China. Abalones were transported to the laboratory within 2 h in small aerated tanks. Healthy abalones of similar size (initial weight 0.9 ± 0.1 g; shell length 21.0 ± 3.4 mm) were picked out in the laboratory and placed in several 100 L tanks filled with filtered seawater and continuously aerated with air stones. The abalones were acclimated to the experimental condition for 7 days, during which they were fed daily on the macroalga Laminaria japonica. All of the seawater in the tanks was completely renewed every day, and the tanks were cleaned every 2–3 days.
The natural seawater used in this study was pumped from Taipingjiao (a clean site with no known pollution history) at Qingdao and sand filtered prior to use in the laboratory. Prior to the experiments, the seawater was also subjected to filtration through a 0.45 mm pore-size cellulose nitrate membrane, boiling for sterilization. The pH of the seawater was measured using a HI991000 pH instrument. Salinity was adjusted to 31 ± 1 ‰ with distilled water as determined using an ATAGO hand-held refractometer.
Haemolytic activity
The preparation of intra- and extra-cellular lipophilic crude extracts: haemolytic activities of the two strains at different growth phases were measured by the protocol adapted from Zhou et al. (Reference Zhou, Fernandez, Chen, You and Yan2011). For each strain, four batches of 3 L were cultured in a modified f/2 medium in 5 L flasks. Initial cell numbers were estimated at 0.5 × 107 cells L−1. Cells were counted every 2 days. Sampling was done at the following algal phases: pre-exponential phase, exponential phase, stationary phase, and the anaphase. At every phase, one batch for each strain was harvested and the cell density was recorded in Table 1. To detect the intra - and extra-cellular toxicity, ultrasonic ruptured cell suspensions (URCS) and cell-free culture supernatants (CFCS) were prepared from the algal culture, respectively. 3 L of culture was filtered through a 0.45 mm Whatman GF/C membrane at a negative pressure of 0.01 MPa. The filters and cells were washed with 100 mL of extraction liquid (chloroform: methanol: water = 13: 7: 5, v/v/v) and then ruptured in a sonicator for 30 min at 200 W, 0 °C, 5 : 5 s. The ruptured medium was observed by microscope to confirm that no cells existed and identified as URCS. URCS was centrifuged at 5000 × g (4 °C) for 10 min. The supernatant was transferred into a separating funnel for the lipophilic liquid parts collection, which was then evaporated to dryness at 40 °C at negative pressure of 0.05 MPa. The dry, lipophilic crude extract was stored in 2 mL absolute methanol (final volume) at −20 °C till use. The filtering medium served as CFCS and was immediately filtered by SPE C18 Cartridges. The SPE was collected and eluted with 10 mL methanol with the concentration of 20%, 40%, 60%, 80%, and 100%, respectively. The final elutes were collected separately and stored at −20 °C until use.
Preparation of rabbit erythrocytes: The rabbit for the haemolytic assay was bought from Qingdao Bio-test and Test-animal Center in Qingdao, China, and acclimated in cages with water and food prior to the experiment. The erythrocytes were withdrawn by needle and syringe from the rabbit's ear and added to 10% sodium citrate to avoid clotting, which was centrifuged at 500 × g (4 °C) for 10 min. After centrifugation, the supernatant was discarded. The erythrocytes were washed three times with phosphate-buffered saline (PBS) and diluted to a final concentration of 4% (v/v) in PBS for use within 24 h (Zhou et al., Reference Zhou, Fernandez, Chen, You and Yan2011).
The hemolytic test: For the standardization of a known hemolytic agent, the standard curve between Saponin (Sigma) concentration and haemolytic activity was conducted prior to the test. The protocol set up nine saponin concentration medium, including 0.5, 1.0, 2.5, 4.5, 5.0, 6.0, 7.0, 8.0, and 9.0 μg mL−1. A triplicate of 150 μL saponin medium and 50 μL erythrocytes suspension were added to Clean 96-well V-bottom plates (Costar). Erythrocytes suspension in PBS buffer was performed as zero blank, and the erythrocytes with 1% Triton X-100 as 100% positive control. The 96-well plates were incubated at 30 °C for 60 min in an incubator, and then centrifuged at 1000 × g (4 °C) for 10 min. 150 μL supernatant were serially collected on other 96-well plates, and haemolysis was measured by a microplate reader at 550 nm absorbance. Haemolytic activity was calculated according to Zhou et al. (Reference Zhou, Fernandez, Chen, You and Yan2011). Thus, the relationship between saponin concentration and haemolytic activity was performed.
The stocks of intra- and extra-cellular lipophilic crude extracts were diluted five-fold by PBS buffer. A triplicate of 150 μL samples and 50 μL erythrocytes suspension were added to Clean 96-welled V-bottom plates (Costar). Other steps were the same as those described above. According to the final haemolytic activity, the relative saponin concentration for each extract was calculated by the standard curve.
Acute toxicity test on abalones
Based on the bloom density occurring in the field, one high algal density (3 × 107 cells L−1) and one relatively low algal density (1 × 107 cells L−1) were used for each algal strain in the experiment. Algal cells were harvested at the early stationary phase. A 1 mL subsample was taken and counted under the microscope after fixation in Lugol's solution. Based on this cell count, the algae were diluted to the designated density.
The experiment was conducted in 3 L beakers. Each beaker contained 10 healthy juvenile individuals in 2.5 L of the prepared algal treatments. As a negative control, 10 individuals were cultured in 2.5 L of seawater alone. Every treatment consisted of three replicates, with 30 individuals in total. Each beaker was individually aerated using air stones and replaced with newly prepared algal treatment every day. To avoid the interaction between K. mikimotoi and the food macroalgae L. japonica, no food was offered during the experiment. The culture conditions were measured as follows: water temperature (19 ± 0.5°C), pH value (7.5–8.2), ammonia concentration(no more than 0.1 mg L−1, and dissolved oxygen (7.3–7.8 mg L−1). Mortality was recorded at 12, 24, 36, and 48 h. A final mortality test was processed according to Reddy-Lopata et al. (Reference Reddy-Lopata, Auerswald and Cook2006). The abalone with no response by repeated stimulation could be judged as the dead. Dead individuals were removed immediately to avoid water contamination.
Constituent toxicity in K. mikimotoi was performed to further identify the algal toxicity origin. Intact cell suspension (ICS), URCS, CFCS, and methanol crude extracts (MCE) were prepared from fresh algal cells with a density of 3 × 107 cells L−1, and the method was in accordance with Li et al. (Reference Li, Yan, Lin, Yu and Zhou2017). Thereafter, the prepared constituents were tested on abalones, respectively. Other conditions for the test were the same as those described above.
To identify whether the abalone mortality resulted from the combination of K. mikimotoi – FJ algal toxic effects and related oxygen depletion during bloom, another experiment was conducted in a non-aeration condition. No air stones were added during the experiment, with DO lower than 2.0 mg L−1. Other environmental conditions and algal densities were the same as those described above.
Abalone gills and haemocyte parameters of K. mikimotoi FJ-strain
To evaluate the possible damage to the gill and haemocyte organs of abalones due to K. mikimotoi FJ-strain, another batch of healthy juvenile individuals was exposed to a low density of 1 × 107 cells L−1 for 48 h under the same experimental conditions described above. After 48 h, five alive abalones from each treatment were randomly sampled for haemocyte parameters, gill configuration, and enzyme activity measurements.
Analysis of haemocyte morphology and function was done on haemolymph extracted from the prepared abalones above. Haemolymph was withdrawn by needle and syringe from the blood sinus by cutting the adductor muscle with a scalpel. During the collection, the haemolymph samples were stored temporarily on ice and immediately used for immune function measurement by flow cytometry (Accuri 6, BD Biosciences, San Diego, CA). According to Hégaret et al. (Reference Hégaret, Wikfors and Soudant2003a, Reference Hégaret, Wikfors and Soudant2003b), we measured three main immune function parameters, including haemocyte characterization, phagocytosis activity, and haemocyte viability.
Configuration of abalone gill and the enzyme activity changes were also examined. Histological photos recorded the effects of K. mikimotoi on abalone gills. The abalone gill specimens were first fixed in 10% buffered formalin and then routinely processed for the preparation of histological slides, which was conducted according to Dutra et al. (Reference Dutra, Rönnau, Sponchiado, Forneck, Freire and Ballester2017). The images of the gills were captured under a microscope (Leica DM2000, Germany). Another batch of fresh abalone gills were collected for the major enzyme activity measurement involved in immune responses and energy transfer, including superoxide dismutase (SOD), Catalase (CAT), Na+-K+-ATPase, Ca2+-Mg2+-ATPase. The enzyme was measured using a commercialized kit (Jiancheng, Nanjing, China), according to the manufacturer's instructions. SOD and CAT were expressed as U/mg protein. Na+-K+-ATPase and Ca2+-Mg2+-ATPase were expressed as μmol Pi/mg protein/hour.
Data analysis
Data were analyzed using the Excel 2010, Origin 8.5, and SPSS 16.0 software packages. Due to the different cell density gradients for every alga, the statistical analysis of the data hardly corresponded to a two-way ANOVA with algal species and algal density as factors. Therefore, one-way analysis of variance (ANOVA) was applied for statistical evaluations. Prior to statistical analysis, all data were tested for normality and homogeneity (SPSS 16.0). Mean differences were considered to be significant at the 0.05 level. If the overall ANOVA results were significant, a Fisher's least significance difference (LSD) post-hoc test was performed to test among experimental combinations.
Results
Various haemolytic activity between K. mikimotoi FJ- and SCS-strain
The preliminary assays showed that ultrasonic ruptured cell suspensions (URCS) and cell-free culture supernatants (CFCS) from the two strains all had haemolytic activity (Figure 1). For the URCS extract, there was no significant difference during the pre- and exponential phases in the two strains (P > 0.05). However, during the stationary phase and the anaphase, the relative concentration of saponin became significantly higher in FJ-strain (P < 0.05), which exceeded 3.27, and 4.38 folds, respectively, to those in the SCS-strain. For the CFCS extract, the relative concentration of saponin remained significantly higher in the SCS-strain than those in FJ-strain (P < 0.05) during the pre- and exponential phase, however, which declined as the population reached the stationary phase and anaphase, with no significant difference in the two strains (P > 0.05). Totally, at the high bloom density in situ (above 3 × 107 cells L−1, around stationary phase), the FJ-strain exhibited more haemolytic toxicity than SCS-strain.

Figure 1. Haemolytic activity of K. mikimotoi FJ- and SCS- strain during different algal phases (URCS: ultrasonic ruptured cell suspensions;CFCS: cell-free culture supernatants; algal phases 1, 2, 3, and 4: pre-exponential phase, exponential phase, stationary phase, and anaphase, respectively; each data point represents the mean percentage ± standard deviation of triplicate cultures).
Differential toxicity of K. mikimotoi FJ- and SCS- strain to abalones
The mortality of H. hannai eventually increased significantly with the increasing bloom densities of K. mikimotoi-FJ during the aeration. The high algal density even led to the sharp fatality in a short period (e.g. mortality rate of 67% in 48 h at 3 × 107 cells L−1). More seriously, all the abalones would rapidly die out at the algal density of 3 × 107 cells L−1 within 24 h through the cascading effect of non-aeration (DO below 2.0 mg L−1, Figure 2). In contrast, K. mikimotoi SC-strain showed weaker lethal effects on abalones. Even at high bloom density (3 × 107 cells L−1), over 90% of individuals still survived at the end of the experiment (up to 48 h) during the aeration (Figure 3).
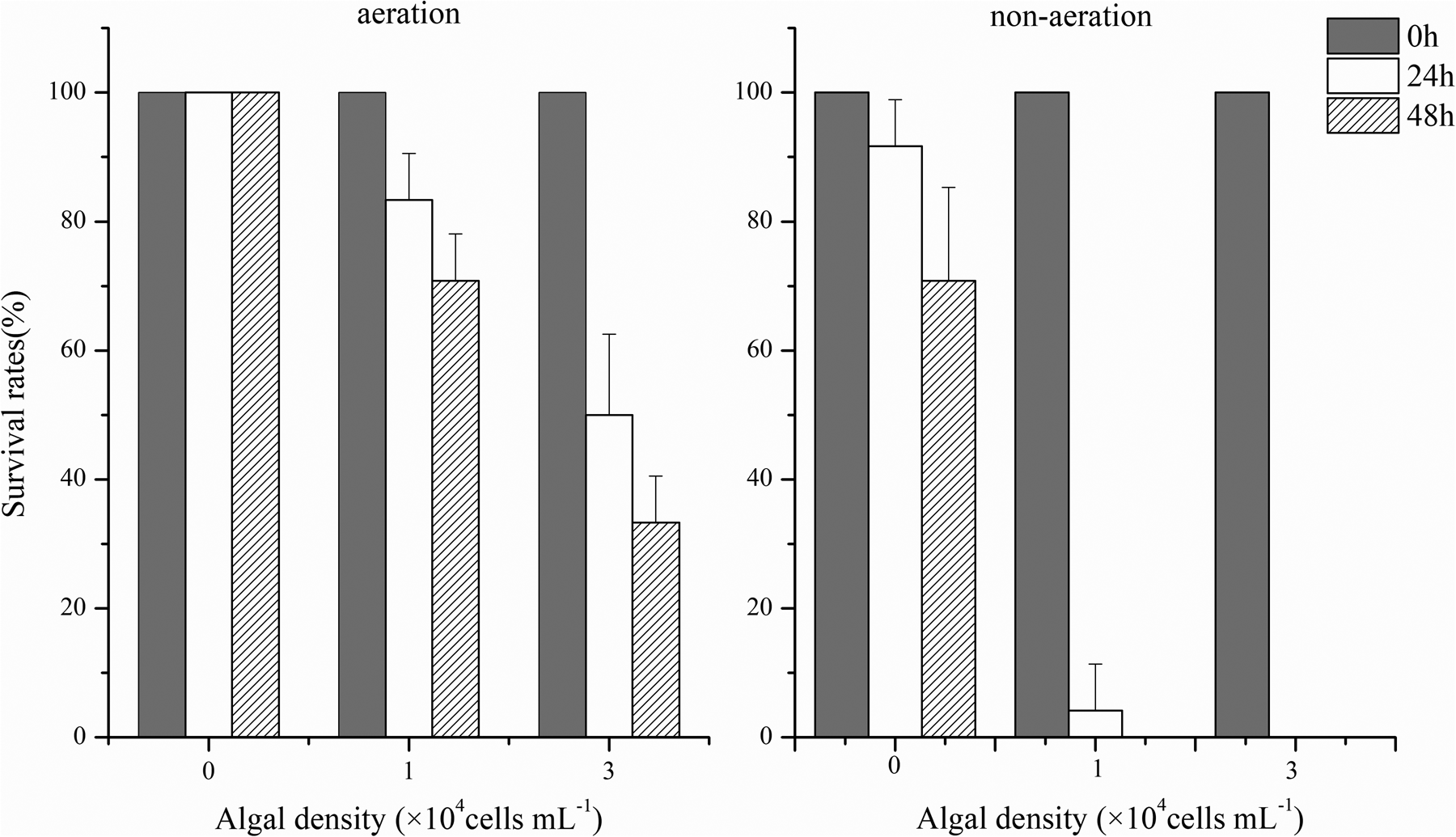
Figure 2. Survival rates of abalones exposed to K. mikimotoi-FJ strain at bloom density during the aeration and non-aeration condition (each data point represents the mean percentage ± standard deviation of triplicate cultures).

Figure 3. Survival rates of abalones exposed to K. mikimotoi-SCS strain at bloom density during the aeration and non-aeration condition (each data point represents the mean percentage ± standard deviation of triplicate cultures).
To further identify the algal toxicity origin in K. mikimotoi-FJ, algal constituent toxicity was performed. Intact cell suspensions (ICS) showed toxic effects on abalones, with mortality rates of 66.67% at 48 h at equal algal density (3 × 107 cells L−1). However, when exposed to CFCS, URCS, and methanol crude extracts (MCE) of algal cells, no abalone individual died at 48 h (Figure 4).

Figure 4. Survival rates of abalones exposed to different algal constituents in K. mikimotoi-FJ strain (ICS: intact cell suspension; URCS: ultrasonic ruptured cell suspensions; CFCS: cell-free culture supernatants; MCE: methanol crude extracts; each data point represents the mean percentage ± standard deviation of triplicate cultures).
Specific effects on abalones by K. mikimotoi FJ-strain
Under the exposure to a relatively low bloom density of K. mikimotoi FJ-strain (1 × 107 cells L−1), abalones exhibited significant histopathological and immunological changes despite high mortality rates. The normal configuration of the gill was disrupted including apparent shrinkage and deformation of the afferent and efferent branchial vessels in comparison to the control groups (Figures 5A, and B). Gill filaments were also found broken (Figure 5C). All major enzymes in the gill involved in immune responses and energy transfer (SOD, CAT, Na+-K+-ATPase, Ca2+-Mg2+-ATPase) showed inhibited activities (Figure 6), which were significantly (p < 0.05) decreased by 21.46%, 62.99%, 46.32%, 50.34% in 48 h, respectively.

Figure 5. The configuration of abalone gills in the control and experimental groups (A: the normal branchial vessel in control group; B: apparent shrink and deformation of afferent and efferent branchial vessel in experimental group at the algal bloom density; C: broken gill filaments in experimental group at the algal bloom density, as the arrow showed).
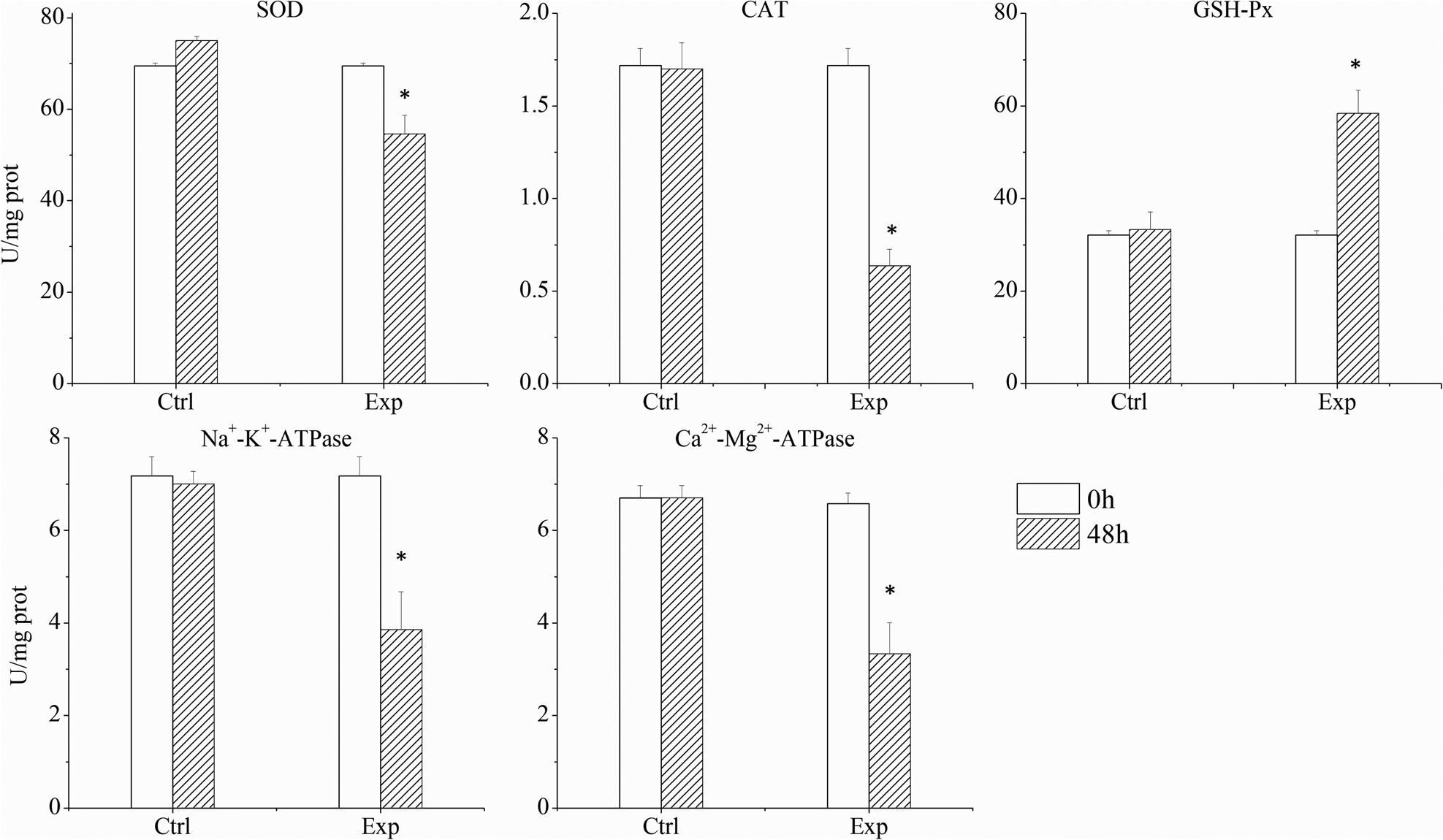
Figure 6. The activity of major enzymes in the gills in the control and experimental groups (each data point represents the mean percentage ± standard deviation of triplicate cultures).
In the response of abalone haemocytes (Figure 7), granulocytes with respect to hyalinocyte type cells and phagocytosis activities declined significantly by 58.96%, and 75.64% (p < 0.05), respectively, with respect to control groups. The rate of haemocyte mortality was significantly increased by 4.8 folds (p < 0.05).
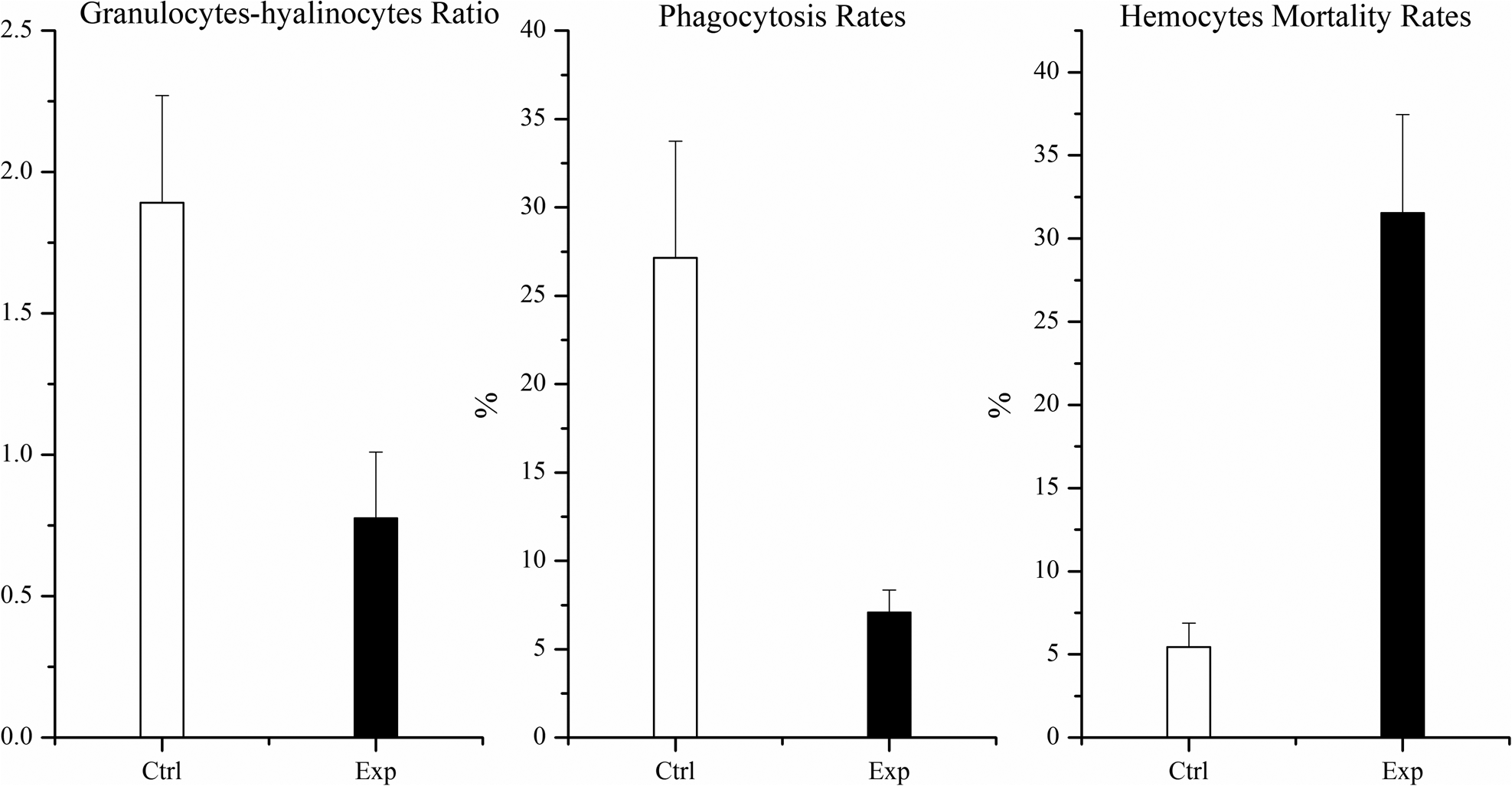
Figure 7. The abalone haemocyte parameters after 48 h exposure (each data point represents the mean percentage ± standard deviation of triplicate cultures).
Discussion
Specific algal toxicity effects among K. mikimotoi strains from various biotopes
K. mikimotoi has dominated coastal waters around Europe, Japan, China, and other countries recently. (Haywood et al., Reference Haywood, Steidinger, Truby, Bergquist, Adamson and MacKenzie2004; Taylor et al., Reference Taylor, Fukuyo, Larsen, Hallegraeff and Hallegraeff2004; Steidinger et al., Reference Steidinger, Wolny and Haywood2008) with these populations being commonly associated with fish and marine invertebrate mortalities. In the present research, we identified the major differences in the toxicity potency and toxic effects of the FJ and SCS strains of K. mikimotoi on abalones. Consistent with these findings, Zou et al. (Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010) also compared the haemolytic activity and effects on rotifers by two K. mikimotoi strains (SUO-1 and FUK) isolated from Japan. The SUO-1 strain (2 × 107 cells L−1) showed strong haemolytic activity and high toxicity to rotifers, whereas the FUK strain (2 × 107 cells L−1) was less toxic. The different effects were also observed on the brine shrimp Artemia salina. Sun et al. (Reference Sun, Yan, Zhou and Ho2010) and Dang et al. (Reference Dang, Li, Liu, Zhang, Yang, Li and Liu2015) reported that the SCS strain (1.5 × 107 cells L−1) led to all A. salina dying at 96 h, however, the mortality rate was just 23% when exposed to FJ strain (3 × 107 cells L−1, Li et al., Reference Li, Yan, Lin, Yu and Zhou2017). In our laboratory, the ITS sequences comparison also identified differences between FJ and SCS strains (Zhang QC, unpublished data). It was possible that there was a range of K. mikimotoi ecotypes with varying toxicities and modes of action.
To test whether the characters of isolates from other geographical areas were discriminating or not, we also documented haemolytic toxin production and the adverse impacts of different K. mikimotoi strain worldwide on a range of marine organisms (Table 2). Although the toxic effects of K. mikimotoi JP isolates were just reported on rotifers, there was much comprehensive data on various marine invertebrates by other K. mikimotoi strains. Interestingly, many similarities were observed among the three K. mikimotoi strains (CH-FJ, NZ, and EU isolates) in the toxic effects. No acute effects of the three K. mikimotoi strains were observed for the brine shrimp A. salina tested, even at the algal density of 5–6 × 107 cells L−1 (Botes et al., Reference Botes, Smit and Cook2003; Neely and Campbell, Reference Neely and Campbell2006; Shi et al., Reference Shi, McNabb, Rhodes, Holland, Webb, Adamson, Immers, Gooneratne and Holland2012; Li et al., Reference Li, Yan, Lin, Yu and Zhou2017). However, lethal effects on abalone juveniles were all observed following exposure to any of the three K. mikimotoi isolates at the density of 1–3 × 107 cells L−1. Due to the similarity in toxicity on marine invertebrates, one possible view is that the FJ strain may be more relative to NZ and EU isolates. Interestingly, Al-Kandari et al. (Reference Al-Kandari, Highfield, Hall, Hayes and Schroeder2011) have proposed the isolates from Europe and New Zealand are more closely related to each other in ITS regions and the rbcl gene. However, the genotype comparison between Chinese strains and other isolates was not conducted, which is more needed to identify the differences among K. mikimotoi strains worldwide in future research.
Table 2. Literature review of the toxic impacts of different K.mikimotoi strains on marine organisms
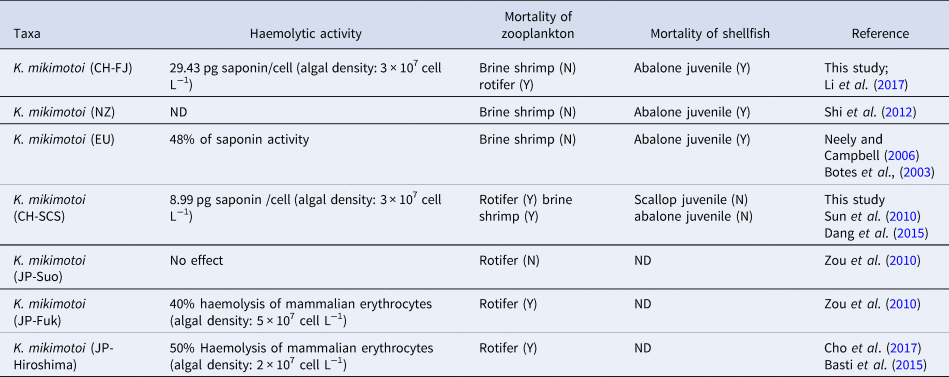
ND, No data; Y, inimical impacts found; N, no inimical impacts found.
For many algal strains, including Karenia spp., Chattonella spp., Heterocapsa spp., and Alexandrium spp., haemolytic toxicity was detected by haemolysis of mammalian erythrocytes (Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010; Basti et al., Reference Basti, Nagai, Go, Okano, Nagai, Watanabe, Suzuki and Tanaka2015; Cho et al., Reference Cho, Kasaoka, Ueno, Basti, Yamasaki, Kim and Oda2017). For example, for K. mikimotoi in Japan, 40% haemolysis of mammalian erythrocytes was detected in JP-Fuk strain with an algal density of 5 × 107 cells L−1 (Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010), however, 50% haemolysis was found from JP-Hiroshima strain with an algal density of 2 × 107 cells L−1 (Cho et al., Reference Cho, Kasaoka, Ueno, Basti, Yamasaki, Kim and Oda2017). Neely and Campbell (Reference Neely and Campbell2006) also proposed a modified assay to determine haemolytic toxicity by the percentage of saponin. However, due to the differences in the algal density during measurement, the haemolytic toxicity was hardly compared among strains in various research. In this study, we conducted an adapted protocol to determine haemolytic toxin variability among Karenia clones isolated from China. Haemolytic toxicity was assessed by relative saponin concentration in each algal cell, based on the standard curve between saponin (Sigma) concentration and mammalian erythrocytes haemolysis. Using this approach, the difference in the production of haemolytic compounds among various Karenia clones will be examined in future research.
Causes of abalone mortality resulted from K. mikimotoi-FJ strain
Although several similar phenomena on abalone mortality were previously reported in Karenia clones (eg. K. mikimotoi, Karenia cristata, and Karenia Concordia) in the laboratory, the lethal mechanisms have not been clarified. In this study, K. mikimotoi was able to kill the abalones during the aeration. The mortality rate significantly increased and lethal time sharply shortened as the dissolved oxygen-depleted during the non-aeration. These results indicated the death of abalones was caused by K. mikimotoi own as well as oxygen depletion secondary-developed.
In Karenia spp., some researchers have demonstrated that haemolytic toxins had toxic effects on fish and shellfish (Yasumoto et al., Reference Yasumoto, Underal, Aune and Graneli1990; Satake and Tanaka, Reference Satake and Tanaka2005; Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010) by primarily destroying the gill structure and immune systems (Ou, Reference Ou2006). However, the lethal effect of K. mikimotoi only occurred in URCS. All of the abalones successfully survive in CFCS. This suggested that the haemolytic toxins killing the abalones originated from active cells of K. mikimotoi FJ-strain. The direct contract of active cells with gills could produce haemolytic compounds, which worked and led to the histopathological changes in gills as the previous reports (Neely and Campbell, Reference Neely and Campbell2006; Zou et al., Reference Zou, Yamasaki, Matsuyama, Yamaguchi, Honjo and Oda2010). In fish, the haemolytic toxin of K. mikimotoi caused gill arch, raker, primary filament, and secondary filament (Ou, Reference Ou2006). Similarly, in this experiment, the afferent and efferent branchial vessels for abalone gills become shrinking and deformed evidently. Many gill filaments were also broken. In this research, the ratio of granulocytes to hyalinocytes type cells and phagocytosis activity of abalones decreased, and the mortality of haemocytes significantly increased, which was constant with the previous studies. Many researchers have reported that shellfish immune systems were attacked by many HAB algal species (Haberkorn et al., Reference Haberkorn, Lambert, Le Goı̈c, Guéguen, Moal, Palacios, Lassus and Soudant2010; Medhioub et al., Reference Medhioub, Lassus, Truquet, Bardouil, Amzil, Sechet, Sibat and Soudant2012; Lassudrie et al., Reference Lassudrie, Soudant, Richard, Henry, Medhioub, Silva, Donval, Bunel, Goïc, Lambert, Montaudouin, Fabioux and Hégaret2014, Reference Lassudrie, Wikfors, Sunila, Alix, Dixon, Combot, Soudant, Fabioux and Hégaret2015). For example, some algal cells of Alexandrium fundyense can increase the shellfish blood cell mortality rate, and increase the proportion of shellfish apoptotic blood cells (Galimany et al., Reference Galimany, Sunila, Hégaret, Ramón and Wikfors2008). Apart from the abalones, it has been tested that K. mikimotoi caused harmful effects on the immune systems of other shellfish species, such as Ruditapes philippinarum and Argopectens irradias (Smolowitz and Shumway, Reference Smolowitz and Shumway1997; Hégaret et al., Reference Hégaret, Da Silva, Wikfors, Lambert, De Bettignies, Shumway and Soudant2007; Hégaret et al., Reference Hégaret, Da Silva, Wikfors, Haberkorn, Shumway and Soudant2011).
In addition, DO depletion was another crucial stressor, which aggravated the mortality of abalones in the experiment. Similar physiological responses were also found in other molluscs when exposed to low oxygen. Cheung et al. (Reference Cheung, Chan, Liu and Shin2008) found that prolonged hypoxia (3.0 mg O2 L−1 and 1.5 mg O2 L−1) could reduce food consumption, respiration, growth, and reproduction in gastropod Nassarius festivus. DO depletion has also been documented to cause depression in the immune system. Under the 24-hour exposure of 2.05 mg O2 L−1, the abalone Haliotis diversicolor supertexta showed a significantly decreased total haemocyte counts and respiratory burst (Cheng et al., Reference Cheng, Li and Chen2004). The green-lipped mussel Perna viridis also exhibited high haemocyte mortality and reduced phagocytosis at the oxygen level of 1.5 mg O2 L−1 (Wang et al., Reference Wang, Hu, Shin and Cheung2011). During harmful algal bloom, the decomposition and respiration of algal cells always lead to the depletion of oxygen (Wang et al., Reference Wang, Yu and Zhou2012). For example, laboratory simulation found that DO could drop to 2.9 mg O2 L−1 after 5 days in the decay stage of Alexandrium catenella (Wang et al., Reference Wang, Li, Yan, Song, Yu and Zhou2021). In the natural environment, DO levels showed a depressed tendency and could persist for weeks during the K. mikimotoi bloom collapse (O'Boyle et al., Reference O'Boyle, McDermott, Silke and Cusack2016). Thus, the extensive death of abalones was largely associated with the combination of algal toxicity and DO depletion in Fujian during K. mikimotoi blooms.
Apart from the oxygen, the survival, growth, and physiological metabolism of abalone could also be obviously influenced by other environmental factors such as pH and ammonia nitrogen. For example, non-ionic ammonia nitrogen in seawater with a concentration exceeding 35 μg L−1, and 9.8 μg L−1, could cause a significant effect on the growth of Haliotis discus hannai, and Haliotis midae, respectively (Matsuyama et al., Reference Matsuyama, Uchida and Honjo1999). In this research, pH, temperature, and salinity were maintained under suitable conditions. Although the total ammonia nitrogen in the water showed some fluctuation, the maximum value reached 0.084 mg L−1. Whereas, according to Bower and Bidwell (Reference Bower and Bidwell1978), the maximum non-ionic ammonia nitrogen can be calculated to 2.50 μg L−1. Therefore, the concentration of non-ionic ammonia nitrogen in the experimental system could not affect the survival of abalone.
Conclusion
The outbreaks of K. mikimotoi caused massive mortalities of abalones and serious economic losses in Fujian. Our experiments verified that K. mikimotoi Fujian-strain could kill the abalones through haemolytic toxicity destroying the afferent, efferent branchial vessels and filaments of abalone gill and decreasing granulocytes respect to hyalinocytes type cells, phagocytosis activity and the survival of haemocytes at the bloom density in situ. The toxic effect just worked for the haemolytic toxicity from active algal cells, which were probably produced under the contract of algal cells and abalone gills. Compared to the K. mikimotoi SCS-strain as ‘fish killer’, the haemolytic toxicity of Fujian strain was stronger, suggesting a new ecotype different from the former one. Apart from the algal toxicity, DO depletion exacerbated the mortality of abalones in the experiment. Since DO could decrease during K. mikimotoi blooms, DO depletion cooperating with the algal toxicity of K. mikimotoi led to the numerous deaths of abalones in Fujian.
Data availability
All data generated or images used during this study are included in this published article.
Author contributions
Lingzhi Liao conducted paper writing. Jianing Lin made project administration and laboratory experiments. Xinshu Ding conducted data analysis. Song Feng edited the paper. Tian Yan made project administration and methodology.
Financial support
This work was supported by Open Fund of CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences (grant number KLMEES201801) and the National Nature Science Foundation of China (NSFC 41476102).
Competing interests
None.
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.











