Introduction
Rhabdomyolysis, a syndrome of skeletal muscle cell damage, engenders the release of toxic intracellular materials into systemic circulation. The pathogenesis of rhabdomyolysis is based on an increase in free ionised calcium in the cytoplasm. Its main complications are acute kidney injury (AKI), which is triggered by renal vasoconstriction and ischemia, myoglobin cast formation in the distal convoluted tubules, and direct renal toxic effect of myoglobin on the epithelial cells of proximal convoluted tubules. Other major complications include electrolyte disorders such as hyperkalaemia, which might cause cardiac arrhythmia, metabolic acidosis, hyperphosphataemia, early hypocalcaemia, and late hypercalcaemia (Chatzizisis et al. Reference Chatzizisis, Misirli, Hatzitolios and Giannoglou2008). The aetiology of rhabdomyolysis is usually identified easily, although it is sometimes elusive. The most common causes of rhabdomyolysis are iliac drugs, alcohol abuse, medical drugs, muscular diseases, trauma, neuroleptic malignant syndrome, seizures, and immobility (Kahn, Reference Kahn2009). Rhabdomyolysis in a psychiatric hospital is typically signalled by overexertion, exacerbation, medication, alcoholism, infections and convulsions. Among medical drugs, the most frequently involved are antipsychotic drugs, statins, zidovudine, colchicine, selective serotonin reuptake inhibitors (SSRIs) and lithium (Melli et al. Reference Melli, Chaudhry and Cornblath2005). Prompt diagnosis requires routine monitoring of serum creatine kinase (SCK) activity in a subject with a suggestive history or clinical features.
At psychiatric hospitals in Japan, 32.1% of patients show increased SCK activity (>200 IU/l; Sakamoto, Reference Sakamoto1995). Markedly increased SCK activity has been observed in 10% of patients treated with antipsychotic drugs (Meltzer et al. Reference Meltzer, Cola and Parsa1996). Rhabdomyolysis shows various features from an asymptomatic illness to a life-threatening condition such as AKI. Although many reviews of rhabdomyolysis aetiologies and pathogenic mechanisms have been published, little is known about the actual frequency of the different aetiologies causing rhabdomyolysis. Furthermore, many studies have been conducted among patients with rhabdomyolysis-induced AKI. Therefore, for this study, we examined 87 patients with rhabdomyolysis and analysed the incidence of different aetiologies, the characteristics of patents with rhabdomyolysis, the laboratory variables that are useful for detecting the disorders, and the incidence of complications.
Patients And Methods
SCK activity was monitored during 2007–2012 at varying intervals at Minamihama Hospital. Any patient for whom SCK activity determination was available during treatment with antipsychotic drugs was included in this study. The 87 patients enrolled in this study comprised 80 inpatients and 7 outpatients including 51 schizophrenia patients, 17 with mood disorder, 6 with personality disorder and 13 with other disorders. The patients satisfied the diagnostic categories of DSM-IV-TR (Sadock et al. Reference Sadock, Kaplan and Sadock2007). The diagnosis of polydipsia was made when the patient was observed by the hospital staff or the patient's family to drink excessive quantities of fluid consistently (Verghese et al. Reference Verghese, de Leon and Josiassen1996).
Case reports, hospital records, and laboratory results from protocol studies were examined for all SCK determinations obtained during the course of treatment. SCK activity was determined by a hospital laboratory in accordance with protocol schedules. A blood sample was taken on admission. Subsequently, blood samples were taken each month. From outpatients, the blood sample was obtained at the first visit to our hospital, and at the next hospital visit, with various durations of 1–6 months. If the SCK level was found to be greater than 1500 IU/l, then the determination of SCK levels was conducted at least until SCK decreased to less than 1000 IU/l and clinical symptoms in the affected patient disappeared.
The respective normal ranges of SCK activities in men and women are 43–272 IU/l and 30–165 IU/l. In fact, SCK concentration greater than five times the upper normal limit is accepted as a diagnostic criterion for rhabdomyolysis (Melli et al. Reference Melli, Chaudhry and Cornblath2005; Walter & Catenacci, Reference Walter and Catenacci2008; Kahn, Reference Kahn2009; Cervellin et al. Reference Cervellin, Comelli and Lippi2010). Therefore, SCK concentration higher than 1500 IU/l was defined for this study as rhabdomyolysis. The abnormal values were re-assayed in duplicate or triplicate to ensure that they were not attributable to laboratory error. Serum aspartate aminotransferase, alanine amino transferase, gamma-glutamate transferase, lactic dehydrogenase, uric acid, calcium, creatinine and blood urea nitrogen, as well as urine myoglobin were determined by the clinical laboratories.
We also attempted to exclude myocardial infarction using clinical signs and electrocardiography, but troponin determination was not routinely available. AKI was diagnosed with increased serum creatinine above the upper normal limit (1.1 mg/dl).
We divided the patients into groups according to their aetiologic factors and assessed their values of SCK activity statistically. Data were recorded on an Excel spreadsheet. Because the observed distribution did not fit the normal distribution by Shapiro–Wilk test for normality even after log transformations, differences in the medians of continuous data were compared using a Mann–Whitney U test. Results for which p < 0.05 were inferred to be statistically significant. The analyses were conducted using freely available software (Easy R; Kanda, Reference Kanda2013).
Results
We diagnosed 87 patients with rhabdomyolysis; they all had SCK higher than 1500 IU/l. These concentrations, which were approximately five times the normal upper limit, were selected to eliminate from consideration those increases in SCK activity that might be related to psychosis or nonspecific effects such as physical activity and trauma. The annual incidence of rhabdomyolysis during the period 2007–2012 was 0.80%–1.45% (Table 1). Among those 87 patients, men were more numerous than women (59 men vs 28 women). The median age was 50.9 years: 17–87 years. The most common age in both sexes was 60 years old. Men were more numerous than women in all age groups, except for that of 80–89 years (Fig. 1).
Table 1 Patients with rhabdomyolysis (SCK higher than 1500 IU/l)
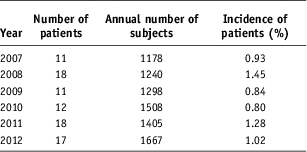

Figure 1 Age distribution of patients with rhabdomyolysis
Peak SCK activities were 1544–186 500 IU/l (median 3566 IU/l), but 45% of the patients showed SCK higher than 5000 IU/l. The increases were 5.2 to 622 times the upper normal limit. SCK activity was not monitored daily, therefore, these might not have been the actual peak levels. In most cases, additional samples taken either immediately before or after the peak value also demonstrated marked increases that were less than the peak levels. SCK activities usually declined promptly within three days after the initial evaluation. The mean SCK fell to 355 IU/l on day 12 (Fig. 2). Peak SCK activities in men were significantly higher than those in women (p = 0.002; Fig. 3). No correlation was found between the age and the highest value of SCK activity.
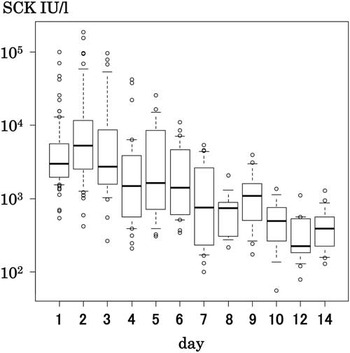
Figure 2 Time course changes in SCK of patients with rhabdomyolysis. Bold horizontal line = median; box area = interquartile range (IQR: 25–75th percentile); whiskers = upper and lower adjacent values; open circles = extreme outlying values.
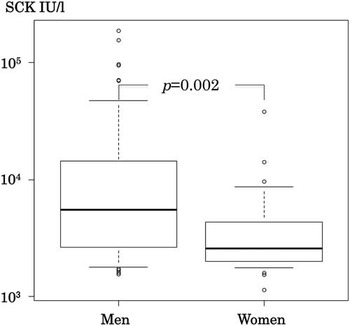
Figure 3 Comparison of peak SCK of men and women. Bold horizontal line = median; box area = interquartile range (IQR: 25–75th percentile); whiskers = upper and lower adjacent values; open circles = extreme outlying values.
Medication, excessive physical activity, exacerbation of psychosis, and psychogenic polydipsia were major aetiological factors, collectively accounting for about 35% of affected patients with rhabdomyolysis. In all categories, men were more frequently included than women (Table 2). In the category of medication, 12 patients (14%) were treated with the following drugs: 3 patients with aripiprazole; 2 fluphenazine; 2 haloperidol; 1 valproate; 1 memantine; 1 timiperone; 1 clomipramine; and 1 fluvoxamine. The possible factors of 47 patients (54.0%) remain to be clarified.
Table 2 Aetiological factors of rhabdomyolysis and patient characteristics
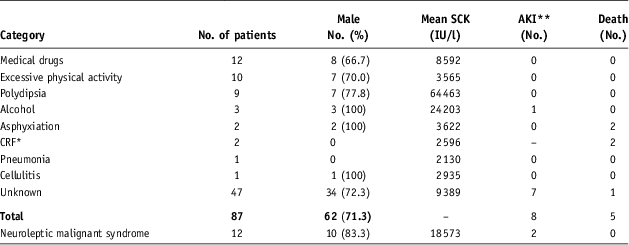
*Chronic renal failure
**Acute kidney injury
Results show recurrence in six patients and triple recurrence in one patient. Polydipsia was presumed to be the causative factor in at least in two cases in a first episode. All six cases showed spontaneous improvement of SCK activity without discontinuation of medical treatment.
Patients with both psychogenic polydipsia and alcoholism had higher SCK activities than those with other groups (Table 2). Peak SCK activities of patients with polydipsia were the most markedly elevated among all categories (p = 0.0003) (Fig. 4); their mean SCK activity was 215 times the upper limit.

Figure 4 Peak SCK of polydipsia patients and others. Bold horizontal line = median; box area = interquartile range (IQR: 25–75th percentile); whiskers = upper and lower adjacent values; open circles = extreme outlying values.
Death occurred in five patients, but only one died of rhabdomyolysis (Table 2). Neuroleptic malignant syndrome developed in 12 cases with mean peak SCK activity of 18 573 IU/l (62 times the upper limit), including 2 of medication, 3 of polydipsia, and 7 of unknown cause. Of those cases, eight men had AKI (9.1%), which accounted for 16.7% of those with neuroleptic malignant syndrome. Six of eight patients with AKI required urgent hospitalisation because of disturbance of movement and increased serum creatinine concentration on admission.
Discussion
The laboratory diagnosis of rhabdomyolysis is based on the determination of SCK activity. The true incidence of increased SCK activity in the psychiatric hospital was obscure because it was not checked routinely during hospitalisation. Elevated SCK activity has been reported in 10% of patients treated with antipsychotics (Meltzer et al. Reference Meltzer, Cola and Parsa1996). In our study, the annual frequency of rhabdomyolysis was approximately 1%. No evidence was found that muscular trauma or hyperactivity contributed to the release of creatine kinase from skeletal muscle in any of these cases. Therefore, the annual incidence of rhabdomyolysis found in this study is regarded as reliable.
Men were not only more affected by rhabdomyolysis, but also showed higher SCK activity more frequently than women, as reported previously (Sakamoto, Reference Sakamoto1995; Meltzer et al. Reference Meltzer, Cola and Parsa1996; Melli et al. Reference Melli, Chaudhry and Cornblath2005), which was attributed to gender differences in muscle quantity.
The peak age was 60 years old, which was approximately equal to that of a population examined in an earlier study (Oshima, Reference Oshima2011). The most common causes of rhabdomyolysis were illicit drugs, medications, muscle disease, and trauma (Melli et al. Reference Melli, Chaudhry and Cornblath2005). Among toxic cases related to medications, lipid modifying agents and HMG-CoA reductance inhibitors were the most frequently cited agents (Oshima, Reference Oshima2011). Rhabdomyolysis in psychiatric patients is expected to result from multiple factors such as agitation, dehydration, and adverse effects of psychotropic medications (Jermain & Crismon Reference Jermain and Crismon1992). In our psychiatric patients, possible causes were medications, exacerbation of psychosis and psychogenic polydipsia. Psychogenic polydipsia is a common clinical feature in psychiatric patients, especially with a psychotic disorder. Acute episodes of polydipsia might develop severe metabolic alteration, such as the calcium–sodium exchange mechanism across the skeletal myocyte or failure of cell volume regulation secondary to extracellular hypo-osmolality (Zaidi, Reference Zaidi2005). Patients with polydipsia in this study had significantly elevated SCK activity. Seven of nine patients (77.7%) with polydipsia also showed hyponatraemia.
Elevated SCK activity was detected because most patients were asymptomatic at the time of diagnosis. A 38-year-old man treated with haloperidol as an outpatient showed SCK activity of 13 130 IU/l. The next day, SCK activity had decreased to 7150 IU/l. Thereafter, it decreased to 895 IU/l at day 7, and 426 IU/l at day 14 without discontinuation of the treatment. Unfortunately, the exact cause of his rhabdomyolysis was not identified.
In the first 12 hr, SCK activity started to rise, reaching a peak on the second or third day. It reverted promptly to baseline values 3–5 days thereafter (Cervellin et al. Reference Cervellin, Comelli and Lippi2010). SCK activities are generally regarded as predictive of the likelihood of developing AKI. Concentrations higher than 5000 IU/l are closely related to AKI development. Actually, SCK has a half-life of 1.5 days; as a consequence, blood levels remain increased longer than myoglobin concentrations, which have a half-life of 2–4 hr. Myoglobin concentrations tend to normalise within 6–8 hr following an event (Cervellin et al. Reference Cervellin, Comelli and Lippi2010). Measurement of plasma and/or urine myoglobin might be useful in the early stages of the syndrome, but such measurements are not necessary for diagnosis (Cervellin et al. Reference Cervellin, Comelli and Lippi2010). The absence of urine myoglobin, as measured by quantitative assay, does not exclude rhabdomyolysis (Melli et al. Reference Melli, Chaudhry and Cornblath2005).
Rhabdomyolysis occurs with a wide spectrum of signs and symptoms, ranging from a completely asymptomatic rise in plasma creatine kinase, through to massive increase in enzyme activity associated with AKI, severe alternations in electrolytes, and disseminated intravascular coagulation (Huerta-Alardin et al. Reference Huerta-Alardin, Varon and Marik2005; Cervellin et al. Reference Cervellin, Comelli and Lippi2010). Furthermore, rhabdomyolysis often progresses to AKI because of tubular obstruction that occurs when myoglobin leaks into the kidney (Rodriguez-Capote et al. Reference Rodriguez-Capote, Balion, Hill, Cleve, Yang and Sharif2009). In the current literature, the percentage of patients developing AKI secondary to rhabdomyolysis varies from 13% to more than 50%, depending on the clinical and organisational setting (Cervellin et al. Reference Cervellin, Comelli and Lippi2010). AKI, the most severe complication, occurred in about 46% of affected patients (Melli et al. Reference Melli, Chaudhry and Cornblath2005).
Factors contributing to rhabdomyolysis-induced AKI include hypovolemia, tubular obstruction, and the nephrotoxic effects of myoglobin. Decreased circulating plasma volume potentiates renal hypoperfusion through renal vasoconstriction. In acidic urine, myoglobin and uric acid precipitates and forms obstructive casts, as myoglobin dissociates into ferrihaemate and globulin. Ferrihaemate has a direct nephrotoxic effect that potentiates acute tubular necrosis (Kahn, Reference Kahn2009). Peak SCK activity can be predictive of the development of renal failure. In fact, SCK activity higher than 5000 IU/l is closely related to the development of AKI (Cervellin et al. Reference Cervellin, Comelli and Lippi2010). At our psychiatric hospital, peak SCK concentrations were 1544–186 500 IU/l. Of the patients, 45% showed SCK activities greater than 5000 IU/l. AKI occurred in eight cases, which accounted for only 9.1% of all patients. Meltzer et al. (Reference Meltzer, Cola and Parsa1996) reported that no patient treated with antipsychotic drugs developed AKI, and suggested a selective increase in sarcolemma permeability rather than muscle necrosis as a cause of the increased SCK activity.
No patient in the polydipsia group developed renal insufficiency. The myoglobin activities in spot urine were, respectively, 998–9999 ng/ml and 6633–540 000 ng/ml in the AKI group and polydipsia group. All patients with AKI were men with a mean age of 47 years. Seven out of nine patients with polydipsia were men with mean age of 45 years. No difference in the backgrounds of these groups was significant. Six of eight patients with AKI required urgent hospitalisation because of the appearance of disturbance of movement and the increase in the serum creatinine concentration on admission. Dehydration worsened their AKI. Excessive water intake served a protective role (Walter & Catenacci Reference Walter and Catenacci2008).
Most psychiatric patients with rhabdomyolysis were asymptomatic. The condition in each was discovered during routine blood examination. Some patients showed increased SCK activity that was unrelated to their drug treatment and which subsided spontaneously without specific treatment (Meltzer et al. Reference Meltzer, Cola and Parsa1996; Terao et al. Reference Terao, Matsuda, Kojima, Okuno, Hori, Kaku, Ueda and Etoh1999). Despite their magnitude, SCK increases in psychiatric patients seemed not always to be related to a higher risk of complication (Meltzer et al. Reference Meltzer, Cola and Parsa1996). Extreme elevation in SCK concentration can be toxic to kidney function. Therefore, regular routine monitoring of SCK is recommended for patients in psychiatric hospitals, especially those treated with novel antipsychotic drugs.
Summary
-
1. SCK concentrations higher than 1500 IU/l were found in 87 psychiatric patients with rhabdomyolysis.
-
2. The annual frequency of rhabdomyolysis was approximately 1%.
-
3. Men are at greater risk than women.
-
4. Patients with psychogenic polydipsia showed the highest SCK activity.
Acknowledgement
We acknowledge Dr. H. Matsukura for critical reading of the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.








