Autism-spectrum disorder is increasingly diagnosed, Reference Baird, Simonoff, Pickles, Chandler, Loucas and Meldrum1 with recent studies estimating that at least 1% of children in South London are affected. Further, it is recognised that people with autism-spectrum disorder are at increased risk of developing comorbid epilepsy, Reference Amiet, Gourfinkel-An, Bouzamondo, Tordjman, Baulac and Lechat2 obsessive–compulsive disorder, Reference Russell, Mataix-Cols, Anson and Murphy3 depression and anxiety. Reference Howlin4,Reference Bolton, Pickles, Murphy and Rutter5
It has been suggested by some that people with autism-spectrum disorder are also at increased risk of developing psychosis. Reference Petty, Ornitz, Michelman and Zimmerman6 Autism was initially thought to be an early manifestation of schizophrenia, and so was often referred to as ‘schizophrenic syndrome of childhood’ or ‘childhood psychosis’. This can be understood from a historical perspective given that there are a number of parallels between psychotic disorders and autism-spectrum disorder. For example, similar brain regions, neurotransmitter systems and genetic markers have been implicated in both disorders. Also, autism-spectrum disorder is characterised by symptoms such as deficits in social behaviour, oddness of speech, unusual responsiveness to the sensory environment, isolated skill areas, and inappropriate affect – which may sometimes be difficult to differentiate from psychosis. Indeed it was only in 1971 that autism-spectrum disorder was finally distinguished from schizophrenia. Reference Kolvin7 Before Kolvin's work it was thought that there was a unitary psychosis of childhood. Kolvin and his colleagues delineated the differences in symptomatology, family history and treatment response between those with suspected psychosis of later childhood (described by Kolvin as more akin to adult schizophrenia) and infantile autism-spectrum disorder.
There are, however, significant difficulties in establishing the true rate for comorbidity of psychosis in autism-spectrum disorder. For example, some have suggested that there is a tendency for some people with autism-spectrum disorder to display paranoid ideation, and so be misdiagnosed with schizophrenia. Reference Wing8 Moreover, previous diagnostic classification systems specifically excluded the presence of both disorders. 9 Finally, given the current division between adult and child services in many clinical settings (i.e. a lack of ‘transitional’ services), lifelong behavioural differences may be mislabelled as psychotic, or new behaviours as ‘autistic’. Reference Konstantareas and Hewitt10
There is growing consensus, however, that the presence of positive psychotic symptoms such as hallucinations or delusions, which are not included among the diagnostic criteria for autism-spectrum disorder, warrants the additional diagnosis of a psychotic disorder. Reference Stahlberg, Soderstrom, Rastam and Gillberg11 Nevertheless, relatively few studies have investigated the comorbidity of autism-spectrum disorder and psychosis. One group (in a case series) Reference Volkmar and Cohen12 reported no increase in psychotic symptoms in people with autism-spectrum disorder. However, almost half of this sample were mute – making the diagnosis of schizophrenia very difficult. In contrast, other studies Reference Stahlberg, Soderstrom, Rastam and Gillberg11 reported that up to 7% of individuals with autism-spectrum disorder have bipolar disorder with psychotic features and 7.8% have schizophrenia. Another group reported that 16.1% of those referred to their clinic in South London from 1983 to 2000 (total n=137) with autism-spectrum disorder and comorbid intellectual disability also fulfilled the criteria for comorbid schizophrenia-spectrum disorders. Reference Tsakanikos, Sturmey, Costello, Holt and Bouras13 Further, a recent case–control study of 61 people with ‘atypical autism’ followed for 45 years in Denmark found that 31 (34%) had been diagnosed with a schizophrenia-spectrum disorder. Reference Mouridsen, Rich and Isager14 Thus, there is recent evidence, albeit preliminary, that people with autism-spectrum disorder may be at increased risk of developing psychosis. However, nobody has yet examined the biological correlates of psychosis in people with autism-spectrum disorder. Therefore we used in vivo magnetic resonance imaging (MRI) to compare the brain anatomy of people with autism-spectrum disorder with and without psychosis. We hypothesised that those with psychosis would differ in proportion of total brain grey matter and in the anatomy of the medial temporal lobe, insular cortex, the basal ganglia and prefrontal regions (i.e. brain regions classically reported as most affected in psychosis in the general population). Reference Gur, Keshavan and Lawrie15
Method
Recruitment
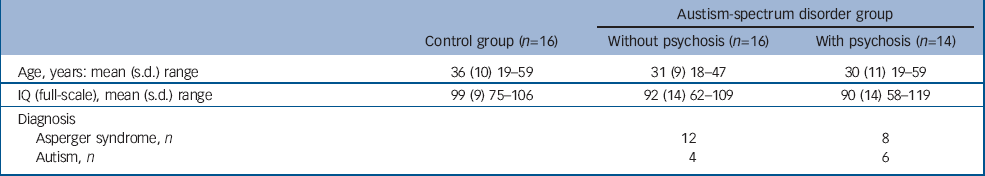
We included 30 adult males (age range 18–59) with autism-spectrum disorder (16 without a history of psychosis and 14 with). We also included 16 healthy control males from the local general population (Table 1).
Table 1 Participant characteristics
| Austism-spectrum disorder group | |||
|---|---|---|---|
| Control group (n=16) | Without psychosis (n=16) | With psychosis (n=14) | |
| Age, years: mean (s.d.) range | 36 (10) 19-59 | 31 (9) 18-47 | 30 (11) 19-59 |
| IQ (full-scale), mean (s.d.) range | 99 (9) 75-106 | 92 (14) 62-109 | 90 (14) 58-119 |
| Diagnosis | |||
| Asperger syndrome, n | 12 | 8 | |
| Autism, n | 4 | 6 | |
The participants with autism-spectrum disorder were identified via a specialist local clinical service for autism-spectrum disorder at the Maudsley Hospital (London, UK); the controls were recruited by advertisement.
People were diagnosed with autism-spectrum disorder using ICD–10 research criteria, 16 and the Autism Diagnostic Instrument (ADI–R, see below). Reference Lord, Rutter and Le Couteur17 Psychosis was defined using ICD–10 research criteria (presence of positive symptoms such as hallucinations and delusions). Diagnosis was made by those with extensive clinical experience with the autism-spectrum disorder population, and use of systematic observation and cross-referencing with information from caregivers. We excluded people with confusional states. Of the 14 people with psychosis, 9 fulfilled the criteria for schizophrenia, 3 for schizoaffective disorder and 2 for psychotic bipolar affective disorder. The presence of either transitory hallucinations or delusions was not considered sufficient for inclusion. Both groups underwent a structured clinical exam and routine clinical blood tests to exclude biochemical, haematological or chromosomal abnormalities. People were excluded if they had a history of head injury, toxic exposure, diabetes, abnormalities in routine blood tests, clinical abnormality on routine MRI, or a medical or genetic disorder associated with autistic symptoms (e.g. epilepsy or fragile-X syndrome). One person with psychosis had a history of substance misuse, therefore the analysis was repeated with and without this individual to determine whether inclusion confounded our results. All participants gave informed consent and/or assent (as approved by the Institute of Psychiatry and the South London and Maudsley NHS Trust research ethics committee).
Pervasive developmental disorder assignment
In all cases diagnosis was based on ICD–10 clinical research criteria. This was achieved via consensus between two psychiatrists and a specialist nurse trained in the Autism Diagnostic Instrument (ADI–R). Reference Lord, Rutter and Le Couteur17 The ADI–R was completed when parental informants were willing/available (26 participants); and in a further 3 participants the diagnosis was confirmed via the Autism Diagnostic Observation Schedule (ADOS) Reference Lord, Rutter, Goode, Heemsbergen, Jordan and Mawhood18 together with collateral information from family members and review of school reports. All assessments were made masked to MRI scan data. Of the 16 people without psychosis, 12 (75%) were diagnosed as having Asperger syndrome and 4 (25%) as having autism. Of the 14 individuals with coexisting psychosis, 8 (57%) were diagnosed as having Asperger syndrome and 6 (43%) as having autism.
Neuropsychological testing
Overall IQ was determined for all participants using an abbreviated Weschler Adult Intelligence Scale (WASI–R). Reference Wechsler19
Magnetic resonance acquisition
All MRI data were obtained using the same scanner, a GE Signa 1.5 T neuro-optimised magnetic resonance system (General Electric, Milwaukee, Wisconsin, USA). Whole-head coronal three-dimensional SPGR images (echo time (TE)=13.8 ms, repetition time (TR)=2.8 ms, 256×192 acquisition matrix, 124×1.5 mm slices) were obtained from all participants. All image analysis was carried out masked to participant status.
Voxel-based morphometry preprocessing
Voxel-based morphometry is a technique whereby differences in grey or white matter tissue volume or density, determined by segmentation of a magnetic resonance image, are compared between individuals following registration into a standard space. In the current study, segmentation was performed using Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neurosciences, University College London, UK). In previous versions of SPM software, a set of processing steps (commonly referred to as optimised voxel-based morphometry and first introduced by Good et al Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak20 ) were needed to ensure high-quality segmentations. The SPM5 combines both registration and tissue classification components into a single model, which also includes the effects of image intensity non-uniformity (so called ‘bias correction’). Reference Ashburner and Friston21 Following segmentation in this manner, grey matter probability images were ‘modulated’ (to compensate for the effect of spatial normalisation, by multiplying each voxel value by its relative volume before and after warping); these modulated results are referred to as ‘tissue volumes’ below, whereas the unmodulated images are referred to as ‘tissue densities’. We then smoothed with a 5 mm×5 mm×5 mm Gaussian kernel (to reduce noise and also allow for the effects of small residual misregistrations). A 5 mm Gaussian kernel was chosen as this is the expected level of accuracy in the cortical regions of the registration and it reflects the thickness of the cortical ribbon. Furthermore, this kernel ensures compatibility with previous studies.
Statistical analysis
Group differences in age and IQ were examined using SPSS version 12.0 for Windows and independent samples t-tests (two-tailed). The level of statistical significance was defined as P<0.05 (two-tailed). For the voxel-based morphometry analyses, grey and white matter volumes were calculated from the normalised images by SPM5 by adding up all voxels in the respective segmented images. Between-group differences in grey and white matter volume were estimated by fitting an analysis of covariance (ANCOVA) model at each intracerebral voxel in standard space.
Given that structural brain changes are likely to extend over a number of contiguous voxels, test statistics incorporating spatial information, such as three-dimensional cluster mass (the sum of suprathreshold voxel statistics), are generally more powerful than other possible test statistics, which are informed only by data at a single voxel. Therefore, our approach is to initially set a relatively lenient P (P=0.05) to detect voxels putatively demonstrating differences between groups. We then search for spatial clusters of such voxels and test the cluster mass of each cluster for significance at a level of P=0.05. Permutation testing is used to assess statistical significance at both the voxel and cluster levels. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer22 At the cluster level, rather than set a single a priori P, below which we regard findings as significant, we calculate, for a range of Ps, the number of clusters that would be expected by chance alone. We then set the statistical threshold for cluster significance for each analysis such that the expected number of false positive clusters is <1 over the brain as a whole, and quote the P at which this occurs. Our main comparison was between people with autism-spectrum disorder only and autism-spectrum disorder with psychosis. However, three voxel-based morphometry analyses were completed to clearly delineate the between-group differences associated with psychosis. Initially, we compared the entire autism-spectrum disorder group with the control group. We then compared the group with autism-spectrum disorder and comorbid psychosis with controls, and finally we compared those with and without psychosis in the autism-spectrum disorder group.
Results
Group characteristics
There was no significant difference in age, gender or IQ between participants with autism-spectrum disorder with and without psychosis and controls.
Analysis of MRI data using computerised voxel-wise analysis
There were no significant differences between any group in volume of total brain grey or white matter (Table 2).
Table 2 Total grey and white matter volumes in native space as calculated by Statistical Parametric Mapping software 5 (SPM5)

| Austism-spectrum disorder group | |||
|---|---|---|---|
| Control group, mean (s.d.) | Without psychosis, mean (s.d.) | With psychosis, mean (s.d.) | |
| Grey matter | 0.71 (0.072) | 0.74 (0.077) | 0.70 (0.064) |
| White matter | 0.48 (0.055) | 0.48 (0.072) | 0.47 (0.045) |
Spatial extent statistics
Autism-spectrum disorder v. controls
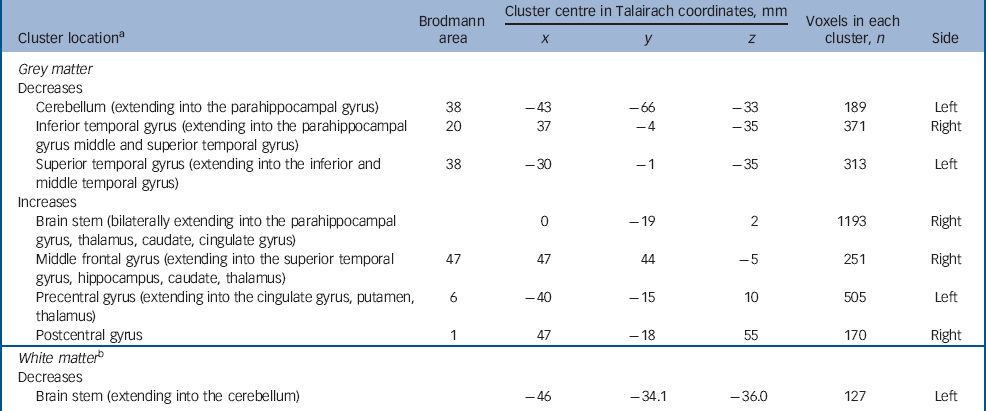
Adults with autism-spectrum disorder had a significant reduction in regional grey matter volume in three clusters centred in the cerebellum (extending into the right parahippocampal gyrus) in addition to reductions in the right inferior temporal gyrus (extending into the middle temporal gyrus) and left superior temporal gyrus (extending into the inferior and middle temporal gyrus). They also displayed increased grey matter in four clusters located bilaterally in the brainstem, the right middle frontal gyrus, and in the precentral and postcentral gyri (extending bilaterally into the thalamus, right caudate, left putamen and bilaterally in the cingulate gyrus). Reductions in white matter were not significant at our stringent (<1) false positive discovery rate, but when explored at less stringent significance (<2) were seen within the brain stem and cerebellum (Fig. 1 and Table 3) (P=0.0039 grey, P=0.01 white, <2 false positives).

Fig. 1 Autism-spectrum disorder v. controls. Relative deficits (blue) and excesses (red) in (a) grey and (b) white matter volume in autism-spectrum disorder participants compared with healthy controls (cluster threshold = 0.05, P = 0.0039 grey corrected <1 false positive, P = 0.01 white uncorrected <2 false positives). The maps are oriented with the right side of the brain shown on the left side of each panel. The z-coordinate for each row of axial slices in the standard space of Talairach and Tournoux is given in millimetres.
Table 3 Autism-spectrum disorder v. controls
| Brodmann area | Cluster centre in Talairach coordinates, mm | Voxels in each cluster, n | ||||
|---|---|---|---|---|---|---|
| Cluster locationa | x | y | z | Side | ||
| Grey matter | ||||||
| Decreases | ||||||
| Cerebellum (extending into the parahippocampal gyrus) | 38 | -43 | -66 | -33 | 189 | Left |
| Inferior temporal gyrus (extending into the parahippocampal gyrus middle and superior temporal gyrus) | 20 | 37 | -4 | -35 | 371 | Right |
| Superior temporal gyrus (extending into the inferior and middle temporal gyrus) | 38 | -30 | -1 | -35 | 313 | Left |
| Increases | ||||||
| Brain stem (bilaterally extending into the parahippocampal gyrus, thalamus, caudate, cingulate gyrus) | 0 | -19 | 2 | 1193 | Right | |
| Middle frontal gyrus (extending into the superior temporal gyrus, hippocampus, caudate, thalamus) | 47 | 47 | 44 | -5 | 251 | Right |
| Precentral gyrus (extending into the cingulate gyrus, putamen, thalamus) | 6 | -40 | -15 | 10 | 505 | Left |
| Postcentral gyrus | 1 | 47 | -18 | 55 | 170 | Right |
| White matter b | ||||||
| Decreases | ||||||
| Brain stem (extending into the cerebellum) | -46 | -34.1 | -36.0 | 127 | Left | |
Autism-spectrum disorder with psychosis v. controls
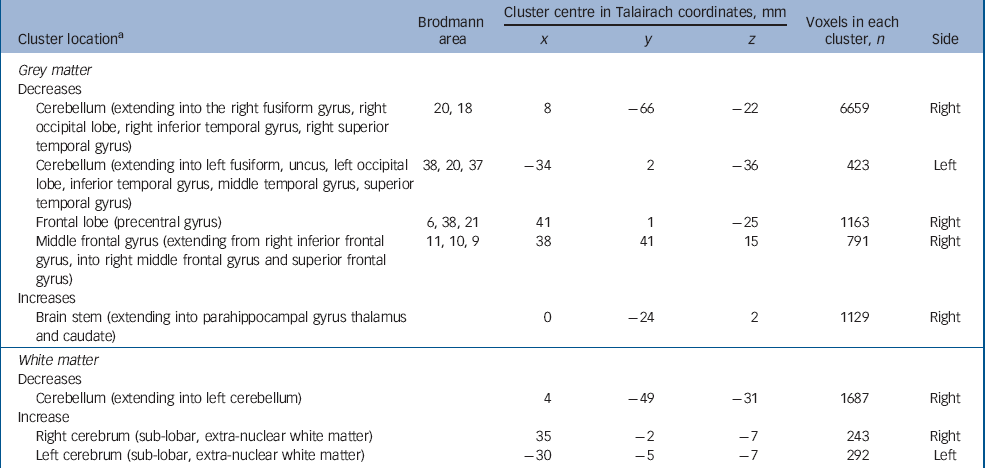
Adults with autism-spectrum disorder and psychosis had a significant reduction in grey matter volume of two clusters centred in the cerebellum (extending bilaterally into the fusiform gyrus, occipital lobe, inferior and superior temporal lobe). In addition, they displayed two clusters of decreased grey matter within the frontal lobe (right precentral and left middle frontal gyrus). They also demonstrated increased grey matter in the thalamus, caudate nuclei and brainstem (extending into the parahippocampal gyrus). Moreover, people with autism-spectrum disorder and psychosis had a significant reduction in white matter volume bilaterally in the cerebellum but bilateral increases in white matter of striatal regions (Fig. 2 and Table 4) (P=0.003 grey, P=0.007 white).

Fig. 2 Autism-spectrum disorder with psychosis v. controls. Relative deficits (blue) and excesses (red) in grey matter volume in (a) autism-spectrum disorder with comorbid psychosis participants compared with healthy controls (cluster threshold = 0.05, P = 0.003) and (b) white matter deficits (blue) (cluster threshold = 0.05, P = 0.007).
Table 4 Autism-spectrum disorder with psychosis v. controls
| Brodmann area | Cluster centre in Talairach coordinates, mm | Voxels in each cluster, n | ||||
|---|---|---|---|---|---|---|
| Cluster locationa | x | y | z | Side | ||
| Grey matter | ||||||
| Decreases | ||||||
| Cerebellum (extending into the right fusiform gyrus, right occipital lobe, right inferior temporal gyrus, right superior temporal gyrus) | 20, 18 | 8 | -66 | -22 | 6659 | Right |
| Cerebellum (extending into left fusiform, uncus, left occipital lobe, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus) | 38, 20, 37 | -34 | 2 | -36 | 423 | Left |
| Frontal lobe (precentral gyrus) | 6, 38, 21 | 41 | 1 | -25 | 1163 | Right |
| Middle frontal gyrus (extending from right inferior frontal gyrus, into right middle frontal gyrus and superior frontal gyrus) | 11, 10, 9 | 38 | 41 | 15 | 791 | Right |
| Increases | ||||||
| Brain stem (extending into parahippocampal gyrus thalamus and caudate) | 0 | -24 | 2 | 1129 | Right | |
| White matter | ||||||
| Decreases | ||||||
| Cerebellum (extending into left cerebellum) | 4 | -49 | -31 | 1687 | Right | |
| Increase | ||||||
| Right cerebrum (sub-lobar, extra-nuclear white matter) | 35 | -2 | -7 | 243 | Right | |
| Left cerebrum (sub-lobar, extra-nuclear white matter) | -30 | -5 | -7 | 292 | Left | |
Autism-spectrum disorder with psychosis v. autism-spectrum disorder without psychosis
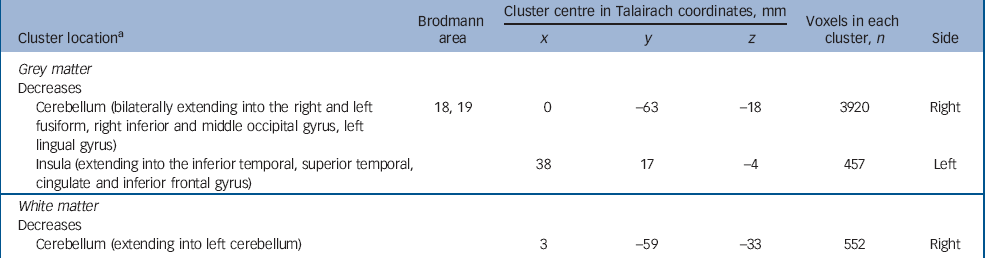
Adults with autism-spectrum disorder with and without psychosis differed mainly in the anatomy of the insular cortex and cerebellum extending into the fusiform gyrus. Those with psychosis had a significant reduction of grey matter centred in the right insular cortex extending into the right inferior and superior temporal gyrus, the right inferior frontal gyrus and cingulate gyrus. In addition, they demonstrated a significant reduction in regional grey matter volume in one large cluster centred bilaterally in the cerebellum and extending into the right and left fusiform gyrus, and the right and left inferior occipital gyrus (including the lingual gyrus). Finally, they also had a significant reduction in white matter volume of the cerebellum bilaterally (Fig. 3 and Table 5) (P=0.003 grey, P=0.007 white).

Fig. 3 Autism-spectrum disorder with psychosis v. autism-spectrum disorder without psychosis. Relative deficits (blue) in (a) grey matter volume in autism-spectrum disorder participants with comorbid psychosis compared with autism-spectrum disorder without psychosis (cluster threshold = 0.05, P = 0.003) and (b) white matter (cluster threshold = 0.05, P = 0.007).
Table 5 Autism-spectrum disorder with psychosis v. autism-spectrum disorder without psychosis
| Brodmann area | Cluster centre in Talairach coordinates, mm | Voxels in each cluster, n | ||||
|---|---|---|---|---|---|---|
| Cluster locationa | x | y | z | Side | ||
| Grey matter | ||||||
| Decreases | ||||||
| Cerebellum (bilaterally extending into the right and left fusiform, right inferior and middle occipital gyrus, left lingual gyrus) | 18, 19 | 0 | -63 | -18 | 3920 | Right |
| Insula (extending into the inferior temporal, superior temporal, cingulate and inferior frontal gyrus) | 38 | 17 | -4 | 457 | Left | |
| White matter | ||||||
| Decreases | ||||||
| Cerebellum (extending into left cerebellum) | 3 | -59 | -33 | 552 | Right | |
The exclusion of the extra participant with a past history of substance misuse did not significantly change the results.
Discussion
We investigated, for the first time, the neuroanatomical associates of psychosis in people with autism-spectrum disorder. We found that both groups of participants with autism-spectrum disorder (i.e. those with and without psychosis) differed from healthy controls in brain regions that are typically reported as abnormal in the disorder (i.e. both groups had significant differences from controls in the anatomy of cerebellar, temporal lobe and striatal regions) (see Toal et al Reference Toal, Murphy and Murphy24 for a review).
In addition, those with psychosis, compared with healthy controls, demonstrated additional reductions in grey matter volume of the frontal and occipital lobes. Within autism-spectrum disorder, however, the diagnosis of psychosis was associated with a significant reduction in grey matter volume of the right insular cortex and bilaterally in the cerebellum extending into the fusiform gyrus, occipital lobe and lingual gyrus. Reduction in white matter volume was observed within the cerebellum and left lingual gyrus.
Thus, people with autism-spectrum disorder and psychosis have significant differences compared with those without psychosis. However, our data suggests that the neurobiological associates of psychosis in autism-spectrum disorder may differ in some regards from those with psychosis in the non-autism-spectrum disorder population.
In the latter group, commonly reported anatomical differences in controls include increased volume of cerebral ventricles, together with a reduction in the total brain and grey matter volume of about 3% (for a review see Shenton et al Reference Shenton, Dickey, Frumin and McCarley25 ). Also, localised volume and grey matter reductions mostly implicate medial temporal lobe structures (including the amygdale and hippocampus), the orbitofrontal and insular cortices and the basal ganglia. There have been fewer studies of white matter. However, diffusion tensor imaging Reference Lim, Hedehus, Moseley, de Crespigny, Sullivan and Pfefferbaum26–Reference Shergill, Kanaan, Chitnis, O'Daly, Jones and Frangou29 and magnetic transfer imaging studies Reference Foong, Maier, Barker, Brocklehurst, Miller and Ron30 and microarray data Reference Hakak, Walker, Li, Wong, Davis and Buxbaum31 suggest that white matter integrity is also compromised in schizophrenia. Studies of those with an affective psychosis (such as bipolar disorder) have generally reported similar findings. At present, the only disease-specific finding is that people with bipolar disorder may not demonstrate the reduction in total brain volume or the amygdala Reference Gur, Keshavan and Lawrie15,Reference McDonald, Zanelli, Rabe-Hesketh, Ellison-Wright, Sham and Kalidindi32 that are found in schizophrenia.
Two studies have investigated changes in brain anatomy in those at high risk of developing psychosis as they transition into the disorder. Pantelis et al Reference Pantelis, Velakoulis, McGorry, Wood, Suckling and Phillips33 reported a significant reduction in grey matter volume of the left parahippocampal and fusiform gyri, as well as other regions including the orbitofrontal, cingulate and cerebellar cortex as people developed psychosis. Job et al Reference Job, Whalley, Johnstone and Lawrie34 reported reductions in grey matter density in right cerebellar cortex and left parahippocampal, uncus and fusiform gyrus. Thus, there is emerging evidence that people with psychosis have differences in brain development prior to the onset of psychosis, and it appears that as a psychotic illness progresses the anatomy of some brain regions continue to change. Moreover, some of theses regions are also implicated in autism-spectrum disorder and (in the case of the fusiform gyrus) are essential to processing facial emotion.
Within our sample of people with autism-spectrum disorder, those with and without psychosis differed mainly in the anatomy of the right insular cortex and the cerebellum extending into the fusiform and lingual gyri. Therefore, the clinical diagnosis of psychosis within people with autism-spectrum disorder is not associated with many of the anatomical differences normally reported as abnormal in cross-sectional studies of psychosis in the general population; but it is associated with differences in brain regions tentatively implicated by studies of young people at increased risk of developing psychosis.
The reason for this is unknown, but potential explanations include the following.
Overlapping developmental brain abnormalities
People with autism-spectrum disorder already have significant developmental abnormalities in brain regions typically affected in psychosis (e.g temporal and frontal lobes), therefore it may be more difficult to detect subtle additional differences associated with further neuropsychiatric disorder.
Alternative ‘entry-point’ into schizophrenia
On the other hand, the presence of autism-spectrum disorder-associated neurodevelopmental abnormalities might represent an alternative ‘entry-point’ into a final common pathway of psychosis. That is, as people with autism-spectrum disorder already have differences in the development of brain regions that are also implicated in schizophrenia (frontostriatal and temporal lobe structures), they may only require relatively subtle additional abnormalities in insular cortex (implicated in sensory and emotional response), the fusiform gyrus and cerebellum (known to be responsible for both inhibitory control and face processing) to develop positive symptoms of psychosis such as delusions and hallucinations. For example, it has been consistently demonstrated that people with autism and those with schizophrenia demonstrate similar deficits in social functioning and social cognition including overlapping deficits in specific theory of mind tasks. Recent work has demonstrated similar reduced neural activation in both autism and schizophrenia in a neural network involved in social cognition. Reference Pinkham, Hopfinger, Pelphrey, Piven and Penn35 Thus, baseline deficits in understanding the mental states of others and processing internal stimuli may render those with autism more susceptible to psychosis in the face of additional insults to specific structures proposed to be specifically related to delusions and hallucinations.
For instance, the insular cortex has been repeatedly implicated in schizophrenia both in structural Reference Makris, Goldstein, Kennedy, Hodge, Caviness and Faraone36,Reference Saze, Hirao, Namiki, Fukuyama, Hayashi and Murai37 and functional studies. Reference Crespo-Facorro, Paradiso, Andreasen, O'Leary, Watkins and Ponto38 Grey matter volume has been demonstrated to be reduced within the insular cortex in those experiencing chronic auditory hallucinations, Reference O'Daly, Frangou, Chitnis and Shergill39 and activation of the insular cortex has been demonstrated in individuals experiencing auditory hallucinations Reference Shergill, Brammer, Williams, Murray and McGuire40 (along with activation of the bilateral inferior frontal, anterior cingulate and temporal cortices bilaterally). The insular cortex provides connections between limbic memory regions as well as the auditory and visual association areas of the sensory system. Abnormal activation of the insula has been hypothesised to result in unbalanced sensory–memory integration contributing to auditory and visual hallucinations.
In addition, the cerebellum has been tentatively proposed to be involved in the development of delusions. Functional MRI studies have demonstrated a potential correlation between cerebellar activation and delusions and suspiciousness/persecution scores. Also, neurobiological theories have directly implicated the cerebellum regarding deficits of ‘internal monitoring’ that are suggested, for example, to underlie the formation of delusions of alien control where the person attributes their own actions to an external agent. Reference Whalley, Gountouna, Hall, McIntosh, Whyte and Simonotto41–Reference Blakemore43
Finally, the fusiform gyrus is known to be a key brain region supporting social perception, including facial identity and emotion recognition. Reference Schultz44,Reference Deeley, Daly, Surguladze, Page, Toal and Robertson45 Anatomical abnormalities in fusiform gyrus may be associated with difficulties in accurate social perception, increasing the likelihood that the benign or neutral actions of others could be construed as hostile – particularly in the presence of impaired mentalising abilities and prior experiences of bullying and social rejection which are common in people with autism-spectrum disorders. Reference Blackshaw, Kinderman, Hare and Hatton46 In other words, it may be hypothesised that fusiform gyrus abnormalities may lower the threshold for the formation of paranoid delusions. Future studies could test this hypothesis by testing for greater facial identity and emotion perception deficits in people with autism-spectrum disorder and psychosis compared with those without psychosis. Alternatively, fusiform gyrus abnormalities could lower the threshold for experiencing visual and auditory hallucinations (although the mechanisms remain unclear). For example, a recent study of people with Parkinson's disease who experienced visual hallucinations demonstrated reduced cerebral blood flow within the fusiform gyrus. Reference Oishi, Udaka, Kameyama, Sawamoto, Hashikawa and Fukuyama47 In addition, reductions in grey matter volume of the fusiform gyrus have been demonstrated to correlate with severity of auditory hallucinations Reference O'Daly, Frangou, Chitnis and Shergill39 in those with schizophrenia. Hence, future studies in larger samples should determine whether frequency and modality of hallucinations in people with autism-spectrum disorder correlates with anatomical abnormalities in the fusiform gyrus.
Abnormality of neurotransmitters
We and others have reported that some people with autism-spectrum disorder also have significant differences from controls in neurochemical pathways (the serotonergic, glutamatergic and gaba-ergic systems) Reference Page, Daly, Schmitz, Simmons, Toal and Deeley48–Reference Yip, Soghomonian and Blatt50 that are also implicated in psychosis. For example, some people with autism-spectrum disorder have platelet hyperserotonaemia, Reference Schain and Freedman51,Reference Hranilovic, Bujas-Petkovic, Vragovic, Vuk, Hock and Jernej52 abnormal brain serotonin synthesis capacity, Reference Chugani, Muzik, Behen, Rothermel, Janisse and Lee53 serotonin receptor dysfunction, Reference Hollander, Novotny, Allen, Aronowitz, Cartwright and DeCaria54 and reduced 5-HT2A (5-hydroxytryptamine) receptors. Reference Murphy, Daly, Schmitz, Toal, Murphy and Curran49 We have previously reported that people with autism-spectrum disorder have significant differences from controls in the glutamate/glutamine concentration of the amygdala–hipocampal complex. Reference Page, Daly, Schmitz, Simmons, Toal and Deeley48 Thus, the combination of pre-existing anatomical, and neurochemical, differences in brain systems also implicated in psychosis may reduce the additional disease burden required to develop comorbid psychotic symptoms. If this were the case, however, we would anticipate a very significantly increased risk of developing psychosis in autism-spectrum disorder, and the evidence to substantiate that is (at best) preliminary. Reference Stahlberg, Soderstrom, Rastam and Gillberg11,Reference Mouridsen, Rich and Isager14
Limitations
This is the first study of its kind. Nevertheless, it was a relatively small observational study of adults. Moreover, we included a person with a prior history of substance misuse. We suggest, however, that the differences we found cannot be fully accounted for by substance misuse as our results were largely unchanged when we excluded this individual. All individuals with psychosis had been treated with antipsychotics at some time in the past and the majority were on antipsychotic medication at the time of scanning (which may affect brain anatomy). However, it is unlikely that this can fully explain our results as most MRI studies of medication effects on brain anatomy in psychosis found differences mainly in the globus pallidus Reference Gur, Keshavan and Lawrie15 and we found no abnormalities in this region.
It may be asserted by some that the differentiation of psychosis in autism-spectrum disorder is inappropriate and/or impossible. However, our diagnosis of autism-spectrum disorder without psychosis was based on evidence of early childhood onset, collateral history from family/friends/teachers and use of reliable diagnostic instruments. In addition, these symptoms present in early childhood were distinct from the frank psychotic symptoms of auditory hallucinations and bizarre delusions that developed in later life. Further, inclusion in the psychosis group did not rely on features such as thought disorder or negative symptoms that (respectively) may be difficult to differentiate within autism-spectrum disorder from pre-existing speech and language abnormalities, and impassivity and lack of emotional awareness.
Another potential limitation of our study is that the psychosis group consisted of a mixed group of people; most fulfilled the diagnostic criteria for schizophrenia, but three had schizoaffective disorder and two had a psychotic bipolar affective disorder. However, increasingly genetic, neuropharmacological and neurophysiological studies are highlighting the overlap between these groups. Reference Gur, Keshavan and Lawrie15,Reference Tamminga and Davis55–Reference Tsuang, Stone and Faraone58
Finally, neuroimaging techniques such as voxel-based morphometry are still evolving and inherent limitations include difficulties with registration of the images and limitations in detecting differences in areas with high interindividual variance. Therefore, we await replication of these findings in larger groups before definitive conclusions can be drawn.
The diagnosis of autism-spectrum disorder is being made much more frequently, and so we are likely to encounter an increased number of people with autism-spectrum disorder diagnosed with psychosis. Our preliminary study suggests that the presence of neurodevelopmental abnormalities normally associated with autism-spectrum disorder might represent an alternative ‘entry-point’ into a final common pathway of psychosis. Future longitudinal studies may further help to elucidate the changes associated with the development of psychosis within this disorder.











eLetters
No eLetters have been published for this article.