Coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic that is ongoing. Reference Wang, Hu and Hu1 Early reports suggested that patients with cancer on active therapy were at higher risk for severe COVID-19–related events. Reference Yu, Ouyang, Chua and Xie2,Reference Liang, Guan and Chen3 In an analysis of 423 cases of symptomatic COVID-19 diagnosed at a cancer center in the United States between March 10 and April 7, 2020, 163 (40%) required hospitalization, 87 (20%) developed severe respiratory illness, and 51 (12%) died within 30 days. Reference Robilotti, Babady and Mead4 Patients with hematologic or lung malignancies, peri–COVID-19 lymphopenia, or baseline neutropenia had worse outcomes. Reference Jee, Foote and Lumish5 Even in the absence of COVID-19, these and other factors (eg, cytotoxic chemotherapy, other immunosuppressive therapy, anatomic derangements, central venous catheters) portend infectious risk for cancer patients.
Since the onset of the COVID-19 pandemic, several studies have reported frequent prescription of broad-spectrum empiric antibiotics despite low rates of microbiologically confirmed bacterial infections diagnosed around the time of SARS-CoV-2 infection. Reference Nori, Cowman and Chen6–Reference Russell, Fairfield and Drake8 As patients extend their hospitalization beyond 48 hours, nosocomial bacterial infections appear to be more frequent, particularly among patients admitted to the intensive care unit (ICU). Reference Russell, Fairfield and Drake8,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia9 However, data on infectious complications specific to cancer patients infected with SARS-CoV-2 are scarce. The purpose of this study was to characterize bacterial infections and antibiotic utilization in hospitalized cancer patients with COVID-19 at a tertiary-care cancer center between March 1, 2020, and May 31, 2020.
Methods
Study population and design
This study was reviewed and approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSKCC), a 498-bed tertiary cancer center in New York, New York. Hospitalized adults ≥18 years of age diagnosed with COVID-19 between March 1, 2020, and May 31, 2020 were included in the study. SARS-CoV-2 was detected by nasopharyngeal polymerase chain reaction (PCR). The institutional electronic health record was used to obtain clinical information, as well as laboratory, microbiology, and radiology results for each hospitalization. Abstracted data included demographics, underlying cancer diagnosis, medical comorbidities, anti-infectives and dates of administration, supplemental oxygen support, intensive care unit (ICU) stay, noninvasive and invasive ventilatory support, and pressor support. The primary outcome was the rate of bacterial infection within 30 days of the date of COVID-19 diagnosis. Secondary outcomes included the proportion of patients receiving antibiotics and antibiotic length of therapy (LOT) initiated within 30 days of COVID-19 diagnosis. Prophylactic antibiotics (eg, trimethoprim-sulfamethoxazole for Pneumocystis jirovecii prevention) and anti-infectives lacking antibacterial activity were excluded. Outpatient antibiotic prescriptions were also excluded since there would not be a way to verify patient’s compliance. Antibiotics were grouped by therapeutic class. 10
Role of the institutional antimicrobial stewardship program (ASP) during the pandemic
At the start of the COVID-19 pandemic, the hospital’s ASP team members along with the infectious disease (ID) service drafted guidelines for therapeutic management of patients with COVID-19. Institutional guidelines, which were posted on the hospital’s intranet, considered risk stratification not only by need for hospitalization and oxygenation status but also the degree of immunosuppression. As data emerged on efficacy and safety of therapeutics (eg, remdesivir or dexamethasone) for COVID-19 management, institutional guidelines were revised in real time. In addition, the ASP maintained stewardship interventions such as prior approval for restricted antibiotics (eg, piperacillin–tazobactam, cefepime, ceftriaxone, carbapenems, fluoroquinolones, and azithromycin) and postprescription review and feedback during the pandemic.
Definitions
Because oxygenation status can vary during the COVID-19 course of illness, we categorized COVID-19 severity based on the maximum oxygen requirement during the hospitalization. Patients were classified as having mild COVID-19 if no supplemental oxygen was used, moderate COVID-19 if a nasal cannula was used, or severe COVID-19 if high-flow oxygen or mechanical ventilation was used.
An infectious episode was defined as the presence of clinical symptoms along with supporting laboratory, microbiologic (if available), and radiographic results documented in admission and daily progress notes by the primary teams. Definitions for bacterial infections were based on standard definitions from national society guidelines (Supplementary Material online). 11–Reference Stevens, Bisno and Chambers19 Patients who were classified as having fever and neutropenia had preceding cytotoxic chemotherapy. Suspected bacterial infections without microbiologic confirmation were also included. Despite testing positive for SARS-CoV-2, patients with respiratory symptoms and radiographic infiltrates warranting empiric antibacterial treatment within 48 hours of admission were considered to have community-acquired pneumonia (CAP). If a patient had clinical worsening combined with a new infiltrate on chest imaging, the patient was considered to have hospital-acquired pneumonia (HAP) if the symptoms were not incubating at the time of admission and worsening occurred ≥48 hours after admission or the patient was considered to have ventilator-associated pneumonia (VAP) if worsening occurred >48 hours after endotracheal intubation.
Antibiotic LOT was defined as the number of days that a patient received systemic antimicrobial agents, irrespective of the number of different drugs. 20 Antibiotic days of therapy (DOT) was defined as the number of days that a patient received an antimicrobial agent; the DOT for a given patient on multiple antibiotics was the sum of DOT for each antibiotic that the patient received. 20
Statistical analysis
Descriptive statistics were used to tabulate demographic characteristics and outcomes. Numeric data were expressed as medians (interquartile range, IQR) and were compared using the Mann-Whitney rank-sum test or the Kruskal-Wallis test across groups as appropriate. Categorical data were compared using the χ Reference Yu, Ouyang, Chua and Xie2 test or the Fisher exact test across groups and are reported as number (percentage). The Cochran-Armitage and Jonckheere-Terpstra tests were used to assess the increasing trends of proportion of patients given antibiotics and the median antibiotic LOT by ordinal COVID-19 severity, respectively.
Results
In total, 358 hospitalized patients were diagnosed with COVID-19 between March 1, 2020, and May 31, 2020. Patient characteristics and outcomes are listed in Table 1. Among the 358 patients, 234 (65%) had solid-tumor malignancies. Also, 166 patients (46%) were evaluated by the infectious disease (ID) service. Patients with mild COVID-19 were significantly younger than those with moderate or severe disease: mild COVID-19 (median age, 62 years) versus moderate COVID-19 (median age, 67 years) versus severe COVID-19 (median age, 67 years) (P = .004). Statistically significant differences in 30-day mortality, median hospital length of stay (LOS), the proportion of patients admitted to the ICU, and the proportion of intubated patients were observed along the spectrum of mild, moderate, and severe COVID-19 (Table 1). Furthermore, 200 patients (56%) were diagnosed with at least 1 bacterial infection, and 42 (21%) of these 200 were neutropenic when diagnosed with the bacterial infection. The proportion of patients diagnosed with a bacterial infection increased with COVID-19 severity: 47 patients (35%) had mild COVID-19, 49 (51%) had moderate COVID-19, and 104 (81%) had severe COVID-19 (P < .0001). Similar results were observed when restricting to patients with microbiologically documented bacterial infections: 16 (12%) had mild COVID-19, 11 (11%) had moderate COVID-19 and 32 (25%) had severe COVID-19 (P = .0052).
Table 1. Comparison of Patient Characteristics and Outcomes by COVID-19 Severity
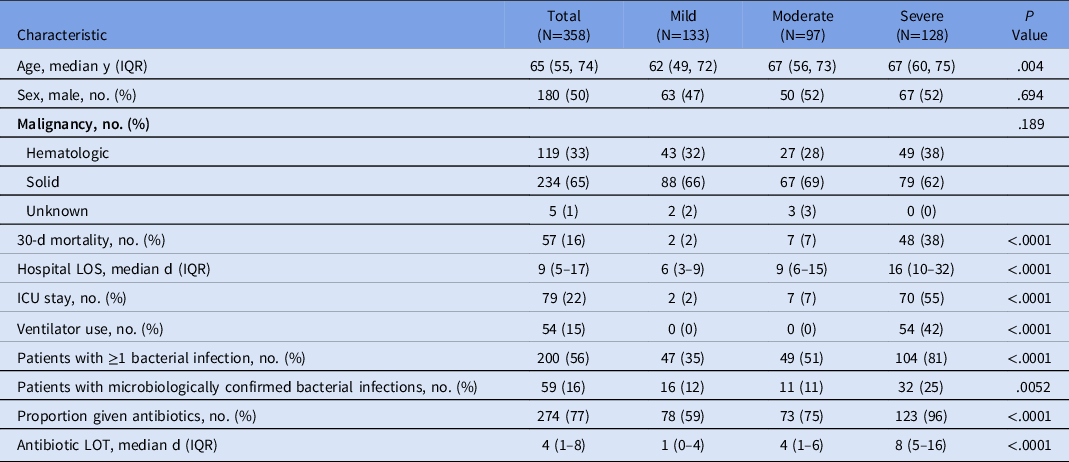
Note. IQR, interquartile range; ICU, intensive care unit; LOS, length of stay; LOT, length of therapy.
In total, 245 bacterial infections were diagnosed in 200 patients; 42 patients had >1 bacterial infection. Types of bacterial infections as well as the rates of microbiologically confirmed infections are shown in Table 2. Of 245 infections, 163 (67%) were diagnosed within 48 hours of admission. The predominant infection involved the lower respiratory tract, which occurred in 140 patients. Among these patients, 19 (14%) had 2 episodes. Neutropenia was present in 12 (8%) of 159 episodes of pneumonia. Furthermore, 105 patients (66%) were classified as having community-acquired pneumonia (CAP), and 88 (84%) of these patients required oxygen supplementation. Of 105 (82%) patients with a CAP diagnosis, 86 also had a respiratory PCR (BioFire FilmArray RP2, Salt Lake City, UT), urine Legionella antigen test (BinaxNow, Abbott, Chicago, IL), a streptococcal urine antigen test (BinaxNow, Abbot, Chicago, IL), and/or sputum culture sent. However, none was positive for a bacterial organism. Of 105 patients, 52 (50%) were evaluated by the ID service, and empiric antibiotics for CAP were deemed appropriate. Also, 2 patients with empyema had a bacterial organism identified from pleural fluid.
Table 2. Classification of Bacterial Infection

a 42 patients had >1 infection.
Of these 159 patients, 19 (12%) had bacteria recovered from a respiratory specimen: Achromobacter xylosoxidans/denitrificans (n = 1), Burkholderia cepacia (n = 1), coagulase-negative Staphylococcus spp (n = 1), Escherichia coli (n = 2), Klebsiella pneumoniae (n = 1), methicillin-resistant Staphylococcus aureus (n = 1), Pseudomonas aeruginosa (n = 5, 2 isolates were multidrug-resistant), Pseudomonas spp (n = 2), Serratia marcescens (n = 1), Streptococcus anginosus (n = 1), Streptococcus pneumoniae (n = 1), and polymicrobial (n = 2), 1 patient had P. aeruginosa and Stenotrophomonas maltophilia, 1 patient had carbapenem-resistant K. pneumoniae and P. aeruginosa. Other types of bacterial infections occurred far less frequently (<10%) (Table 2).
Table 3 lists the median antibiotic LOT by infection type for patients with a single infection. In patients without a diagnosed bacterial infection, the median antibiotic LOT was 0 days (IQR, 0–2), whereas the median antibiotic LOTs were 7 days (IQR 4, 9) for those with 1 infection and 20 days (IQR, 14–29) for patients with multiple infections (P < .0001). Differences in the median antibiotic LOT were also observed as COVID-19 severity increased (Table 1). The median antibiotic LOT was short in patients with mild COVID-19 (1 day; IQR, 0–4) in comparison to those with moderate COVID-19 (4 days; IQR, 1–6) and severe COVID-19 (8 days; IQR, 5–16) (P < .0001). Figure 1 shows the cumulative antibiotic days of therapy by therapeutic class. The 5 most frequently administered antibiotics were piperacillin-tazobactam, cefepime, ceftriaxone, azithromycin, and meropenem.
Table 3. Comparison of Antibiotic Length of Therapt (LOT) by Infection Type for Patients with a Single Bacterial Infection

Note. IQR interquartile range.

Fig. 1. Cumulative antibiotic days of therapy. Extended-spectrum penicillins: piperacillin-tazobactam; fourth-generation cephalosporins: cefepime; third-generation cephalosporins: cefixime, ceftazidime, ceftazidime-avibactam, ceftolozane-tazobactam, ceftriaxone; macrolides: azithromycin; carbapenems: meropenem; glycopeptides (iv): vancomycin; tetracyclines: doxycycline; glycopeptides (oral): vancomycin; monobactams: aztreonam; quinolones: ciprofloxacin, levofloxacin; aminopenicillins: amoxicillin-clavulanate, ampicillin, ampicillin-sulbactam; first generation cephalosporins: cefadroxil, cefazolin, cephalexin; amebicides: metronidazole; oxazolidoinones: linezolid; fifth-generation cephalosporins: ceftaroline; lincomycins: clindamycin; second generation cephalosporins: cefuroxime; sulfonamides: trimethoprim-sulfamethoxazole; urinary anti-infectives: nitrofurantoin; aminoglycosides: amikacin; cyclic lipopeptides: daptomycin. Note. gen, generation; IV, intravenous.
Discussion
We describe bacterial infections and antibiotic utilization in hospitalized cancer patients diagnosed with COVID-19 between March 1, 2020, and May 31, 2020, in New York City, an early epicenter of the COVID-19 pandemic in the United States. More than half of the study patients were diagnosed with a bacterial infection during the hospitalization, and pneumonia accounted for the bulk of infections. Most patients received antibiotics, but the median durations were within recommended lengths for the types of infection observed. Rates of bacterial infection and antibiotic prescribing, as well as antibiotic durations increased with increasing COVID-19 severity.
Rates of microbiologically confirmed bacterial respiratory and bloodstream infections in hospitalized patients during the initial COVID-19 surge range from 2.3% to 8.2%, but data specific to cancer patients are limited. Reference Nori, Cowman and Chen6,Reference Russell, Fairfield and Drake8,Reference Arentz, Yim and Klaff21–Reference Vaughn, Gandhi and Petty24 Our investigation of SARS-CoV-2–infected cancer patients revealed an overall higher rate of microbiologically confirmed bacterial infections (27%), although other infection types were included. Pneumonia was commonly diagnosed, but only 19 (12%) of 159 cases had positive respiratory cultures. In the only other study examining hospitalized patients with cancer and COVID-19, the rate of microbiologically documented bacterial infections was 17%. Reference Gudiol, Dura-Miralles and Aguilar-Company25 Gudiol et al Reference Gudiol, Dura-Miralles and Aguilar-Company25 also reported that lower respiratory tract infections (67 of 167, 40%) were common in their patient cohort, but there was a higher diagnostic yield, with only 17 (25%) of 67 cases having no organisms identified. Reference Gudiol, Dura-Miralles and Aguilar-Company25 Notably, bacterial infection rates, particularly for pneumonias, may be underestimated if only microbiologically confirmed infections are counted.
Among the 140 lower respiratory tract infections without a bacterial microbiologic diagnosis in our study, 103 were CAP and 37 were HAP or VAP. Among the 37 HAP and VAP cases, 2 were diagnosed with a probable invasive fungal infection (1 pulmonary aspergillosis, 1 pulmonary coccidiomycosis). Of the 35 remaining HAP and VAP cases, these patients had clinical worsening in the setting of a new radiographic infiltrate, suggesting nosocomial superinfection. We acknowledge that the 103 cases designated as CAP could have solely been from SARS-CoV-2 infection. Clinical symptoms such as fever, cough, and shortness of breath combined with abnormal chest imaging (eg, lobar consolidation, ground glass opacities, reticular infiltrates) have been well described in patients with lower respiratory tract involvement with COVID-19. Reference Metlay and Waterer26 This clinical picture, however, is nonspecific because bacterial pneumonia can also present similarly. Reference Kalil, Metersky and Klompas15,Reference Mandell, Wunderink and Anzueto16
Several studies have noted reduced respiratory sampling (7.7%–17.7%) during the pandemic because patients may not have been able to produce phlegm and/or fewer diagnostic procedures (eg, bronchoscopy) may have been performed due to concerns about healthcare worker safety. Reference Russell, Fairfield and Drake8,Reference Vaughn, Gandhi and Petty24 The poor sensitivity of cultures and incomplete use of diagnostic tests are other well-recognized limitations. Reference Kalil, Metersky and Klompas15,Reference Jain, Self and Wunderink27 Even under circumstances in which all available tests are submitted, diagnostic yield remains low. In a large population-based surveillance study for CAP, blood, urine, and respiratory specimens were systematically collected for culture, serologic testing, antigen detection, and molecular diagnostic testing, but a bacterial pathogen was detected in only 247 (11%) of 2,259 individuals. Reference Jain, Self and Wunderink27 According to the 2019 American Thoracic Society and Infectious Diseases Society of America guidelines in the pre–COVID-19 era, routine testing is recommended only for patients with severe CAP or if there are concerns for multidrug-resistant pathogens. Reference Metlay, Waterer and Long28 Thus, CAP diagnosis does not rely on microbiologic confirmation. One area that warrants further investigation is whether SARS-CoV-2 can predispose a patient to bacterial pneumonia, similar to what has been demonstrated with influenza virus. Reference Rowe, Meliopoulos, Iverson, Bomme, Schultz-Cherry and Rosch29 Because bacterial coinfection in critically ill patients has ranged from 23% to 30% in past influenza epidemics, antibacterial treatment is recommended in adults with clinical and radiographic evidence of CAP who test positive for influenza. Reference Metlay, Waterer and Long28,Reference Shah, Greenberg and McNulty30 A recent case series showed that coinfection with bacterial pathogens (including Mycoplasma and Chlamydophila) did occur in patients who died from COVID-19 pneumonia. Reference Du, Tu and Zhu31 The true picture of bacterial coinfection is thus not clear because it has been challenging to definitively include or exclude other concomitant bacterial etiologies in SARS-CoV-2–infected patients with lower respiratory tract findings.
Compared with the general patient population, cancer patients have a higher infectious morbidity and mortality due to the underlying malignancy and/or its treatment. This disparity is borne out in our study by an additional 77 infections (31%) not involving the respiratory tract or bloodstream and included fever and neutropenia, gastrointestinal infections, skin and soft-tissue infections, and urinary tract infections. In contrast, only 1,002 (2%) of 48,092 hospitalized patients infected with SARS-CoV-2 from the United Kingdom were diagnosed with other bacterial infections in addition to pneumonia or bacteremia. Reference Russell, Fairfield and Drake8
It is not surprising that 274 (77%) of 358 patients in this study were exposed to antibiotics during their COVID-19 admission. Many other studies have noted high rates (ie, >70%) of antibiotic administration in hospitalized SARS-CoV-2-infected patients. Reference Nori, Cowman and Chen6,Reference Rawson, Moore and Zhu7,Reference Cong, Poudel, Alhusein, Wang, Yao and Lambert32 However, the proportion of patients diagnosed with a bacterial infection and given antibiotics differed by severity of illness. Although this finding seems intuitive, no prior study has systematically assessed both parameters (bacterial infection and antibiotic utilization) along with COVID-19 severity. In patients with mild COVID-19, 47 (35%) of 133 were diagnosed with a bacterial infection and 78 (59%) received empiric antibiotics for a median of 1 day. Thus, the data suggest that hospitalized cancer patients with mild COVID-19 can avoid or have early discontinuation of empiric antibiotics with active antimicrobial stewardship oversight. Other hospital ASPs have shown that they could discontinue or narrow antibiotics for CAP in patients with COVID-19 within 72 hours of admission. Reference Pettit, Nguyen and Lew33,Reference Evans, Davidson, Low, Basarab and Arnold34 This finding contrasts with a report by Cong et al, Reference Cong, Poudel, Alhusein, Wang, Yao and Lambert32 who reviewed 118 publications and stratified patients with COVID-19 into mild-to-moderate or severe-to-critical illness categories. Antibiotic prescribing during the first 6 months of the COVID-19 pandemic was high (>75%) in both groups. This highlights the potential role that ASPs can play to better optimize anti-infectives during pandemics.
In more severely ill patients, the risk and rate of bacterial infection are higher. Because COVID-19 in patients with cancer is marked by substantial rates of hospitalization, severe outcomes, and additional difficulty in discriminating bacterial infection from severe COVID-19 inflammation, the administration of empiric antibiotic therapy under these circumstances is understandable. Reference Robilotti, Babady and Mead4,Reference Lucien, Canarie and Kilgore35 In out study, nearly all patients who required high-flow oxygen or mechanical ventilation (severe COVID-19) were prescribed antibiotics, and 104 (81%) of 128 patients were diagnosed with a bacterial infection. Even so, the median antibiotic LOT for study patients with severe COVID-19 was 8 days. Martin et al Reference Martin, Shulder, Dobrzynski, Quartuccio and Pillinger36 similarly noted that ICU stay and the necessity for mechanical ventilation were predictors for antibiotic prescribing in hospitalized patients with COVID-19.
In examining antibiotic duration by infection type, the median antibiotic LOT for bacterial infections was not overly long and was consistent with standard duration of therapy. Both ASP members and ID consultation teams strived to maintain standard duration of therapy for suspected or documented bacterial infections and actively discontinued empiric antibiotic therapy in cases where bacterial etiology was unlikely. For example, the median antibiotic LOT for respiratory infections was 7 days, which was in line with national guidelines for CAP, HAP, and VAP. Reference Kalil, Metersky and Klompas15,Reference Metlay, Waterer and Long28 Of the 5 patients who had a bloodstream infection, the median antibiotic LOT was short because 3 patients died. Similar to other studies, broad-spectrum antibiotics (eg, piperacillin/tazobactam, cefepime, meropenem) as well as agents used for respiratory infections were the most commonly prescribed agents between March 2020 and May 2020. Reference Andrews, Budd and Hendrick37–Reference Dieringer, Furukawa and Graber39
This study had several limitations. In this is single-center retrospective study, we also included clinically diagnosed bacterial infections without microbiologic confirmation. This method may have led to an overestimation of infection rates, specifically lower-respiratory-tract infections of which CAP was predominant. Although fever, respiratory symptoms, and lung infiltrates could all be due to COVID-19, there is conversely insufficient high-quality evidence to safely withhold antibacterial therapy in hospitalized cancer patients, most of whom required supplemental oxygen in our study. Patients with multiple bacterial infections can have overlapping antibiotic therapy, so we looked at patients with a single infection so that we could clearly identify antibiotic duration by infection type. Although the exclusion of patients with multiple bacterial infections could lead to the underestimation of antibiotic LOT, the number of patients with multiple infections was small. Antibiotic LOT could also have been underestimated because we did not include outpatient prescriptions given upon hospital discharge. The results of this study may not be generalizable to noncancer settings or institutions that lack a robust ASP. Also, these findings may not be generalizable to later surges from SARS-CoV-2 viral variants of concern [eg, δ (delta) and (omicron) varaints]. The development of COVID-19 therapeutics (eg, antivirals, monoclonal antibodies) and a higher proportion of vaccinated individuals should reduce the proportion of cancer patients who require hospitalization and/or who develop severe COVID-19.
In conclusion, hospitalized cancer patients with COVID-19 had a high rate of bacterial infections. Although antibiotic prescribing was common, the proportion of patients diagnosed with a bacterial infection and given antibiotics increased as patients developed more severe COVID-19. Median durations of antibiotic therapy were within recommended lengths for the types of infections seen. In mild COVID-19 cases, antibiotic therapy was short, suggesting that empiric antibiotics can be safely avoided or stopped in this group.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.129
Acknowledgments
Financial support
This research was funded in by the NIH/NCI Cancer Center (grant no. P30 CA008748).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







