Introduction
As one of the neglected tropical diseases identified by the World Health Organization, schistosomiasis or bilharzia is a widespread parasitic disease caused by blood flukes of the genus Schistosoma (World Health Organization, 2015). Among the species of Schistosoma, S. japonicum, S. mansoni and S. haematobium inflict most infections in humans, with minimal cases caused by S. intercalatum and S. mekongi (Addisu and Tekeste, Reference Addisu and Tekeste2016). The disease is commonly seen among agricultural and fishing populations due to exposure to infested water (World Health Organization, 2015). The parasite is said to affect more than 240 million people worldwide in 78 countries, mostly in Africa, the Middle East and some endemic areas in the Americas and South-east Asia, with mortality rates as high as 200,000 per year in Africa alone (Colley et al., Reference Colley2014; World Health Organization, 2016; Gomes Casavechia et al., Reference Gomes Casavechia2018). Of all the cases of schistosomiasis needing medical intervention, 91.4% are from sub-Saharan Africa, which is endemic for both S. mansoni and S. haematobium (World Health Organization, 2016).
A number of factors have been investigated previously regarding their effect on the pathophysiological mechanism of human schistosomiasis, such as parasite egg intensity, organs or tissues affected, immunoregulatory mechanism of the host against the parasite, and even the presence of specific blood group and host antigens (Pearce and MacDonald, Reference Pearce and MacDonald2002; Alemu et al., Reference Alemu2011). Among the suggested factors, the possible effect of the ABO blood group on the susceptibility and severity of schistosomiasis has been the most promising. It has been studied as early as 1979 (Pereira et al., Reference Pereira1979; Trangle et al., Reference Trangle1979), but its pathophysiologic mechanism in the disease is not yet well understood. A number of studies have shown that the incidence of schistosomiasis due to S. mansoni and S. haematobium among people with blood type O is less than among those with other blood types, suggesting that ABO blood group antigens may affect the severity of infection (Deribew et al., Reference Deribew, Tekeste and Petros2012; Igbeneghu and Olisekodiaka, Reference Igbeneghu and Olisekodiaka2014; Degarege et al., Reference Degarege, Hailemeskel and Erko2015; Addisu and Tekeste, Reference Addisu and Tekeste2016). Reports on the association of the ABO blood group with schistosomiasis susceptibility are limited, and their results are inconsistent. Hence, we performed a systematic review and meta-analysis to obtain more precise estimates by pooling the results of the individual studies available.
Materials and methods
Search strategy and study selection
An online search for relevant literature was initially carried out in PubMed up to 21 August 2018 using the MeSH (medical subject heading) terms “ABO blood group” and “schistosomiasis”. Titles and abstracts of each of the studies returned in the search were screened manually by the authors (RET and NAP). Only studies with available abstracts were included in the initial screening. After removal of duplicates and irrelevant studies, the full text of the resulting articles was checked manually to determine their relevance. The following inclusion criteria were used: (1) studies that contain the incidence of schistosomiasis among cases and controls; (2) studies that grouped their participants depending on their ABO blood type; (3) studies that used microscopic examination in the identification of schistosomiasis; and (4) studies that are written in English. References cited in the selected studies were also checked for additional eligible articles. All studies identified were investigated for eligibility independently by RET and NAP.
Data extraction
Two of the authors (RET and NAP) independently extracted data and reached a consensus on all the items. For each study included, the following information was obtained: (1) the first author's last name; (2) year of publication; (3) country of the participants; (4) species of Schistosoma infecting the study population; (5) specimen used for schistosomiasis identification; (6) sources of controls; (7) total number of participants included; (8) total number of participants per ABO blood type; (9) total number of participants with schistosomiasis; and (10) total number of participants with schistosomiasis per ABO blood type.
Methodological quality assessment of the included studies
The Newcastle–Ottawa Scale (NOS) was used in assessing the methodological quality of the retrieved studies. All included articles were rated based on three perspectives: selection (maximum of 4 points), comparability (maximum of 2 points), and exposure (maximum of 3 points). The total scoring system used has ratings ranging from 0 to 9, i.e. from worst to best, respectively. An accumulated score of ≤ 4 points indicates low-quality studies, 5–6 points indicates moderate-quality studies, and ≥ 7 points indicates high-quality studies (Wells et al., Reference Wells2013).
Meta-analysis protocol
Statistical analysis for this study was carried out using Review Manager 5.3 (Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration, 2014) and Meta-Essentials (Erasmus Research Institute of Management, 2017) (Suurmond et al., Reference Suurmond, van Rhee and Hak2017). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using either the fixed- (absence of heterogeneity) or random-effects model (presence of heterogeneity) (Mantel and Haenszel, Reference Mantel and Haenszel1959; DerSimonian and Laird, Reference DerSimonian and Laird1986). Heterogeneity among the studies was determined using a χ2-based Q test (Lau et al., Reference Lau, Ioannidis and Schmid1997), and its degree was measured using I 2 statistics (Higgins et al., Reference Higgins2003). Due to the low power of the test, the P-value (PH) for heterogeneity testing was set at < 0.10 (Higgins and Thompson, Reference Higgins and Thompson2002), whereas the P-value (PA) for association was two-sided, with the significance threshold set at < 0.05. Subgroup analysis was also performed and was based on the species of Schistosoma and the ethnicity of the participants.
Other analysis
Robustness of the overall summary effects was determined using sensitivity analysis. In this test, the impact of the individual studies on the pooled ORs was examined by systematic removal of one study at a time. A study was considered robust if P-values for association and heterogeneity remained the same throughout. Publication bias was no longer estimated, given the low sensitivity of the test when the number of studies is < 10 (Ioannidis and Trikalinos, Reference Ioannidis and Trikalinos2007).
Results
Search results
Figure 1 summarizes the study selection process utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., Reference Moher2015). The initial search yielded a total of 43 studies that were thoroughly checked. After omitting duplicates and irrelevant studies following the set inclusion criteria, a total of 10 articles were included in this systematic review and meta-analysis (Pereira et al., Reference Pereira1979; Trangle et al., Reference Trangle1979; Kassim and Ejezie, Reference Kassim and Ejezie1982; Deribew et al., Reference Deribew, Tekeste and Petros2012; Degarege et al., Reference Degarege2014, Reference Degarege, Hailemeskel and Erko2015, Reference Degarege2017; Igbeneghu and Olisekodiaka, Reference Igbeneghu and Olisekodiaka2014; Addisu and Tekeste, Reference Addisu and Tekeste2016; Igbeneghu et al., Reference Igbeneghu2018).
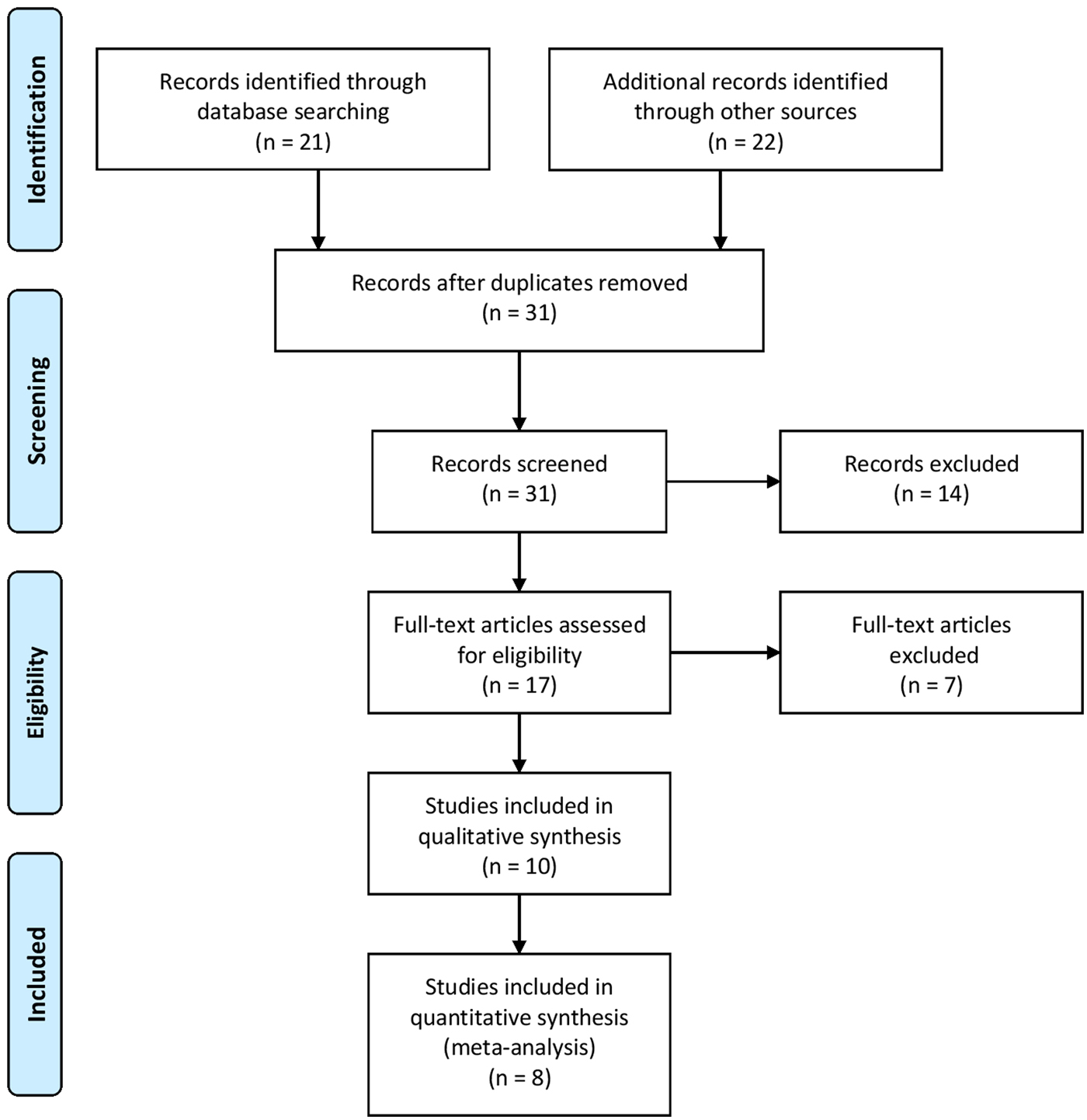
Fig. 1. Literature search summary following the PRISMA guideline.
Characteristics of the included studies
Table 1 summarizes the characteristics of the included studies. Year of publication ranged from 1979 to 2018. Overall, the total sample size from the 10 publications covered is 6472, with a wide range of total sample sizes across all the studies (200 to 2310). Subjects in nine of the articles were predominantly of sub-Saharan African origin (Trangle et al., Reference Trangle1979; Kassim and Ejezie, Reference Kassim and Ejezie1982; Deribew et al., Reference Deribew, Tekeste and Petros2012; Degarege et al., Reference Degarege2014, Reference Degarege, Hailemeskel and Erko2015, Reference Degarege2017; Igbeneghu and Olisekodiaka, Reference Igbeneghu and Olisekodiaka2014; Addisu and Tekeste, Reference Addisu and Tekeste2016; Igbeneghu et al., Reference Igbeneghu2018), whereas one was of Western origin (Pereira et al., Reference Pereira1979). As for the method of Schistosoma identification, four studies performed stool microscopy (Degarege et al., Reference Degarege2014, Reference Degarege, Hailemeskel and Erko2015, Reference Degarege2017; Addisu and Tekeste, Reference Addisu and Tekeste2016), another four performed urine microscopy (Kassim and Ejezie, Reference Kassim and Ejezie1982; Deribew et al., Reference Deribew, Tekeste and Petros2012; Igbeneghu and Olisekodiaka, Reference Igbeneghu and Olisekodiaka2014; Igbeneghu et al., Reference Igbeneghu2018), one performed both stool and urine microscopy (Trangle et al., Reference Trangle1979), and another performed tissue biopsy (Pereira et al., Reference Pereira1979). The majority (9 out of 10 studies) of the participants in the study were recruited from a population-based setting (Trangle et al., Reference Trangle1979; Kassim and Ejezie, Reference Kassim and Ejezie1982; Deribew et al., Reference Deribew, Tekeste and Petros2012; Degarege et al., Reference Degarege2014, Reference Degarege, Hailemeskel and Erko2015, Reference Degarege2017; Igbeneghu and Olisekodiaka, Reference Igbeneghu and Olisekodiaka2014; Addisu and Tekeste, Reference Addisu and Tekeste2016; Igbeneghu et al., Reference Igbeneghu2018). NOS scoring showed the mean and standard deviation to be 5.5 ± 0.5, with a median of 6, indicating that the included studies were of moderate quality.
Table 1. Characteristics of the included studies.

Overall analysis for the association of non-O blood type and type O with schistosomiasis
Summary of overall effects
A total of 10 studies were included in the overall analysis for the relationship of non-O blood type with schistosomiasis susceptibility. The random-effects model (fig. 2a) showed significant associations (OR: 1.39; 95% CI: 1.02–1.91; PA = 0.04), with a high degree of heterogeneity (I 2 = 81%, PH < 0.001). Based on the resulting pooled ORs, individuals with non-O blood type are more susceptible to schistosomiasis. On the other hand, the relationship of blood type O with schistosomiasis susceptibility was also tested using the random-effects model (fig. 2b) and also showed a significant association (OR: 0.72; 95% CI: 0.52–0.98; PA = 0.04), with a high degree of heterogeneity (I 2 = 81%, PH< 0.001). The resulting pooled ORs for this comparison suggest that individuals with blood type O are less susceptible to schistosomiasis.
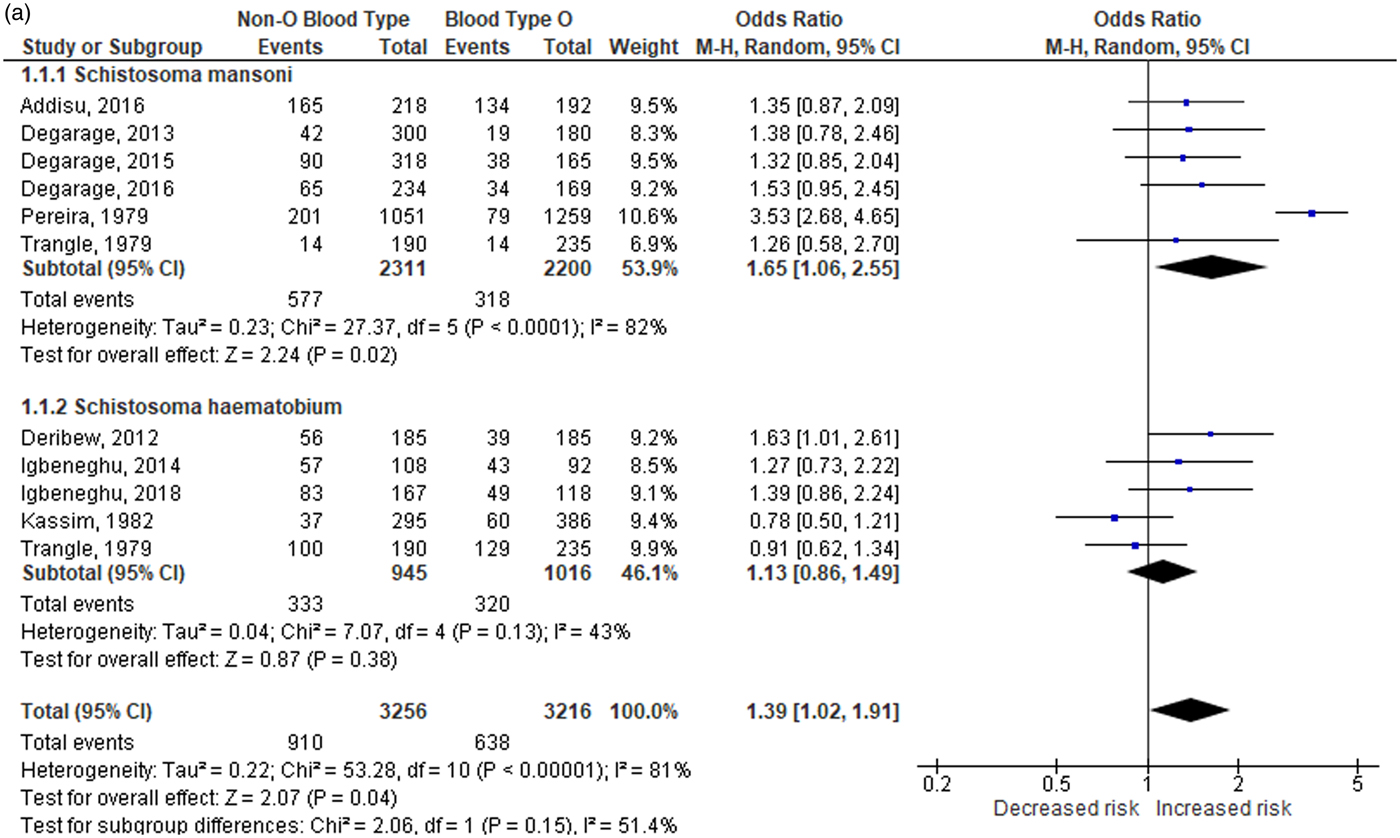
Fig. 2a. Overall and subgroup analysis of the association of non-O blood type with schistosomiasis. CI: confidence interval; df: degrees of freedom.

Fig. 2b. Overall and subgroup analysis of the association of blood type O with schistosomiasis. CI: confidence interval; df: degrees of freedom.
Outlier analysis outcomes
Significant heterogeneity was discovered among the two comparisons (I 2 = 81–82%, PH < 0.001), which warranted an investigation into its cause. The source of inconsistency was determined using the Galbraith plot (fig. 3), which identified three outlier studies. A significant loss in the level of heterogeneity was observed when the studies of Pereira et al. (Reference Pereira1979), Kassim and Ejezie (Reference Kassim and Ejezie1982), and one of the data from the study of Trangle et al. (Reference Trangle1979) were omitted from the overall analysis. The reduction in the I 2 value to 0% (homogeneity) in the overall analysis after their omission indicates that these studies are responsible for the heterogeneity. According to the fixed-effects model (fig. 4a, b), the resulting pooled ORs after outlier analysis still indicate that individuals with non-O blood type are more susceptible to schistosomiasis (OR: 1.40; 95% CI: 1.17–1.67; PA < 0.001) than those who are type O (OR: 0.71; 95% CI: 0.60–0.85; PA < 0.001). Outcomes from this comparison were found to be robust, indicating the stability of our findings (data not shown).

Fig. 3. Identification of outlier studies using the Galbraith plot.
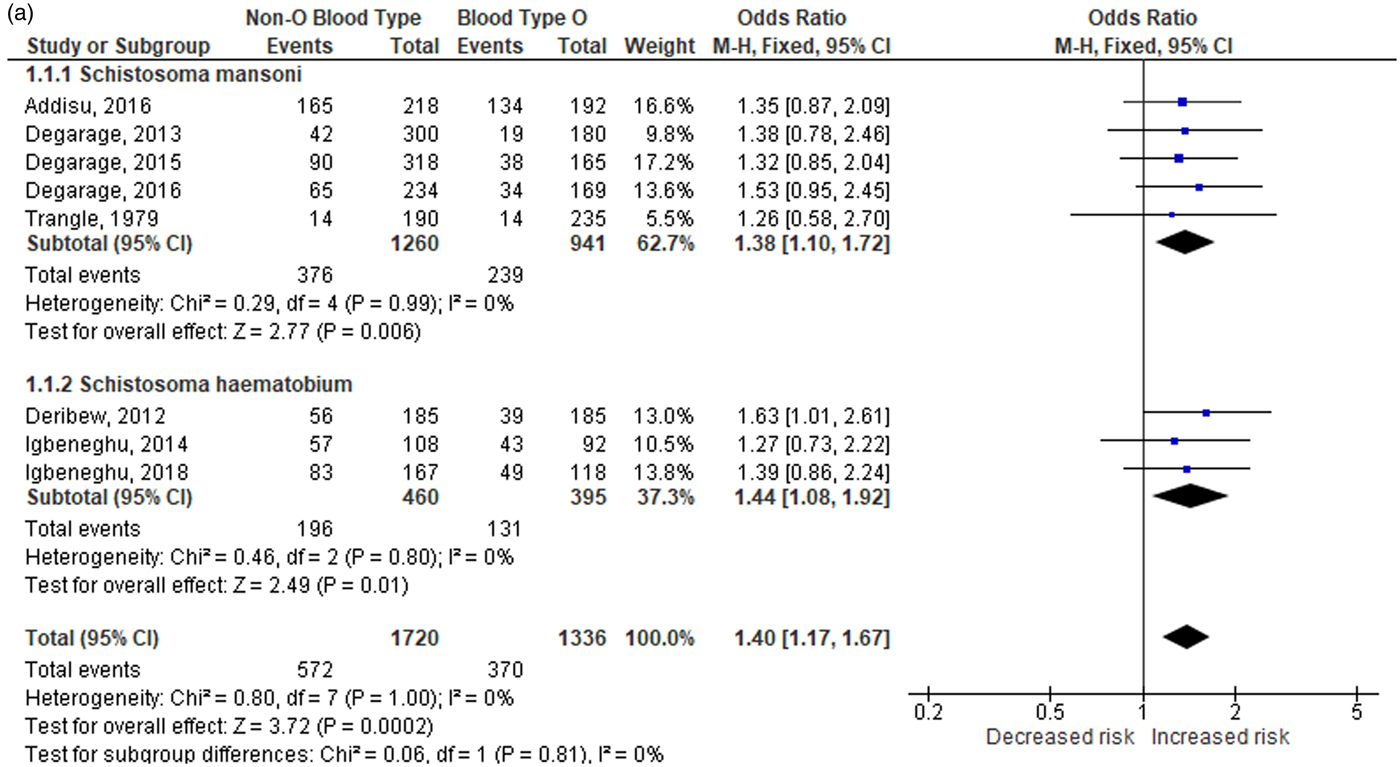
Fig. 4a. Overall and subgroup analysis of the association of non-O blood type with schistosomiasis without the outlier. CI: confidence interval; df: degrees of freedom.

Fig. 4b. Overall and subgroup analysis of the association of blood type O with schistosomiasis without the outlier. CI: confidence interval; df: degrees of freedom.
Post-outlier subgroup analysis
Subgroup analysis was also performed by stratifying the studies based on the species of Schistosoma (S. mansoni and S. haematobium) and ethnicity (sub-Saharan African) of the participants. Based on the results of the post-outlier outcomes (table 2a), stratification showed the odds of acquiring schistosomiasis are high (OR: 1.38–1.44; CI: 1.08–1.92; PA < 0.01) for individuals with non-O blood type, and low for those with blood type O (OR: 0.69–0.73; CI: 0.55–0.93; PA < 0.01) regardless of the species of schistosome infecting the individual or the participant’s ethnicity.
Table 2a. Overall and subgroup analysis of the association of blood type O with schistosomiasis.

N: number of studies included; OR: odds ratio; CI: confidence interval; AM: analysis model; R: random-effects; F: fixed-effects
*P-value significant at < 0.05
**P-value significant at < 0.10
Table 2b. Overall and subgroup analysis of the association of blood types A, B and AB with schistosomiasis.

N: number of studies included; OR: odds ratio; CI: confidence interval; AM: analysis model; R: random-effects; F: fixed-effects
*P-value significant at < 0.05
**P-value significant at < 0.10
Post-outlier analysis for the association of other ABO blood types with schistosomiasis
A total of eight studies were included in the post-outlier analysis for this comparison (table 2b). The fixed-effects model showed a significant association between blood type A and schistosomiasis susceptibility, based on the resulting pooled ORs (OR: 1.32; 95% CI: 1.07–1.63; PA < 0.01). For the subgroup analysis, significant findings were observed for those infected with S. haematobium rather than those infected with S. mansoni. Subgroup analysis by sub-Saharan African ethnicity also yielded significant associations (OR: 1.32; 95% CI: 1.07–1.63; PA < 0.01). These findings suggest that individuals with blood type A are more susceptible to schistosomiasis than those with blood type O.
The resulting pooled ORs using the fixed-effects model also showed significant associations between blood type B and schistosomiasis susceptibility (OR: 1.52; 95% CI: 1.20–1.89; PA < 0.001). Subgroup analysis, on the other hand, showed significant findings for the S. mansoni comparison (OR: 1.57; 95% CI: 1.19–2.06; PA < 0.01) than the S. haematobium comparison. Subgrouping by sub-Saharan African ethnicity also yielded significant results (OR: 1.51; 95% CI: 1.20–1.89; PA < 0.001). These findings indicate that increased susceptibility to schistosomiasis is also observed for those who are blood type B.
On the other hand, no significant associations were observed for individuals with blood type AB when compared to individuals with blood type O.
Discussion
Summary and interpretation of findings
The present meta-analysis summarizes the result of eight studies, involving 3056 participants, although 10 studies were included in the systematic review. By pooling all the ORs and 95% CIs from the individual articles, we showed that ABO blood group antigens are associated with increased susceptibility to schistosomiasis. Overall, increased susceptibility to the disease is observed among those who have ABO antigens on their red cell surface (blood types A and B) (OR: 1.40; 95% CI: 1.17–1.67; PA < 0.001) compared to those individuals who do not have red cell surface antigens (blood type O) (OR: 0.71; 95% CI: 0.60–0.85; PA < 0.001). Subgroup analysis indicated homogeneity (I 2 = 0%, PH = 0.81) across all the strata, indicating that the association is the same regardless of the species of Schistosoma infecting the individual and the participant's ethnicity. Significant associations (PA < 0.01) were also noted for both comparisons.
The significant findings observed provide strong evidence of the possible role of ABO blood group antigens in the pathophysiology of schistosomiasis. This is supported by the homogeneity of the post-outlier results indicating combinability of the individual studies. Moreover, a high degree of significance, the consistent precision of effects, and the robustness of the post-outlier outcomes enhance the level of evidence presented in this meta-analysis.
Comparison with other studies
The findings of this meta-analysis are similar to the results of previous studies (Degarege et al., Reference Degarege, Hailemeskel and Erko2015, Reference Degarege2017; Addisu and Tekeste, Reference Addisu and Tekeste2016). According to Degarege et al., the odds of being infected with S. mansoni are higher among school-aged children with non-O blood type compared to those with blood type O. Also, significant associations were observed between non-O blood type individuals and egg intensity when compared to children with blood type O (Degarege et al., Reference Degarege2017). In another study by Degarege et al. they noted that severity of S. mansoni infection was significantly higher among individuals with blood type A than those with blood type O. Also, the odds of S. mansoni infection were comparable to those with blood types B and AB (Degarege et al., Reference Degarege, Hailemeskel and Erko2015), which is similar to the findings of another study (Trangle et al., Reference Trangle1979). In terms of the degree of infection, Addisu and Tekeste showed that lighter schistosomiasis infections are more likely to be seen among those with blood type O than those with non-O blood type. Interestingly, those with a moderate to high degree of schistosomiasis were about twice as likely to have non-O blood type (Addisu and Tekeste, Reference Addisu and Tekeste2016). Results are still variable regarding which specific blood type is more associated with schistosomiasis. Two of the studies (Degarege et al., Reference Degarege, Hailemeskel and Erko2015; Addisu and Tekeste, Reference Addisu and Tekeste2016) showed that schistosomiasis is more associated with individuals with blood type A than those with blood type B, whereas in another study (Degarege et al., Reference Degarege2017) the opposite was observed, which is consistent with our findings.
Association of human blood groups with infection and host susceptibility
The relationship of different blood groups with pathologic processes in the human body has been studied for a long time. Several studies have noted the association of different blood groups with susceptibility to various conditions, such as cardiovascular disease (Dentali et al., Reference Dentali2012; Franchini and Mannucci, Reference Franchini and Mannucci2014) and even cancer (Franchini et al., Reference Franchini2012; Liumbruno and Franchini, Reference Liumbruno and Franchini2014). Aside from that, the association of different blood groups with infectious disease has also been noted. Blood group antigens may play an essential role in various infectious processes by serving as receptors and co-receptors for different microorganisms and parasites (Cooling, Reference Cooling2015). These blood group antigens also facilitate various invasion mechanisms, such as intracellular uptake of pathogens (Amano and Oshima, Reference Amano and Oshima1999; Marionneau et al., Reference Marionneau2002), signal transduction (Gupta and Surolia, Reference Gupta and Surolia2010; Pontier and Schweisguth, Reference Pontier and Schweisguth2012; Nakayama et al., Reference Nakayama2013), and adhesion to different cells (Bensing et al., Reference Bensing, Lopez and Sullam2004; Yang et al., Reference Yang2014). Aside from those mentioned, blood group antigens can also modify the innate immune system response to the invading microorganism, as seen in the case of Schistosoma (Sell and Dean, Reference Sell and Dean1972; Goldring et al., Reference Goldring1976).
Acquisition of human blood group antigens by Schistosoma
Years before this meta-analysis was conducted, studies investigated the possible acquisition of human blood group antigens by Schistosoma (Clegg et al., Reference Clegg, Smithers and Terry1971; Dean, Reference Dean1974; Goldring et al., Reference Goldring1976). The first investigation started in 1971, wherein after being cultured in human blood of various specificities, schistosomes showed specificity for anti-A or anti-B upon testing. This preliminary experiment suggested that the schistosomula acquired human blood group antigens during culture (Clegg et al., Reference Clegg, Smithers and Terry1971). Similarly, both the studies of Dean and Goldring et al. demonstrated that the schistosomula of S. mansoni acquired ABO blood group antigens A and B from the host after being cultivated in human blood (Dean, Reference Dean1974; Goldring et al., Reference Goldring1976). Therefore, these findings led to the hypothesis that the schistosomula can passively adsorb ABO blood group antigens and possibly even other host antigens. The exact mechanism by which ABO blood group antigens attach to the surface of the schistosome is still unknown, but Gardas and Koscielak (Reference Gardas and Koscielak1973) were able to shed some light regarding the matter. In their view, glycolipids in the plasma, such as the A, B and H antigens, have the unique property of combining with cells and conferring upon them appropriate specificity (Gardas and Koscielak, Reference Gardas and Koscielak1973). The attachment of these antigens in the schistosome surface is made possible by the presence of at least two types of O-linked oligosaccharides, which can interact with different glycolipids (Nyame et al., Reference Nyame, Cummings and Damian1987). There is also evidence that the presence of the poly-N-acetyllactosamine-containing oligosaccharides synthesized by the schistosomes contributes to host–pathogen interactions. A good example of this is observed in the interaction of Mycoplasma pneumoniae with human erythrocytes containing poly-N-acetyllactosamine chains of Ii antigen type (Makaaru et al., Reference Makaaru1992).
Immune evasion by Schistosoma
This elusive trait may enable the schistosome to mask some of its antigenic sites, rendering it unrecognized by the host (Sell and Dean, Reference Sell and Dean1972), a highly effective disguise that contributes to the parasite's successful invasion. Moreover, the evasive ability of the schistosome may infer the high survivability of the adult fluke in human blood (Clegg et al., Reference Clegg, Smithers and Terry1971), as seen in some cases where the parasite can live up to 40 years inside its human host (Colley et al., Reference Colley2014). Vaccines against the very early schistosomula and/or stimulation of effector mechanisms, such as macrophages, which does not require recognition or interaction of the parasite surface antigens, may be an effective counter treatment for schistosomiasis, considering its evasive capability (Pearce and Sher, Reference Pearce and Sher1987). Therefore, it is vital to understand the nature of host antigen acquisition and the evasive mechanism of schistosomes in progress towards the development of the necessary vaccine and/or treatment in combatting this clinically important parasite.
Limitations of the study
Overall findings of this meta-analysis suggest the possible association of ABO blood group antigens with schistosomiasis susceptibility. However, interpreting these results warrants awareness of the study's limitations: (1) a wide range of year of publication and patient sample sizes across the individual studies; (2) only two species of Schistosoma (S. mansoni and S. haematobium) were emphasized; (3) lack of representation of S. japonicum in the study; (4) lack of representation of other areas where schistosomiasis is endemic; (5) no representation of the different ethnic groups present in the study population; and (6) failure to include other risk factors of schistosomiasis. Given these limitations, the findings of this meta-analysis should be treated with caution when applied clinically.
To our knowledge, this is the first meta-analysis that investigated the association of ABO blood group antigens and schistosomiasis susceptibility. With this approach, we hope we have contributed to a better understanding of the pathophysiology of schistosomiasis and the possible role of the ABO blood group in disease susceptibility, and to establishing new ways of treating and controlling schistosomiasis. Generally, based on the resulting pooled ORs, our findings suggest that individuals with blood type B or A are more likely to be infected with schistosomiasis than those with blood type O. Further studies regarding the interaction of Schistosoma spp. with the different ABO and other host antigens may help better understand their role in the pathophysiology of schistosomiasis and to develop a treatment against the disease.
Conflict of interest
None.
Author ORCIDs
R.E. Tiongco 0000-0002-7793-6558











