Introduction
The end-Permian mass extinction event (~251.9 Ma; Burgess et al., Reference Burgess, Bowring and Shen2014) was the most severe biotic crisis of the Phanerozoic, marking a significant change within marine and continental biocoenoses (e.g., Raup, Reference Raup1979; Sepkoski, Reference Sepkoski1984). In the aftermath of this catastrophic event, the surviving and newly evolved clades went through a series of subsequent crises during the Early Triassic, which selectively affected their recovery (e.g., Galfetti et al., Reference Galfetti, Hochuli, Brayard, Bucher, Weissert and Vigran2007b; Orchard, Reference Orchard2007; Brayard et al., Reference Brayard, Escarguel, Bucher, Monnet, Brühwiler, Goudemand, Galfetti and Guex2009, Reference Brayard, Vennin, Olivier, Bylund, Jenks, Stephen, Bucher, Hofmann, Goudemand and Escarguel2011, Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017; Song et al., Reference Song, Wignall, Chen, Tong, Bond, Lai, Zhao, Jiang, Yan, Niu, Chen, Yang and Wang2011; Hofmann et al., Reference Hofmann, Hautmann and Bucher2013a, Reference Hofmann, Hautmann, Wasmer and Bucherb; Hochuli et al., Reference Hochuli, Sanson-Barrera, Schneebeli-Hermann and Bucher2016).
Bony fishes (Osteichthyes) displayed relatively lower diversity during the Paleozoic, but radiated extensively after the end-Permian mass extinction event (e.g., Tintori et al., Reference Tintori, Hitij, Jiang, Lombardo and Sun2014a; Friedman, Reference Friedman2015; Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a). The Triassic fossil record bears testimony to the first diversification event of the Neopterygii, the group to which over half of all living vertebrate species belongs. Whereas most neopterygians were small during the Triassic, the ‘Palaeopterygii’ (non-neopterygian actinopterygians) were predominantly represented by large species, suggesting that this group was important at higher trophic levels (Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a).
Despite recent advances, the detailed pattern and tempo of recovery of the various clades of fishes remain unsettled because Early Triassic taxa are not as well studied as Middle Triassic ones (Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014; Tintori et al., Reference Tintori, Hitij, Jiang, Lombardo and Sun2014a; Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a, Reference Romano, Ware, Brühwiler, Bucher and Brinkmannb). Although Early Triassic fish assemblages have been described from numerous localities around the world (Brinkmann et al., Reference Brinkmann, Romano, Bucher, Ware and Jenks2010), some large paleogeographical domains and time intervals still suffer from a scanty record (e.g., López-Arbarello, Reference López-Arbarello2004; Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a, Reference Romano, Ware, Brühwiler, Bucher and Brinkmannb). For example, little is known about low-latitude Early Triassic vertebrate faunas (e.g., Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014; Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a)—a circumstance that led Sun et al. (Reference Sun, Joachimski, Wignall, Yan, Chen, Jiang, Wang and Lai2012) to speculate about an ‘equatorial vertebrate eclipse’, which they linked to extreme temperatures during the Smithian. Furthermore, the fossil record of Osteichthyes is marked by an extended interval with only a few, scattered occurrences during the Spathian (late Early Triassic). This Spathian gap in the osteichthyan fossil record potentially overlaps with the onset of the first neopterygian radiation (Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a).
Here we describe for the first time articulated fish remains from the Early Triassic of Nevada (USA)—a paleogeographic domain situated near the paleoequator. The presented material notably improves the Early Triassic record of fishes from the United States and provides new information on low-latitude vertebrate faunas during the essentially warm Early Triassic (Goudemand et al., Reference Goudemand, Romano, Brayard, Hochuli and Bucher2013; Romano et al., Reference Romano, Goudemand, Vennemann, Ware, Schneebeli-Hermann, Hochuli, Brühwiler, Brinkmann and Bucher2013). One of the presented fossils is derived from strata of Spathian age, thus adding a new occurrence to this interval.
Localities, ages, and depositional settings
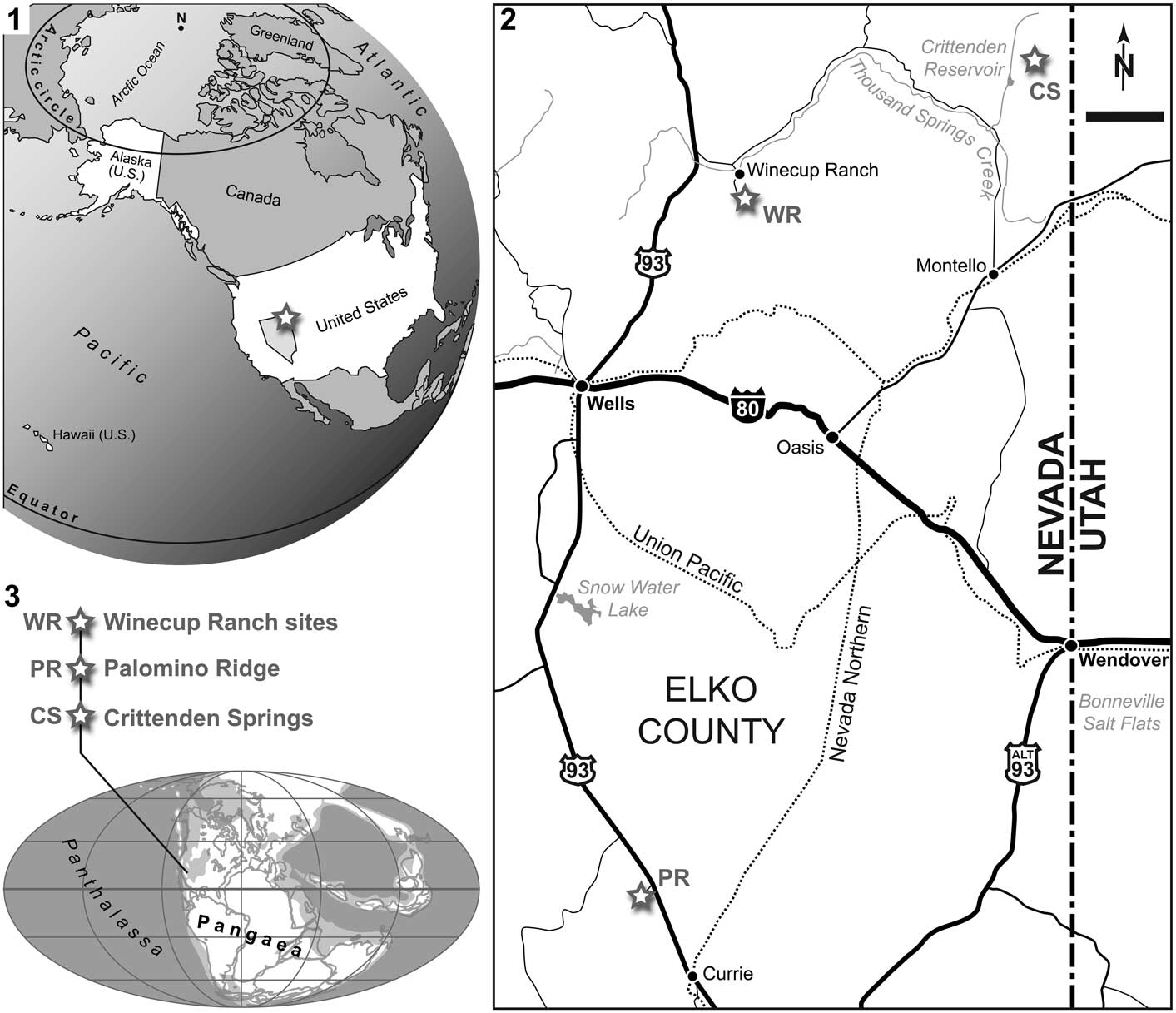
The fossils described below were collected from three distinct localities in Elko County, northeastern Nevada, USA: (1) Winecup Ranch, (2) Palomino Ridge, and (3) Crittenden Springs.
Winecup Ranch
Two specimens (NMMNH P-66225, NMMNH P-77117) were found near the Winecup Ranch (Elko County, Nevada, USA; Fig. 1). The Winecup Ranch (e.g., Oversby, Reference Oversby1972), or Wilkins Ranch of the older literature (e.g., Clark, Reference Clark1957), is located ~43 km north of Wells, and ~7.2 km southeast of Wilkins Junction, on the south side of Thousand Springs Valley (Fig. 1.2). Lower Triassic exposures, designated as ‘Wilkins Ranch (V)’ in Clark (Reference Clark1957), crop out in two separate, but nearly contiguous, large areas encompassing ~7.7 km² each, and beginning ~1.6 km and ~3.2 km south of the ranch, respectively. These exposures occur in T40N, R64E and 65E and extend southward for ~5.5 km, reaching into the northern end of the Windermere Hills (Clark, Reference Clark1957; Oversby, Reference Oversby1972; Coats, Reference Coats1987).
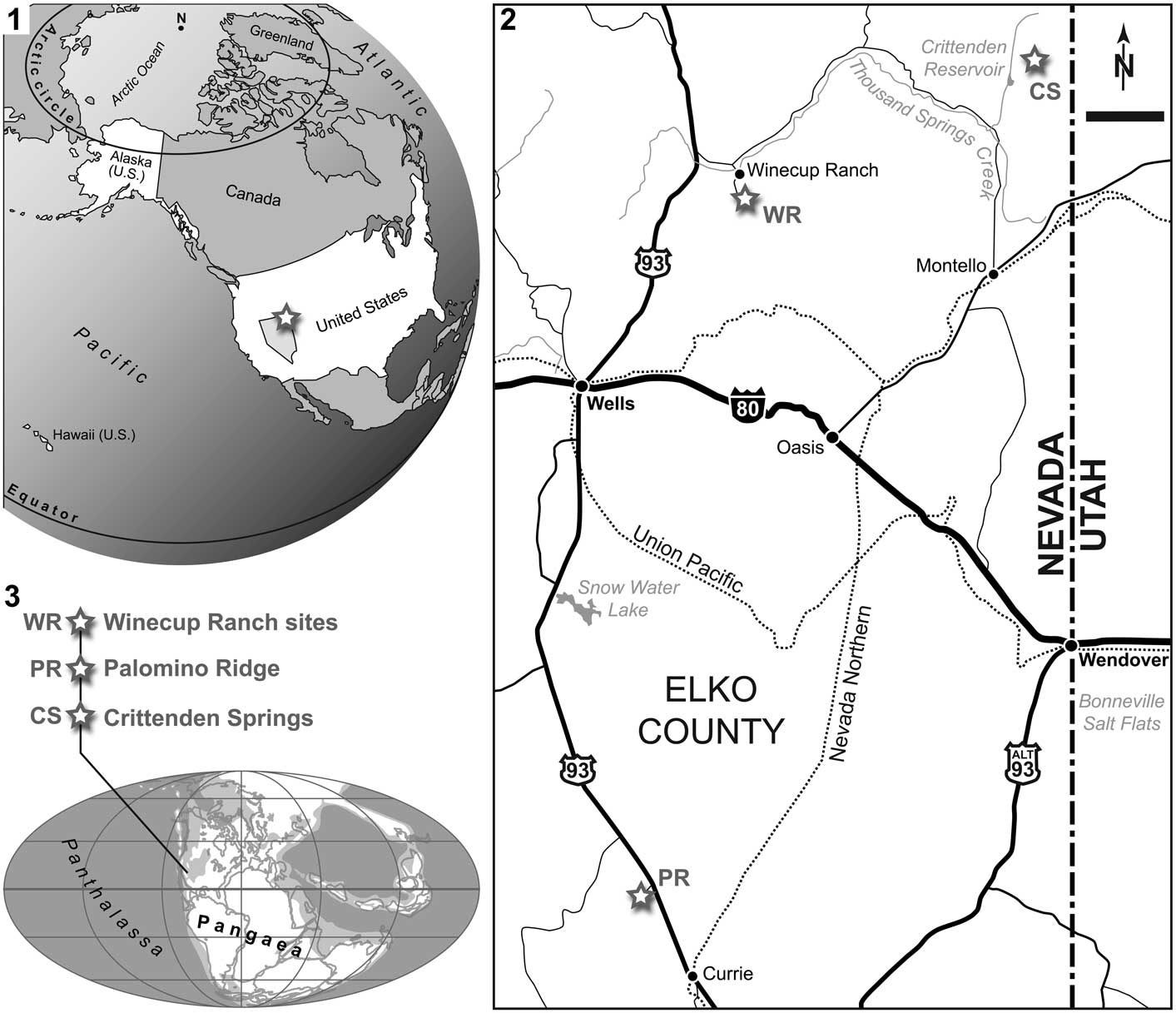
Figure 1 (1) Map of the United States of America (State of Nevada highlighted in bright gray), with indication of the study area, Elko County, in northeast Nevada (white star). (2) Locality map of eastern Elko County, with indication of the three localities where the presented material was found (white stars); scale bar=10 km. (3) Early Triassic world map (modified from PALEOMAP project, www.scotese.com), with approximate location of the study sites.
Compared to most other Lower Triassic exposures in northeastern Nevada, relatively little is known regarding this particular section, especially the portion that represents strata of Smithian age. According to Clark (Reference Clark1957), the section, consisting of vertical and overturned beds in isolated outcrops, is highly faulted, with outcrops abutting against other outcrops with differing attitudes, making detailed logging impossible. Contact with Permian rocks is not visible and alluvial material covers much of the upper beds (Clark, Reference Clark1957). Approximately 460 m of probable Spathian sediments, consisting of massive gray limestone and gray shaly limestone yielding bivalves, brachiopods, and ammonoids crop out in the higher hills ~6 km south of the ranch (Clark, Reference Clark1957).
A succession of Smithian, dark-brown limestone overlain by 60–90 m of black shaly limestone is repeated several times on the east side of an unimproved road ~3 km south of the ranch (Clark, Reference Clark1957). These rocks are relatively incompetent and, consequently, the topography in this particular area consists of sagebrush flats and a few, very low rolling hills. Outcrops are few—weathered shale covers most of the area and small pieces of dark-brown limestone occasionally found lying on the surface usually indicate the position of subsurface beds. A few well-preserved middle Smithian ammonoids (e.g., Inyoites oweni) belonging to the ‘Meekoceras zone’ of Clark (Reference Clark1957) have been found by JJ and HB in these limestone beds of the Thaynes Group at two sites located 340 m apart. Oversby (Reference Oversby1972) mentioned the occurrence of bones (large vertebrae and fish remains) in the study area, but neither described nor depicted them.
The discovery sites of NMMNH P-66225 (N41°22'58.2'', W114°40'11.9'') and NMMNH P-77117 (N41°22'49.5'', W114°40'24.8'') are located ~2.75 km south-southeast of the Winecup Ranch. P-77117 was found at the most prolific ammonoid locality together with Pseudaspidites, Procurvoceratites, proptychitid indet., and prionitid indet., which, combined with I. oweni, indicate a late early Smithian to early late Smithian age (Brayard and Bucher Reference Brayard and Bucher2008; Brühwiler et al., Reference Brühwiler, Bucher, Brayard and Goudemand2010; Brayard et al., Reference Brayard, Bylund, Jenks, Stephen, Olivier, Escarguel, Fara and Vennin2013). P-66225 was found more or less on strike ~300 m northeast of the P-77117 locality. Conodonts or ammonoids have not been detected in the matrix surrounding P-66225, but a similar age as P-77117 is likely. Fish remains and ammonoids sampled in this area all occur in organic-rich, unbioturbated, alternating thin-bedded limestone and shale, indicative of anoxic bottom waters.
Palomino Ridge
Specimen PIMUZ A/I 4397 was found on the eastern slopes of Palomino Ridge (Elko County, northeast Nevada; Fig. 1.2). Palomino Ridge, a ~5 km long and ~2.5 km wide, north-northwest to south-southeast trending structure, is located ~15 km northwest of Currie (southern Elko County) and ~2.5 km west of US Route 93, in T29N, R63E.
The backbone of Palomino Ridge is formed by steeply dipping, highly competent, medium- to thick-bedded limestone of the Permian Gerster Formation, which strikes more or less parallel to the ridge. The base of the Lower Triassic Thaynes Group crops out intermittently on the lower eastern slopes of Palomino Ridge. The Thaynes Group is also exposed in other, nearby localities north of Currie (Lucas and Orchard, Reference Lucas and Orchard2007). At Palomino Ridge, Smithian strata directly overly a 4 m thick chert conglomerate in sharp unconformable contact with the underlying Gerster Formation. The Smithian succession consists of a thick series of shale, interbedded shale and thin-bedded limestone, and thicker limestone beds. Most of these beds are fossiliferous and, consequently, the section represents a much-expanded Smithian ammonoid succession (Jattiot et al., in press), similar in scope but slightly less complete than the successions described by Brayard et al. (Reference Brayard, Bylund, Jenks, Stephen, Olivier, Escarguel, Fara and Vennin2013) from the Confusion and Pahvant ranges of western Utah.
Referring to Jattiot et al. (in press), the oldest ammonoid-bearing beds at Palomino Ridge are of late early Smithian age. The next overlying strata contain middle Smithian ammonoids, including the Inyoites oweni fauna of late middle Smithian age. Above the Inyoites beds are several, 5–10 cm thick limestone beds, which yield an early late Smithian cosmopolitan ammonoid assemblage characterized by Anasibirites (Jattiot et al., Reference Jattiot, Bucher, Brayard, Monnet, Jenks and Hautmann2015). Ammonoids are less abundant in the next overlying beds that yield Xenoceltites subevolutus and Pseudosageceras augustum. PIMUZ A/I 4397 was found in situ in these latest Smithian P. augustum beds (layer PLR 35, Unitary Association Zone 6 of Jattiot et al., in press) in Section 2 (N40°23'00.0'', W114°50'09.9''; Fig. 2) of Jattiot et al. (in press). The P. augustum beds are typically represented by black shales with minor, very thin-bedded (cm), dark limestone. Here again, local anoxic bottom waters favored a good preservation of the fish fossil.
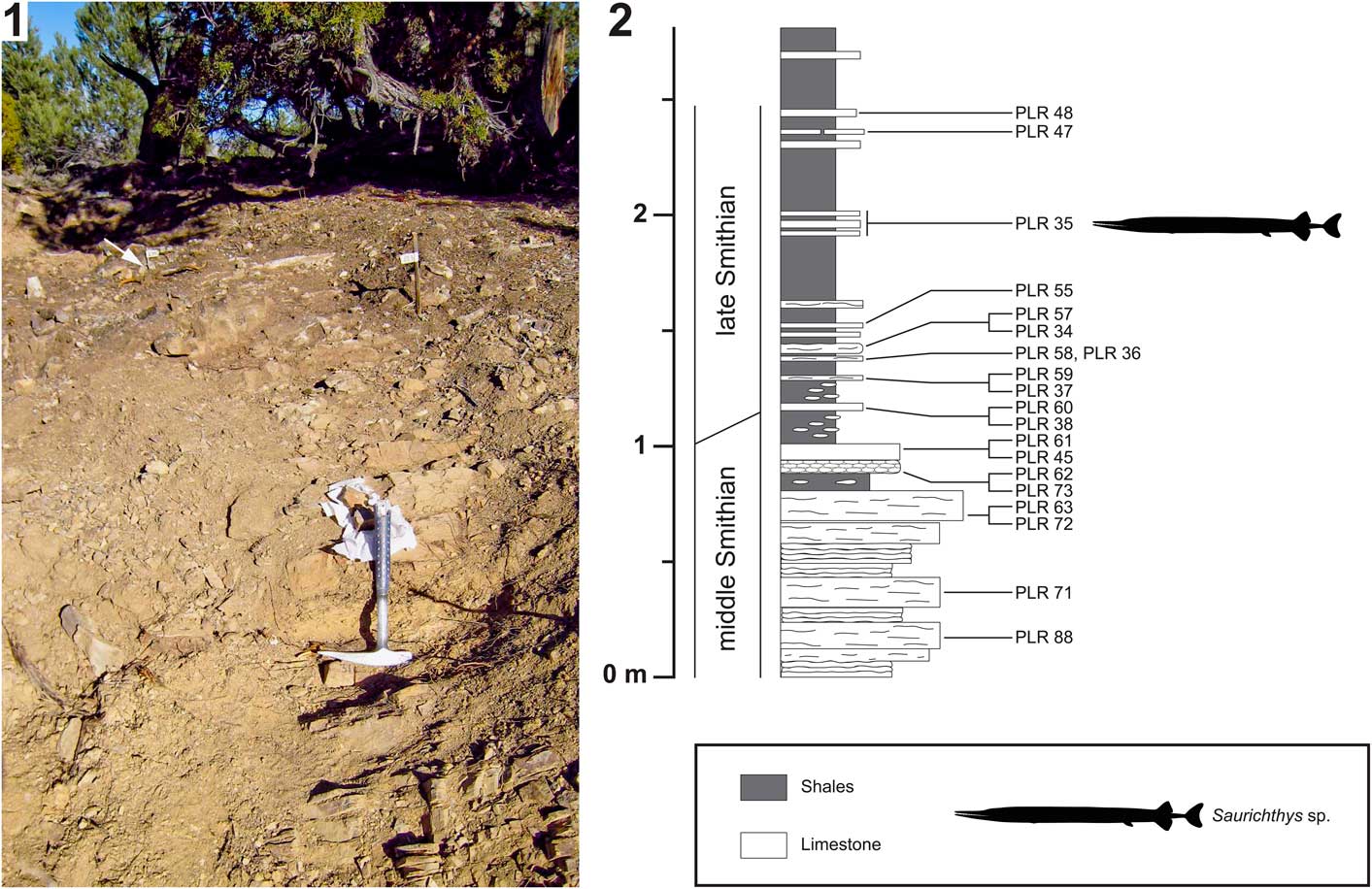
Figure 2 Palomino Ridge locality, Elko County, Nevada, USA. (1) Photo of Section 2 of Jattiot et al. (in press); (2) stratigraphic log of Section 2 (modified from Jattiot et al., in press), with indication of bed PLR 35, in which PIMUZ A/I 4397 (Saurichthys sp.) was discovered (indicated by the upper marker flag/white arrow in 2.1). See text for details.
Crittenden Springs
Specimen NMMNH P-77357 was found at Crittenden Springs (northeast Elko County, Nevada). The classical ammonoid collecting site referred to as Crittenden Springs (e.g., Kummel and Steele, Reference Kummel and Steele1962; Jenks, Reference Jenks2007; Jenks et al., Reference Jenks, Brayard, Brühwiler and Bucher2010) is located on the north side of Long Canyon (township/range coordinates: W½SW¼ Sec 3, T42N, R69E) ~29 km north of Montello, Elko County (Fig. 1.2). Thousands of exceptionally well-preserved Smithian ammonoids, many of which retain relict color bands, and other fossils have been collected from the site since it was discovered in the early 1950s (Mullen, Reference Mullen1985; Jenks et al., Reference Jenks, Brayard, Brühwiler and Bucher2010). Disarticulated vertebrate bones are occasionally found in association with the ammonoids of the ‘Meekoceras beds’ (personal observation, JJ, KGB, TMS, HB, 2010–2016).
Lower Triassic marine sediments, consisting of the Dinwoody Formation and units belonging to the overlying Thaynes Group, crop out in the hills immediately north of the Long Canyon road and extend to the northeast for ~8 km, covering an area of ~33 km2 (Clark, Reference Clark1957; Mullen, Reference Mullen1985). Good exposures of Spathian shales are limited to a few seasonal storm drainage channels. One such channel cuts numerous, gently northward dipping layers with early diagenetic limestone nodules, most of which are barren, but a few yield ammonoids (e.g., Stacheites) typical of the early late Spathian Fengshanites/Prohungarites fauna (Bucher, Reference Bucher1989; Guex et al., Reference Guex, Hungerbühler, Jenks, O’Dogherty, Atudorei, Taylor, Bucher and Bartolini2010). A float concretion found in the same channel contains a partial skeleton of an actinopterygian (NMMNH P-77357), which is described herein. Additional, articulated cranial material (PIMUZ A/I 4641; Birgeria?) has recently been recovered from the Spathian of Crittenden Springs and is currently undergoing preparation for eventual study.
Materials and methods
The described material is curated in part by the NMMNH, and in part by the PIMUZ. PIMUZ A/I 4397 from Palomino Ridge and one specimen from the Winecup Ranch (NMMNH P-66225) were prepared using air pens under a binocular microscope. The fragile bones were consolidated with liquid glue (low-viscosity cyanoacrylate) during preparation.
For consistency, the anatomical terminology used herein follows that of previous publications (e.g., Stensiö, Reference Stensiö1921, Reference Stensiö1925, Reference Stensiö1932; Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952; Ørvig, Reference Ørvig1978; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Romano and Brinkmann, Reference Romano and Brinkmann2009). However, no homology with similarly named bones of other vertebrates is necessarily implied (cf. Schultze, Reference Schultze2008). When describing the scales, we refer to length as their longitudinal (anteroposterior) extent and depth as their dorsoventral dimension. Open nomenclature is used in accordance with the recommendations of Bengtson (Reference Bengtson1988).
The following comparative material was consulted: ZMUC VP 3176 (Birgeria groenlandica, holotype; Stensiö, Reference Stensiö1932); MNHN.F MAE 605, MAE 606 (B. nielseni; Beltan, Reference Beltan1980); PMU P 1421 (B. aldingeri, holotype; Stensiö, Reference Stensiö1932; Schwarz, Reference Schwarz1970); PMU P 349, P 1422 (B. cf. aldingeri; Stensiö, Reference Stensiö1932); PIMUZ A/I 4301 (Birgeria sp.; Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014); PIMUZ material of B. stensioei (as listed in Romano and Brinkmann, Reference Romano and Brinkmann2009); PIMUZ A/I 3900 (Saurichthys cf. elongatus; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012); and PIMUZ A/I 4135, A/I 4144 (S. madagascariensis; Kogan and Romano, Reference Kogan and Romano2016a). Additionally, a photograph of MPUM 9334 (Birgeria sp.; Lombardo and Tintori, Reference Lombardo and Tintori2005, fig. 1b) was obtained for this study.
We employ Tozer’s (Reference Tozer1965) stage subdivisions of the Early Triassic: Griesbachian (early Induan), Dienerian (late Induan), Smithian (early Olenekian), and Spathian (late Olenekian). These stages are well defined by global biotic events (e.g., Galfetti et al., Reference Galfetti, Hochuli, Brayard, Bucher, Weissert and Vigran2007b; Hochuli et al., Reference Hochuli, Sanson-Barrera, Schneebeli-Hermann and Bucher2016). Recent biochronostratigraphic datings indicate a duration of maximally 1.4 ± 0.4 myr (million years) for the Griesbachian–Dienerian interval, ~0.7 ± 0.6 myr for the Smithian, and ~3 myr for the Spathian (Ovtcharova et al., Reference Ovtcharova, Bucher, Schaltegger, Galfetti, Brayard and Guex2006; Galfetti et al., Reference Galfetti, Bucher, Ovtcharova, Schaltegger, Brayard, Brühwiler, Goudemand, Weissert, Hochuli, Cordey and Guodun2007a).
Repositories and institutional abbreviations
Types, figured, and other specimens examined in this study are stored in the following institutions: New Mexico Museum of Natural History and Science (NMMNH), Albuquerque, New Mexico, USA; Paleontological Institute and Museum, University of Zurich (PIMUZ), Zurich, Switzerland; Museum National d’Histoire Naturelle (MNHN.F), Paris, France; Museo del Dipartimento di Scienze della Terra ‘A. Desio’ dell’Università degli Studi di Milano (MPUM), Milan, Italy; Museum of Evolution (PMU, formerly Paleontological Museum), Uppsala University, Uppsala, Sweden; Zoological Museum, Natural History Museum of Denmark (ZMUC), Copenhagen, Denmark.
Systematic paleontology
Anatomical abbreviations
Af, anal fin (lepidotrichia); An, angular (external plate); Aop, antoperculum; Ar, Meckel’s cartilage (ossified as the articular); Br, branchiostegal ray; Cb, ceratobranchial; Cf, caudal fin (lepidotrichia); Cl, cleithrum; De, dentary (external plate); Df, dorsal fin (lepidotrichia); Dh, dermohyal; Dmp, ‘dermometapterygoid’; Dp, dermopterotic; Dpl, dermopalatine; Ds, dermosphenotic; Eb, epibranchial; Ect, ectopterygoid; Ent, entopterygoid; eth.ca., ethmoidal canal; ex.na., external narial openings; F, frontal; Ff, fringing fulcrum; Hm, hyomandibula; int.lam.an., internal lamina of the angular; int.lam.de., internal lamina of the dentary; int.lam.mx., internal lamina of the maxilla; io.ca., infraorbital sensory canal; L, lachrymal; lc.dh., longitudinal crest on the dermohyal; Mx, maxilla (external plate); md.ca., mandibular sensory canal; Nao, nasaloantorbital; Op, operculum; P, parietal; pBf, paired basal fulcrum; p.ca., postotic section of the lateral line sensory canal; Par, prearticular; Pop, preoperculum; pop.ca., preopercular sensory canal; Q, quadratum portion of the palatoqudratum; Rpm, rostro-premaxilla; Sa, surangular; Sc, scale; Scu, scute; so.ca., supraorbital sensory canal; Sob, suborbital; Sop, suboperculum; uBf, unpaired basal fulcrum.
Class Osteichthyes Huxley, Reference Huxley1880
Subclass Actinopterygii Cope, Reference Cope1887, emend. Rosen et al., Reference Rosen, Forey, Gardiner and Patterson1981
Family Birgeriidae Aldinger, Reference Aldinger1937, emend. Nielsen, Reference Nielsen1949
Remarks
The family includes only Birgeria Stensiö, Reference Stensiö1919. Two other genera formerly referred to Birgeriidae, the Early Jurassic Ohmdenia Hauff, Reference Hauff1953 and the Early Cretaceous Psilichthys Hall, Reference Hall1900, have been shown to be unrelated (Waldman, Reference Waldman1971; Friedman, Reference Friedman2012).
Genus Birgeria Stensiö, Reference Stensiö1919, emend. Romano and Brinkmann, Reference Romano and Brinkmann2009
Type species
Birgeria mougeoti (Agassiz, 1843) from the Middle Triassic of Bayreuth, Germany.
Remarks
Stensiö (Reference Stensiö1919) erected the genus based on a maxilla from the Middle Triassic of Germany that contains teeth resembling those of the type material of ‘Saurichthys’ mougeoti Agassiz, 1843, which henceforth became the type species of Birgeria. The type material from the Muschelkalk of France and Germany is composed of several isolated teeth and dentigerous maxilla fragments, which clearly cannot be attributed to Saurichthys Agassiz, Reference Agassiz1834, because in this taxon the maxilla lacks such large teeth (e.g., Stensiö, Reference Stensiö1925; Rieppel, Reference Rieppel1985; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Maxwell et al., Reference Maxwell, Romano, Wu and Furrer2015; Kogan and Romano, Reference Kogan and Romano2016a). The material of Agassiz (1833–Reference Agassiz1843) and Stensiö (Reference Stensiö1919) is too fragmentary for identification at the species level, but bears close resemblance to material of other, much better known species referred to Birgeria. Additional, more complete material from the Early Triassic of Spitsbergen (Svalbard, Arctic Norway), supposedly belonging to B. mougeoti (Agassiz, 1843), was figured and described by Stensiö (Reference Stensiö1919, Reference Stensiö1921, Reference Stensiö1932). One specimen of Stensiö’s (Reference Stensiö1932) material, however, was later considered as a separate species, B. aldingeri Schwarz, Reference Schwarz1970. Although Schwarz (Reference Schwarz1970) did not explicitly comment on the taxonomic status of other material from Spitsbergen (Stensiö, Reference Stensiö1919, Reference Stensiö1921, Reference Stensiö1932), we agree that it is likely not conspecific with that from the Middle Triassic Germanic Basin, due to notable differences in the angle of the anterior margin of the postorbital maxillary blade (see Discussion). Pending a proper revision of the type species and the species from Spitsbergen, we follow previous authors and treat B. mougeoti from the Muschelkalk Sea as a valid species, whereas Stensiö’s (Reference Stensiö1919, Reference Stensiö1921, Reference Stensiö1932) material from Spitsbergen is herein provisionally referred to as B. cf. aldingeri (except for the holotype of B. aldingeri).
Occurrence
Marine Triassic, global: from the Griesbachian (earliest Triassic) of Greenland to the Rhaetian (latest Triassic) of Europe.
Birgeria americana new species

Figure 3 Birgeria americana n. sp. (NMMNH P-66225, holotype) from the Smithian of the Winecup Ranch, Elko County, Nevada, USA. (1) Fossil in dorsal view (above) and in right aspect (below); (2) schematic drawing of 3.1 with interpretations of skeletal features; anterior is right. Scale bar=50 mm (total).
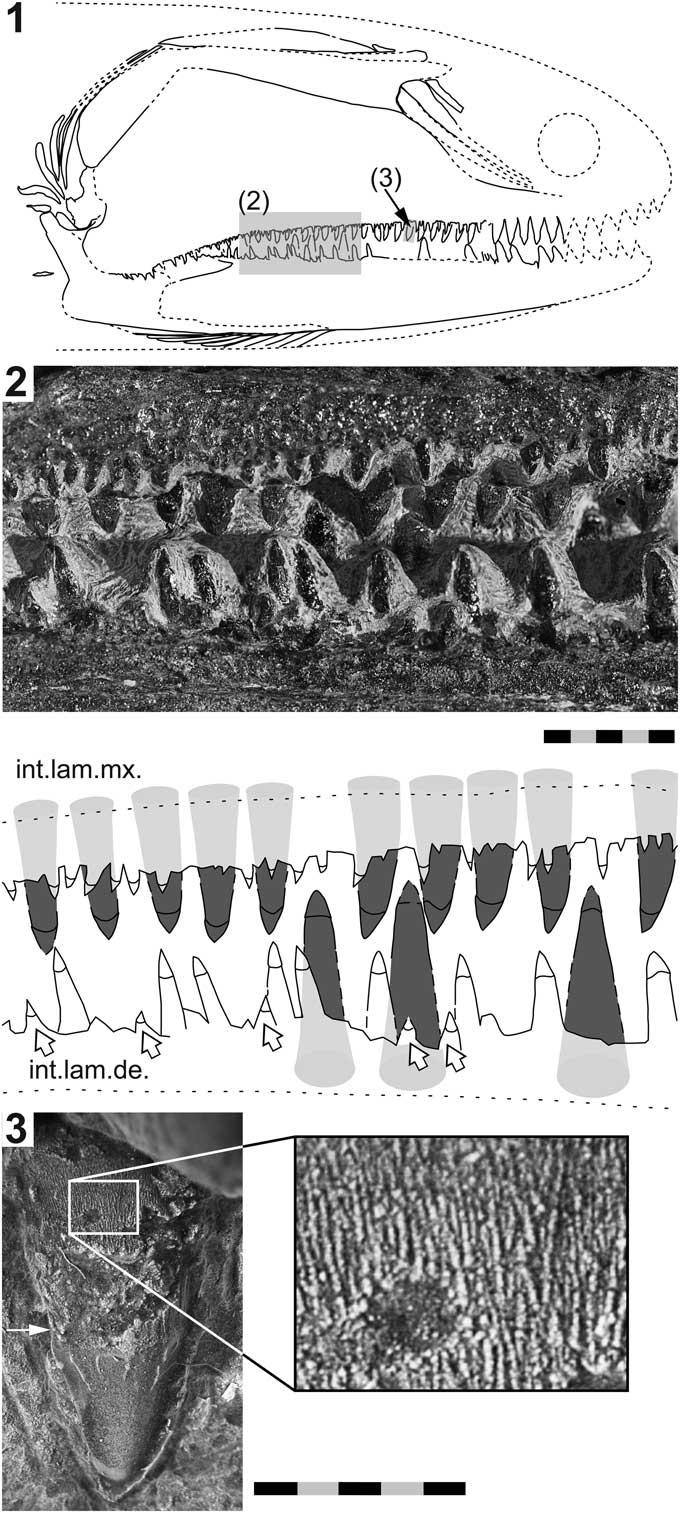
Figure 4 Birgeria americana n. sp. (NMMNH P-66225, holotype) from the Smithian of the Winecup Ranch, Elko County, Nevada, USA. (1) Tentative restoration of the skull of P-66225; (2) close-up view (position indicated in 4.1) of the dentition (above), and drawing thereof (below); the large lingual teeth growing on the internal lamina of the maxilla and the dorsal internal lamina of the dentary, respectively, are highlighted in dark gray; teeth of the labial row (white arrows) and intermediate row are colored white; (3) close-up view of a lingual tooth of the maxilla (position shown in 4.1) illustrating the surface ornamentation of the base and acrodin cap (photographed using a Leica MZ16F camera mounted on a stereomicroscope, contrast enhanced with ammonium chloride); the white arrow in 4.3 points to the demarcation between the tooth base and the acrodin cap; anterior is right (1–3). Scale bars=10 mm (total) (2), or 5 mm (total) (3).
Holotype
NMMNH P-66225 (Figs. 3, 4), from upper lower Smithian to lower upper Smithian beds (Thaynes Group), ~2.75 km south-southeast of the Winecup Ranch, east-central Elko County, Nevada, USA (Fig. 1). P-66225 is a partial skull preserved within a large limestone nodule, with its right side exposed. A digital 3D surface scan of P-66225 is available at MorphoMuseuM (Romano et al., Reference Romano, Jenks, Jattiot, Scheyer, Bylund and Bucher2017).
Differential diagnosis
Very large (>150 cm) birgeriid (larger than B. groenlandica Stensiö, Reference Stensiö1932, B. nielseni Lehman, Reference Lehman1948, B. liui Jin, Reference Jin2001, B. guizhouensis Liu, Yin, and Luo in Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006, and B. acuminata [Agassiz, 1843]); postorbital blade of maxilla elongate, anteriorly low, posteriorly high, with inclined anterior border (relatively shorter, anteriorly about as high as posteriorly, with steep anterior margin in B. mougeoti [Agassiz, 1843], B. stensioei Aldinger, Reference Aldinger1931, and B. acuminata); antoperculum present; suboperculum dorsoventrally elongate (absent or weakly ossified in B. stensioei; dorsoventrally shorter in B. groenlandica and B. nielseni); five to six postmandibular branchiostegal rays (four to five in B. nielseni, three to five in B. groenlandica, maximum one in B. stensioei); teeth of the intermediate row on the maxilla and dentary mostly widely spaced, varying in size, but mostly high (small, equal-sized, close-set intermediate teeth in B. stensioei and B. acuminata); labial teeth distinct (smaller in B. stensioei, absent in B. acuminata).
Description
NMMNH P-66225 (Figs. 3, 4) shows the portion between the cleithrum posteriorly, and the level of the hind margin of the orbital opening anteriorly. The preserved part has a length of 26 cm. Most bones are still in situ. Parts of the maxilla, the preoperculum, the suborbitals, and the lower jaw were damaged due to weathering.
Upper jaw
The maxilla is the dominant bone of the upper jaw (Fig. 3). It consists of a cleaver-shaped external plate (composed of a low suborbital portion and a high, elongate postorbital blade) and an internal lamina. The postorbital blade of the maxillary bone is confined by a slanted, concave anterior margin, a nearly straight dorsal border, a posterior margin that is straight in its upper segment and distinctly sigmoid in its lower part, and a dentigerous ventral margin, which is largely straight except for its posteriormost portion, which is concave. The postorbital plate is mostly flat except for the posteroventral part, which is laterally convex. The ornamentation of the maxilla is only preserved in proximity to the tooth-bearing margin, where it consists of minute teeth (odontodes; Ørvig, Reference Ørvig1978). The internal lamina extends from the rostral end of the maxilla until the level of the posterior end of the dentigerous margin of the dentary (Fig. 3).
Lower jaw
The mandible is largely complete, with only the anteriormost part missing (Fig. 3). Most of the lateral surface is weathered, meaning that the margins between the dentary, angular, and surangular represent the medial ones (the dentary usually covers a large area of the angular laterally; e.g., Romano and Brinkmann, Reference Romano and Brinkmann2009).
The dentary is composed of an external plate as well as a dorsal and a ventral internal lamina. The external plate is bounded by a gently convex ventral margin and a nearly straight, tooth-bearing dorsal border. The dorsal internal lamina runs close to the upper confinement of the external plate, whereas the ventral internal lamina parallels the lower margin of the bone. The angular forms the posterior and posteroventral borders of the lower jaw. This bone, too, is composed of an external plate and a large internal lamina. The plate-like part of the angular is confined by a long, convex border ventrally, and by an S-shaped margin posteriorly, which is dorsally concave and ventrally convex. The posterior and ventral margins run suborthogonally and together form a rounded posteroventral corner on the mandible. The suture between the external plate of the dentary and the surangular runs obliquely from anterodorsal to posteroventral, except in the most dorsal segment; there, the posterior margin of the dentary forms a recess, which houses the pointed anterior part of the surangular (Fig. 3). The upper portion of the boundary between the external plates of the angular and dentary is vertical, whereas the lower portion is distinctly inclined, running from posterodorsal to anteroventral. The wedge-shaped surangular exhibits a coronoid process (Fig. 3), without contribution of the dentary. The mandibular sensory canal traverses the external plate of the angular near its caudal and ventral borders and continues through the external plate of the dentary near its ventral margin, probably along the base of the ventral internal lamina.
The conspicuous internal lamina of the angular projects from the medial side of the plate-like part (Fig. 3). The base of this internal lamina follows the ventral and posterior borders of the external plate, but is offset with regard to these margins. In the posterior region of the lower jaw, the internal lamina is oriented mediocaudally, forming an obtuse angle with the external plate. In contrast, the external plate and internal lamina run suborthogonally within the ventral part of the angular. The ventral component of the medial lamina is lateromedially less broad than the posterodorsal portion of the lamina. The posterior part of the lamina becomes successively broader from the posteroventral angle of the angular to the level of the jaw joint, forming a sizeable posteromedial projection at the caudal end of the mandible (Fig. 3). The internal lamina is also curved medially.
Dentition
Macroscopic teeth are developed on the dentary and the maxilla, whereas the surangular lacks such teeth. The dentition of the maxilla and dentary consists of conical teeth that are arranged in three longitudinal rows: a lingual, an intermediate, and a labial one. The lingual row consists of fairly large, stout teeth growing on the internal lamina of the maxilla, and the dorsal internal lamina of the dentary, respectively. In the rostral part of these bones, the large lingual teeth are fully exposed (but partly weathered), whereas farther posteriorly only the apical parts are visible (Figs. 3, 4). The lingual teeth are aligned almost perfectly, forming a palisade (although gaps may occur where teeth fell out in vivo). The largest lingual teeth of the maxilla are located near the rostral end of the bone. From there, they slowly decrease in size in caudal direction. Conversely, the lingual teeth of the dentary slightly increase in height in posterior direction. The posteriormost lingual teeth of the dentary could not be reached with the air pen.
The intermediate series of teeth line the ventral margin of the external plate of the maxilla, and the dorsal margin of the external plate of the dentary, respectively (they are lost in the anterior parts of both of these bones due to weathering). The teeth of the intermediate row are always smaller than the neighboring teeth of the lingual row. They are irregularly distributed, but consecutive teeth are mostly separated by wide interspaces. The intermediate teeth of the maxilla decrease in size posteriorly, becoming minute at the level of the caudal end of the dentigerous margin of the dentary. Farther posteriorly, within the convex part of the maxilla that laterally covers the lower jaw, the intermediate teeth of the maxilla are larger again (Fig. 3). The intermediate teeth in the anterior segment of the dentary are about the same size as their antagonists on the maxilla, whereas those in the posterior segment of this bone are distinctly larger (Fig. 4). Close to the caudal end of the dentigerous margin of the dentary, the intermediate teeth rapidly decrease in size.
The teeth of the labial row are smaller than those of the intermediate row (Fig. 4). Like the intermediate teeth, the labial teeth are irregularly distributed along the jaw margins. They are quite frequent in the posterior part of the dentary, but a few labial teeth are also intermittently developed on the maxilla, especially in the anterior segment of this bone. The labial teeth are bordered by minute teeth laterally (odontodes; Ørvig, Reference Ørvig1978), forming the ornamentation of the maxilla and the dentary.
Whereas the apicobasal axes of the lingual teeth are inclined medially (and gently caudally), those of the intermediate teeth are more or less oriented dorsoventrally (except in the caudal part of the maxilla, where they are medially tending). The apicobasal axes of the labial teeth are inclined laterally, whereas the lateral leaning varies between individual labial teeth. The apicobasal axis of some labial teeth is nearly perpendicular to that of the lingual teeth (Fig. 4.2). The teeth of the three longitudinal rows are all linguolabially compressed and curved medially.
All teeth comprise an acrodin cap (Ørvig, Reference Ørvig1978) and a high base (Fig. 4.2, 4.3). The acrodin cap usually makes up only a small part of the total tooth height in lingual teeth (probably about one-fifth to one-sixth), whereas in teeth of the intermediate and labial rows the acrodin cap measures at least one-third of the total height. The surface of the acrodin cap is mostly smooth, except for its basal segment, which shows a few widely spaced, meandering, apicobasally oriented ridges (Fig. 4.3). Anterior and posterior cutting edges are present on teeth of all three rows. The surface of the tooth base, where preserved, is ornamented with very fine, subvertical striae. The striae are close-set and frequently anastomose (Fig. 4.3).
Preoperculum and operculogular series
The incompletely preserved preoperculum (Fig. 3) adjoins the postorbital plate of the maxilla dorsally and posteriorly. The boomerang-shaped preoperculum is composed of a long anterior shank and a short posteroventral branch, which meet in an obtuse angle. The rostral margin of the anterior shank is deeply concave and the anterodorsal part of the bone protrudes rostrally. The posteroventral branch of the preoperculum adjoins the straight upper part of the posterior margin of the maxilla, but does not reach farther ventrally, thus exposing the quadratum laterally. The caudal margin of the preoperculum is convex. A section of the preopercular sensory canal is visible in the lower shank. The canal traverses the bone centrally (Fig. 3).
The bones of the operculogular series (Fig. 3) are arranged in the characteristic manner for Birgeria. The operculum is situated dorsally to the anterior shank of the preoperculum. It is tilted in a way that its external side now faces dorsally. The operculum is incompletely preserved, but had an oblong, ovoid outline with acute anterior and posterior ends. Rostral to the operculum is another small, plate-like bone, interpreted as the antoperculum. Caudally, the operculum reaches to the knee of the preoperculum, where it is still articulated with the suboperculum (sensu Romano and Brinkmann, Reference Romano and Brinkmann2009). The vertically arranged suboperculum is elongate and slender, being morphologically indistinguishable from the posteriorly adjoining branchiostegal rays. The suboperculum has a triangular outline and is confined by very long anterior and posterior borders and a short dorsal margin contacting the operculum. The lateral side of the suboperculum is convex.
The branchiostegal series is divided into a postmandibular and a submandibular series, which are separated by a gap (Fig. 3). Five postmandibular radii branchiostegii are preserved ventrocaudal to the suboperculum. The postmandibular branchiostegals are coalesced neither with each other nor with the suboperculum. The postmandibular rays are slender, elongate elements. The first postmandibular branchiostegal ray is dorsoventrally almost as long as the suboperculum. The caudally following postmandibular rays are much shorter and their length consecutively decreases towards the last of these bones. Moreover, whereas the first and second rays are dorsoventrally oriented, like the suboperculum, the subsequent ones are more and more caudally inclined, with the fourth and fifth ray being somewhat anteroposteriorly arranged. Each ray has an acute ventral/anterior end and a rounded dorsal/posterior termination (except for the posteriormost of these elements, which has a pointed caudal end). Their lateral surface is convex. In addition to the five postmandibular rays situated behind the suboperculum, a small, thin, sixth postmandibular radium branchiostegium is seen isolated about halfway between the postmandibular and the submandibular series (Fig. 3). Mediolaterally, this ray is situated at the same level as the other branchiostegals.
Nine slender submandibular branchiostegal rays are preserved medioventral to the lower jaw (Fig. 3), though their original number was higher. Each of these elements is plate-like and has a quadrangular outline, whereas their anterolateral corner protrudes rostrad. The anteroposterior width of these bones subsequently decreases from the first (anterior) to the last one. Furthermore, while the anterior submandibular branchiostegals are only moderately posteriorly inclined, the caudally following ones are more distinctly so.
Suborbital and infraorbital series
The bones of the suborbital series are preserved in situ, albeit mostly in a poor state of preservation. Their morphology and arrangement agree with those of other species of Birgeria, meaning that they are elongate, obliquely and serially arranged bones with a broad posterodorsal part and a slender anteroventral portion. About three suborbitals are visible (Fig. 3), the posterodorsal parts of which are still intact and nested below the anterior process of the preoperculum. The anteroventral parts of the two, posteriormost suborbitals of P-66225 are fragmentarily preserved, but it is evident that they extended rostrad until the level of the third lingual tooth of the maxilla (which would mark the level of the posterior border of the orbital opening; Fig. 4.1).
A subtriangular bone fragment located anterodorsal to the suborbitals (Fig. 3) may belong to the dermosphenotic (this bone is usually subdivided into two or more elements in Birgeria, see Stensiö, Reference Stensiö1932; Romano and Brinkmann, Reference Romano and Brinkmann2009). Other elements of the circumorbital series and the sclerotic ring are perhaps visible at the rostral end of the fossil, but they are too poorly preserved for accurate description.
Splanchnocranium
The quadratum and articular are preserved in situ (Fig. 3). The quadratum is partly covered by the maxilla laterally, but the articulation condyle is exposed. The articular is mostly hidden by the angular and only its dorsalmost portion can be seen. The hyomandibula is only partly visible, being exposed where the preoperculum is damaged (Fig. 3). Some additional bones situated just medial to the postmandibular branchiostegal rays possibly belong to the hyomandibula or the symplectic, but they are not well exposed. The dermohyal is preserved in situ between the preoperculum, the operculum, and the antoperculum (Fig. 3). This bone is elongate and wedge-like, with a pointed posterior end, and a rounded, club-shaped anterior termination. The dermohyal is marked by a longitudinal crest running along its dorsal side. A few elements of the branchial skeleton are partly exposed (Fig. 3). For instance, a portion of an epibranchial is probably visible medially to the operculum. A segment of a possible ceratobranchial is seen at the posterior end of the concretion. Several rod-like bones preserved anteroventral to the lower jaw likely belong to the hyoid arch or the branchial arches.
Pectoral girdle
The right cleithrum is the only bone of the shoulder girdle that is visible (Fig. 3). Only the large lower branch of the cleithrum is preserved, the anterodorsal portion of which could not be prepared. The cleithrum fragment has a roughly triangular outline, being confined by a long, gently convex ventral border, and a long dorsal margin, being deeply concave in its caudalmost segment. The posterodorsal corner of the cleithrum fragment tapers, marking the transition to the missing upper branch of this bone. The external surface of the cleithrum is divided into a large lower portion, facing laterally, and a subhorizontally oriented upper portion.
Etymology
The species name refers to its provenance.
Remarks
The morphology of NMMNH P-66225 agrees well with that of the Triassic genus Birgeria Stensiö, Reference Stensiö1919. Species of Birgeria are essentially differentiated by cranial features, but also by a few postcranial traits, such as the arrangement pattern of the dorsal fin pterygiophores (Romano and Brinkmann, Reference Romano and Brinkmann2009). Diagnostic characters justifying the erection of a new species for the Nevada material are the less reduced dermal gill cover (i.e., presence of an antoperculum), very elongate suboperculum, and relatively numerous postmandibular branchiostegal rays, including a rudimentary ray situated within the gap of the branchiostegal series (see Discussion).
Based on the length of the preserved part (26 cm) of P-66225, corresponding to ~70–75% of the snout to shoulder girdle length in birgeriids with elongate postorbital skull portions (e.g., Nielsen, Reference Nielsen1949), a snout to shoulder girdle length of ~34.5–37 cm for P-66225 is estimated. In large species of Birgeria, the skull plus shoulder girdle length usually corresponds to ~20% of the total length (see Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006; Romano and Brinkmann, Reference Romano and Brinkmann2009), suggesting a total length of ~172–185 cm for P-66225.
Birgeria sp.
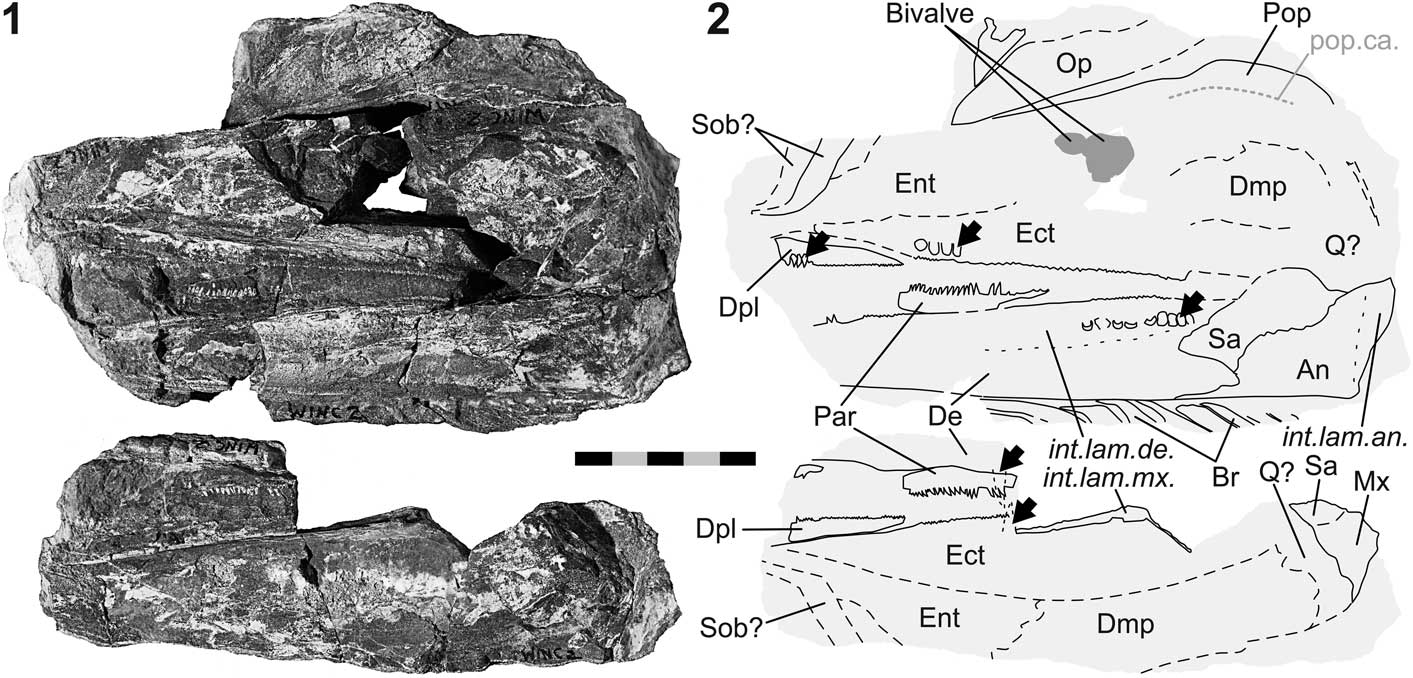
Figure 5 Birgeria sp. (NMMNH P-77117) from the Smithian of the Winecup Ranch, Elko County, Nevada, USA. (1) Part a (above) and counterpart b (below); (2) schematic drawing of 5.1 with interpretations of skeletal features; black arrows point to macroscopic teeth on the jaw bones (except prearticular); anterior is left. Scale bar=50 mm (total).
Occurrence
From upper lower Smithian to lower upper Smithian strata (Thaynes Group), ~2.75 km south-southeast of the Winecup Ranch, east-central Elko County, Nevada, USA (Fig. 1).
Description
NMMNH P-77117 (Fig. 5) is a cranial fragment with a length of 17 cm. The fossil is seen in left aspect and preserved in a limestone nodule as part (P-77117 a) and counterpart (P-77117 b), with the fracture surface going through the bones of the left cheek. The specimen is associated with bivalves, one of which resembles Crittendenia (personal communication to CR, M. Hautmann, 2016).
Palatoquadratum and its dermal bones.—Several upper jaw bones are exposed: the maxilla, dermopalatine, entopterygoid, ectopterygoid, and ‘dermometapterygoid’ (Fig. 5). The quadratum is probably present, though not well visible. The maxilla is only fragmentarily preserved, with portions of the postorbital blade and the internal lamina discernible. The dermopalatine is an elongate, low element situated in the anteroventral region of the upper jaw. This bone exhibits its maximum depth rostrally. The height of this element tapers caudally. Only one dermopalatine is visible. Dorsal to the dermopalatine is the entopterygoid, of which only the rostral portion is preserved. The ectopterygoid lies posterior to the dermopalatine and posteroventral to the entopterygoid. The ectopterygoid is a large, rostrocaudally elongate element that extends posteriorly until the jaw joint area. The boundary between the ectopterygoid and the entopterygoid runs obliquely from anteroventral to posterodorsal. Another, albeit incompletely preserved, element situated posterior to the entopterygoid and posterodorsal to the ectopterygoid, represents the ‘dermometapterygoid’ (terminology of Nielsen, Reference Nielsen1949; but see Arratia and Schultze, Reference Arratia and Schultze1991).
Lower jaw
The mandible is incompletely preserved. Its anterior tip is missing and the lateral surface is broken off (Fig. 5). The exposed margins between the dentary, angular, and surangular represent the medial ones, and the morphologies of these bones are similar to those of NMMNH P-66225 (holotype of B. americana n. sp.; Fig. 3). The angular of P-77117 (Fig. 5) is equipped with a well-developed internal lamina. The dorsal and ventral internal laminae of the dentary are well visible, delimiting the space occupied by Meckel’s cartilage. A fragment of the dentigerous upper margin of the prearticular is preserved.
Dentition
Teeth are preserved on several jaw bones (Fig. 5). The basal portions of close-set lingual teeth are preserved in the posterior segment of the dentary. One tooth of the maxilla and one of the dentary is each visible in transverse section on part b of the fossil. In addition, longitudinal rows of close-set, macroscopic teeth are present on the dermopalatine and the ectopterygoid in the upper jaw, and on the prearticular in the lower jaw. At least one row of large teeth is observed on both the dermopalatine and the ectopterygoid. These teeth are smaller than the lingual teeth of the maxilla. The medial surface of the dermopalatine and ectopterygoid is covered with minute teeth, cross sections of which are seen along the oral margins of these bones. At minimum one row of teeth lines the dorsal margin of the prearticular. These teeth are smaller than the larger teeth of the dermopalatine and the ectopterygoid. Macroscopic teeth are not developed on the surangular.
Preoperculum, operculogular series, and suborbitals.—The fragmentarily preserved preoperculum is composed of two shanks—an anterior and a posteroventral one (Fig. 5). Mainly the dorsal and posterior bone margins are visible. The dorsal border is straight and subvertical, whereas its caudal confinement is convex. At the level of the knee of the preoperculum, a weak notch is discernible. A section of the preopercular sensory canal traverses the posteroventral shank centrally in anterodorsal direction, then continues rostrad near the straight dorsal margin of the bone. The most anterior part of the canal is not preserved. The operculum is situated dorsal to the anterior shank and has an elongate, ovoid shape. The rostral end of this bone is pointed. Nine submandibular branchiostegal rays are preserved ventral to the dentary. The anterior rays are rostrocaudally broader than the posterior ones. The anterolateral corner of each ray protrudes rostrally. One or two suborbitals are possibly seen near the anterior end of the fossil.
Remarks
The overall anatomy of NMMNH P-66225 supports its referral to Birgeria Stensiö, Reference Stensiö1919. However, attribution at the species level is not possible due to the absence of diagnostic characters. Based on comparison with B. groenlandica Stensiö, Reference Stensiö1932 (cf. Nielsen, Reference Nielsen1949), a total length of ~145–165 cm is estimated for P-77117.
Family Saurichthyidae Owen, Reference Owen1860, emend. Stensiö, Reference Stensiö1925
Genus Saurichthys Agassiz, Reference Agassiz1834
Type species
Saurichthys apicalis Agassiz, Reference Agassiz1834 from the Ladinian (Middle Triassic) of Bayreuth, Germany, by original designation.
Occurrence
From the latest Permian of China to the Late Triassic of Europe and possibly China.
Saurichthys sp.
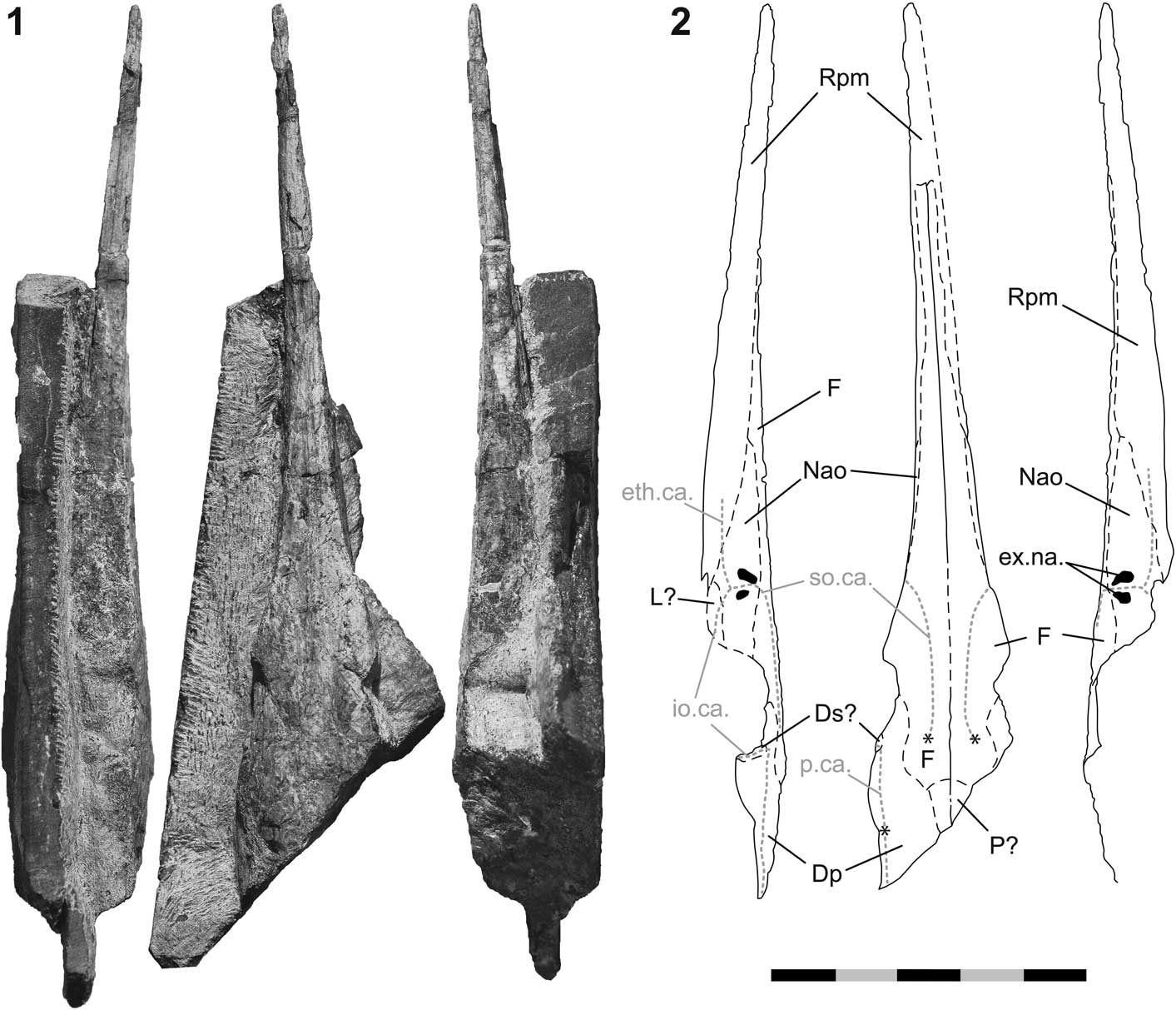
Figure 6 Saurichthys sp. (PIMUZ A/I 4397) from the latest Smithian of Palomino Ridge, Elko County, Nevada, USA. (1) fossil in left, dorsal, and right view (from left to right); (2) interpretive drawings of 6.1; asterisks in 6.2 mark the positions of the ossification centers of the frontals and the dermopterotic; anterior is up. Scale bar=50 mm (total).
Occurrence
Bed PLR 35 (Jattiot et al., in press), latest Smithian Pseudosageceras augustum Zone, Thaynes Formation (Fig. 2), Palomino Ridge, Section 2 (Jattiot et al., in press), southern Elko County, Nevada, USA (Fig. 1).
Description
PIMUZ A/I 4397 (Fig. 6) is a three-dimensional skull fragment preserved in a limestone slab. The posterior part of the specimen, as well as the maxilla, preoperculum, lower jaw, and operculogular series are missing. The superficial layer of the dermal bones, carrying the ornamentation, is mostly weathered and the suture lines are visible.
Dermal skull roof
The dermal cranial roof is mainly formed by the large, paired frontals, and the large, paired dermopterotics. The medial boundaries between these bones are intermittently visible from near the caudal end of the skull fragment up to a short distance posterior to the tip of the rostrum. The frontals are oriented mostly horizontally, except for the part anterodorsal to the orbital opening, where the bone exhibits a small, laterally facing flange. The ossification centers of both frontals are well visible and located at about the level of the hind margin of the orbit. The supraorbital sensory canal runs from these ossification centers up until the level of the external narial openings, where the canal turns laterally.
The ossification center of the dermopterotic is situated close to the posterolateral margin of the dermal skull roof (Fig. 6). The boundary between the frontal and the dermopterotic is scarcely visible, but judging from the circumferences of the growth lines radiating from the ossification centers of both of these bones, their mutual boundary runs sinuously from anterolateral to posteromedial. Anteriorly, the dermopterotic forms the posterior part of the dorsal margin of the orbit. The dermopterotic forms an extended lateral flange posterodorsal to the orbital opening. The remains of a poorly preserved dermosphenotic may be visible along the anterior margin of this lateral flange. A sensory canal is traceable along the lateral border of the postorbital portion of the dermal skull roof, running through the dermopterotic in the rostrocaudal direction. Posterodorsal to the orbital opening, the canal turns ventrolaterally. Some tubercles are visible along the sensory canal in the posterolateral part of the left dermopterotic. A pair of small parietals is developed medially to the dermopterotics and posteriorly to the frontals, but the boundaries between these elements are mostly unclear.
Rostrum
The margins between the bones composing the rostrum are only partly visible. The tip of the rostrum is formed by the large, unpaired rostropremaxilla (Fig. 6). Posterolaterally, the rostropremaxillary bone tapers on both sides. Caudal to the rostropremaxilla lies the small lachrymal, which is possibly preserved on the left side. The nasaloantorbital forms the anterior border of the orbit. This bone is wedged between the lachrymal and the posterolateral part of the rostropremaxilla ventrally, and the frontal dorsally. Its anterior end is elongate and probably acute. The nasaloantorbital contains both external narial openings, which are well visible on the right side of the skull (Fig. 6). The anterior naris is deeper than the posterior one. The nasaloantorbital also includes the junction of three sensory canals, which is situated ventral to the nares. The supraorbital canal enters the nasaloantorbital dorsally and runs between the narial openings. The ethmoidal canal passes laterally through the rostropremaxilla and pierces the nasaloantorbital through its anteroventral margin. The infraorbital canal enters the nasaloantorbital coming from the lachrymal.
Remarks
PIMUZ A/I 4397 is a saurichthyid but, identification to the species level is complicated due to its incomplete preservation. The majority of diagnostic traits differentiating species of Saurichthys pertain to the postcranium, which is not preserved in the specimen from Palomino Ridge (see Discussion).
The skull has a preserved length of ~132 mm, suggesting that it belonged to a medium-sized saurichthyid (sensu Tintori, Reference Tintori2013). The rostrum is subcomplete and long (~100 mm from the tip of the snout until the anterolateral margin of the orbit).
Actinopterygii indet.

Figure 7 Actinopterygii indet. (NMMNH P-77357) from the early late Spathian of Crittenden Springs, Elko County, Nevada, USA. (1) The fossil (enhanced using ammonium chloride); (2) interpretive drawing of 7.1; anterior is left. Scale bar=50 mm (total).
Occurrence
Early late Spathian Fengshanites/Prohungarites beds (Thaynes Group), Crittenden Springs, northeast Elko County, Nevada, USA (Fig. 1).
Description
NMMNH P-77357 (Fig. 7) is a posterior body portion of a medium-sized actinopterygian (estimated total length: ~25–30 cm). Preserved are the scales, most of the caudal fin, and the basal portions of some lepidotrichia of the dorsal and anal fins. The skeletal elements are largely in situ and mostly preserved as imprints, which have been affected weakly to strongly by surface erosion.
Squamation
The squamation consists of rhombic scales arranged in oblique vertical rows (Fig. 7). Scales are also developed on the proximal part of the dorsal caudal lobe. About 31 scale rows are counted along the lateral midline of the trunk, from the anterior end of the fossil to the caudal inversion. Dorsally, some of the vertical scale rows seem to split into two rows. The scales along the flank are about as long as they are deep, whereas those in the dorsal and ventral areas are less deep. The scale ornamentation and the lateral line sensory canal are not preserved, and a peg-and-socket articulation is not visible.
Fins
The proximal parts of at least six lepidotrichia of the dorsal fin are preserved in situ near the rostral end of P-77357 (Fig. 7). The fin rays are subdivided into short segments. The anal fin, although incomplete as well, seemingly inserts a few scale rows posterior to the dorsal fin. At least seven anal fin rays are discernible. Each fin ray is segmented into several short, close-set units, and some of the lepidotrichia bifurcate at least once.
The caudal fin is abreviated-heterocercal, exhibiting a reduced, scaled body lobe (Fig. 7). The distal ends of the dorsal and ventral caudal lobes are not preserved. Most of the caudal fin web is not well visible, but the lepidotrichia are clearly segmented into short elements and at least the central fin rays are distally branched. The leading margin of the dorsal caudal fin ramus is preceded by one lanceolate scute, followed posteriorly by three unpaired basal fulcra and ~12 paired basal fulcra (Pattern II of Arratia, Reference Arratia2009). The first (anterior) unpaired basal fulcrum has a weakly concave anterior margin, whereas the two caudally following ones have distinctly concave anterior borders. Small fringing fulcra are observed along the leading margin of the ventral caudal lobe.
Remarks
Preservation of NMMNH P-77357 is not sufficient for an attribution at low taxonomic rank, and it is thus left in open nomenclature.
Taxonomy and comparative anatomy
Birgeria Stensiö, Reference Stensiö1919
Birgeria is known from most marine Triassic fish localities, with up to 11 nominal species (Stensiö, Reference Stensiö1919, Reference Stensiö1921, Reference Stensiö1932; Boni, Reference Boni1937; Lehman, Reference Lehman1948, Reference Lehman1952; Nielsen, Reference Nielsen1949; Savage and Large, Reference Savage and Large1966; Schwarz, Reference Schwarz1970; Beltan, Reference Beltan1980; Bürgin and Furrer, Reference Bürgin and Furrer1992, Reference Bürgin and Furrer1993; Jin, Reference Jin2001; Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006; Romano and Brinkmann, Reference Romano and Brinkmann2009; this study). Its occurrence in the Permian of Bolivia is questionable (Beltan et al., Reference Beltan, Freneix, Janvier and López-Paulsen1987; Cione et al., Reference Cione, Gouiric-Cavalli, Mennucci, Cabrera and Freije2010). Contrary to Saurichthys (Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012), species richness of Birgeria remained relatively low and steady during the Triassic. Fossils of Birgeria are often (but not always) relatively rare within fish assemblages (e.g., Lombardo and Tintori, Reference Lombardo and Tintori2005; Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014), a fact that has been ascribed to the anatomically inferred offshore habitat of this taxon (e.g., Schwarz, Reference Schwarz1970).
From the eastern Panthalassan rim, Birgeria has thus far been described from the Early Triassic of western Canada (Schaeffer and Mangus, Reference Schaeffer and Mangus1976; Neuman, Reference Neuman2015), Greenland (Stensiö, Reference Stensiö1932; Nielsen, Reference Nielsen1949; Jessen, Reference Jessen1972; Ørvig, Reference Ørvig1978; Bartsch, Reference Bartsch1988), Spitsbergen (Stensiö, Reference Stensiö1921, Reference Stensiö1932; Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014), and Arctic Russia (Berg et al., Reference Berg, Kazantseva and Obruchev1964). The Canadian and Russian birgeriids are poorly known. Birgeria was also reported from Lower Triassic exposures near Bear Lake, Idaho, USA (Dunkle cited in Schaeffer and Mangus, Reference Schaeffer and Mangus1976, p. 552), but this material has not been described in the literature and the repositories are unknown. Lastly, cranial remains from the Late Triassic of California described as Xenestes velox Jordan, Reference Jordan1907 have been reassigned to Birgeria by Stensiö (Reference Stensiö1932); however, this poorly known species needs revision. The specimens described herein represent the first fossil evidence for the occurrence of Birgeria in the Early Triassic of the western USA.
Birgeria encompasses species of large size that, together with some species of Saurichthys Agassiz, Reference Agassiz1834, pertained to the actinopterygian apex predators of the Triassic (Lombardo and Tintori, Reference Lombardo and Tintori2005). One of the largest individuals of Early Triassic age is PIMUZ A/I 4301 from Spitsbergen, a few fragments of which were illustrated by Scheyer et al. (Reference Scheyer, Romano, Jenks and Bucher2014). This specimen had an estimated total length of >2 m (skull without shoulder girdle length is ~35 cm). Specimens NMMNH P-66225 and NMMNH P-77117 from Nevada are both large individuals. With an estimated total length of 1.72–1.85 m, P-66225 had a size comparable to the birgeriids from the Smithian of Spitsbergen (Stensiö, Reference Stensiö1921, Reference Stensiö1932; Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014), the Middle Triassic of Monte San Giorgio and Besano (Swiss-Italian borderland; Schwarz Reference Schwarz1970; Romano and Brinkmann, Reference Romano and Brinkmann2009), the Middle–Late Triassic of China (Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006; Jiang et al., Reference Jiang, Ni, Sun and Jiang2016; Sun et al., Reference Sun, Jiang, Ji and Hao2016), and possibly the Middle?–Late Triassic of Europe (B.? costata; Münster, 1839; Boni, Reference Boni1937; Bürgin and Furrer, Reference Bürgin and Furrer1993). Others were distinctly smaller (~1 m or less), for example individuals from the Early Triassic of Greenland (Stensiö, Reference Stensiö1932; Nielsen, Reference Nielsen1949) and Madagascar (Lehman, Reference Lehman1952; Guffroy, Reference Guffroy1956; Beltan, Reference Beltan1980), the Middle–Late Triassic of China (Jin, Reference Jin2001; Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006; Sun et al., Reference Sun, Jiang, Ji and Hao2016), and the Late Triassic of Slovenia (Jurkovšek and Kolar-Jurkovšek, Reference Jurkovšek and Kolar-Jurkovšek1986), Italy (e.g., Boni, Reference Boni1937), and Switzerland (Bürgin and Furrer, Reference Bürgin and Furrer1992). A clear evolutionary trend in body size is not apparent, except that very large birgeriids (>120 cm) are as yet unknown from strata older than Smithian.
As a member of the ‘Palaeopterygii,’ Birgeria mostly retained the plesiomorphic ‘paleoniscoid’ bauplan, being characterized, for instance, by the possession of an extended, cleaver-shaped maxilla (reflecting the large gape size and the far forward location of the eyes), the arrangement of jaw teeth in parallel rows, the remote position of the dorsal fin, and the heterocercal caudal fin (e.g., Aldinger, Reference Aldinger1931, Reference Aldinger1937; Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952). Like many other early actinopterygians, Birgeria possesses a dermohyal (Romano and Brinkmann, Reference Romano and Brinkmann2009; this study), but the presence of this bone remained undetected in earlier descriptions. In B. americana n. sp., B. nielseni, B. aldingeri, and B. stensioei, the dorsal side of the dermohyal is marked by a longitudinal crest (Stensiö, Reference Stensiö1932; Schwarz, Reference Schwarz1970; Beltan, Reference Beltan1980; Romano and Brinkmann, Reference Romano and Brinkmann2009; this study).
Apart from the aforementioned plesiomorphic traits, Birgeria stands out by a set of derived features, such as the advanced reduction of the squamation, the presence of an unpaired rostropremaxilla, the posterior extension of the frontals medially separating the parietals, or the large internal lamina on the angular (e.g., Stensiö, Reference Stensiö1921, Reference Stensiö1932; Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952; Schwarz, Reference Schwarz1970; Jin, Reference Jin2001; Liu et al., Reference Liu, Yin, Luo, Wang and Wang2006; Romano and Brinkmann, Reference Romano and Brinkmann2009). A further, autapomorphic condition is the arrangement of the elements of the operculogular series. The gill cover of Birgeria consists of a low, anteroposteriorly elongated operculum, located dorsal to the maxillary blade, a narrow, vertically arranged suboperculum (sensu Romano and Brinkmann, Reference Romano and Brinkmann2009; i.e., first ray of the ‘suboperculum’ of Nielsen, Reference Nielsen1949; Beltan, Reference Beltan1980), and a branchiostegal series that is divided into separate postmandiular and submandibular series (e.g., Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952; Beltan, Reference Beltan1980; Romano and Brinkmann, Reference Romano and Brinkmann2009). These highly specialized modifications are attributable to the extreme obliquity of the suspensorium (Aldinger, Reference Aldinger1937; Schwarz, Reference Schwarz1970), related with the tremendous enlargement of the gape size (Nielsen, Reference Nielsen1949), pushing the jaw joint close to the pectoral girdle.
Interspecific variation within the operculogular series concerns chiefly the number of postmandibular branchiostegal rays (sensu Romano and Brinkmann, Reference Romano and Brinkmann2009; i.e., second to last ray of the ‘suboperculum’ of Nielsen, Reference Nielsen1949; Beltan, Reference Beltan1980). Whereas three to five postmandibular branchiostegals are developed in B. groenlandica (cf. Nielsen, Reference Nielsen1949) and four to five in B. nielseni (cf. Beltan, Reference Beltan1980), none or at most one is usually preserved in B. stensioei (Romano and Brinkmann, Reference Romano and Brinkmann2009). In these species, all postmandibular branchiostegals are vertically arranged, like the suboperculum, whereas in B. americana n. sp. the posterior ones are caudally tending (Fig. 3). The operculogular series of B. americana n. sp. also displays a rudimentary branchiostegal ray between the main postmandibular and the submandibular series, which has thus far not been observed in other species. In addition, an antoperculum is developed in the Nevada taxon, a bone that is present in several other early actinopterygians, but until now was unknown in Birgeria (e.g., Aldinger, Reference Aldinger1937; Lehman, Reference Lehman1952; Gardiner and Schaeffer, Reference Gardiner and Schaeffer1989). The gill cover of B. americana n. sp. is less reduced compared to other birgeriids.
Other interspecific differences within the skull concern, among others, the outlines of the parasphenoid and maxilla (Boni, Reference Boni1937; Schwarz, Reference Schwarz1970). Many Early Triassic birgeriids possess a maxilla with a relatively low, elongate postorbital blade (e.g., B. groenlandica, B. aldingeri, B. cf. aldingeri, and B. americana n. sp.). Furthermore, the anterior margin of the postorbital blade is much more oblique (e.g., B. groenlandica, B. aldingeri, B. cf. aldingeri, B. nielseni, and B. americana n. sp.) than, for instance, in the Middle Triassic B. mougeoti or B. stensioei (Agassiz, Reference Agassiz1834–1843; Stensiö, Reference Stensiö1919, Reference Stensiö1932; Aldinger, Reference Aldinger1931; Nielsen, Reference Nielsen1949; Schwarz, Reference Schwarz1970; Romano and Brinkmann, Reference Romano and Brinkmann2009; Fig. 3). Additionally, the dorsal and ventral margins of the postorbital blade are subparallel in Middle and Late Triassic species (B. mougeoti, B. stensioei, and B. acuminata) and some Early Triassic species (B. nielseni and Birgeria sp. from British Columbia; Lehman, Reference Lehman1952; Schaeffer and Mangus, Reference Schaeffer and Mangus1976), whereas in most Early Triassic forms they clearly converge rostrad (e.g., B. americana n. sp., B. groenlandica, B. aldingeri, B. cf. aldingeri, Birgeria sp. from Russia, and some specimens referred to B. nielseni; Stensiö, Reference Stensiö1919, Reference Stensiö1932; Boni, Reference Boni1937; Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952; Berg et al., Reference Berg, Kazantseva and Obruchev1964; Schwarz, Reference Schwarz1970; Beltan, Reference Beltan1980; Romano and Brinkmann, Reference Romano and Brinkmann2009; Fig. 3). In general, Griesbachian–Smithian birgeriids have low, elongate skulls, whereas Middle–Late Triassic species possess higher, shorter crania (see Schwarz, Reference Schwarz1970). A reduction of the postorbital skull length has also been documented in Saurichthys during the Early–Middle Triassic transition (Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012), being a possible case of parallelism between these two predatory actinopterygians.
Some comments are necessary concerning the number of tooth rows and the size distribution pattern of teeth on the maxilla and dentary. Stensiö (Reference Stensiö1921, Reference Stensiö1932), Nielsen (Reference Nielsen1949), Lehman (Reference Lehman1952), Savage and Large (Reference Savage and Large1966), and Bürgin and Furrer (Reference Bürgin and Furrer1992) described two rows of teeth on the maxilla and/or dentary of B. groenlandica, B. nielseni, B. aldingeri, B. cf. aldingeri, and B. acuminata, whereas Schwarz (Reference Schwarz1970) observed three rows in B. stensioei. Three discrete rows of teeth are also developed in B. americana n. sp. (Fig. 4) and in the poorly known Middle?–Late Triassic B.? costata (Münster, Reference Münster1839), whose generic attribution has been questioned (Boni, Reference Boni1937; Bürgin and Furrer, Reference Bürgin and Furrer1993). Based on unpublished computer tomography generated images of the holotype of B. groenlandica (ZMUC VP 3176; personal communication to CR, T. Argyriou, 2016), it is evident that most of the large lingual teeth are not exposed on the surface, but two rows of teeth are confirmed here. Regarding B. aldingeri, only the lateral imprints of the intermediate and labial teeth of the maxilla are visible, with the internal lamina and lingual teeth not preserved (personal observation, C. Romano, 2016; Fig. 8; Stensiö, Reference Stensiö1932), thus three rather than two rows are developed in this species.

Figure 8 Interspecific variation in the dentition of Birgeria Stensiö, Reference Stensiö1919. Whereas species with two rows of teeth on the maxilla and dentary occur throughout the Triassic, species with three discrete rows are restricted to the Early Triassic. See text for details. Specimens shown: Birgeria americana n. sp. from the Smithian of Elko County, Nevada, USA (NMMNH P-66225, holotype; posterior part of dentary, middle part of maxilla); B. aldingeri Schwarz, Reference Schwarz1970 from the Smithian of Spitsbergen (PMU P 1421, holotype, cf. Stensiö, Reference Stensiö1932; suborbital part of maxilla—the internal lamina and the lingual teeth are not preserved because this is an external mold); B. stensioei Aldinger, Reference Aldinger1931 from the Anisian and Ladinian of Monte San Giorgio and Besano, Swiss-Italian borderland (PIMUZ T 1; posterior part of dentary); Birgeria sp. (B. acuminata?) from the middle Norian of Cene, Bergamo, Italy (MPUM 9334, cf. Lombardo and Tintori, Reference Lombardo and Tintori2005; posterior part of dentary). Anterior is right in all photographs. Specimens not to scale. Arrows point to teeth of the lingual (black arrow), the intermediate (gray arrow), and the labial row (white arrow). U/Pb ages for the Early Triassic: a after Burgess et al. (Reference Burgess, Bowring and Shen2014), b after Galfetti et al. (Reference Galfetti, Bucher, Ovtcharova, Schaltegger, Brayard, Brühwiler, Goudemand, Weissert, Hochuli, Cordey and Guodun2007a), c/d after Ovtcharova et al. (Reference Ovtcharova, Bucher, Schaltegger, Galfetti, Brayard and Guex2006, Reference Ovtcharova, Goudemand, Hammer, Guodun, Cordey, Galfetti, Schaltegger and Bucher2015). Abbreviations of intervals: Di.=Dienerian, G.=Griesbachian, I.=Induan, Ladin.=Ladinian, Olenek.=Olenekian, Sm.=Smithian.
Notably, only some Early Triassic birgeriids (B. aldingeri and B. americana n. sp.) possess intermediate teeth that are variable in size but predominantly high, and chiefly widely spaced, whereas in the Middle Triassic B. stensioei and the Late Triassic B. acuminata and Birgeria sp. they are close-set and of relatively small, uniform size throughout the length of the jaw (Woodward, Reference Woodward1889; Boni, Reference Boni1937; Savage and Large, Reference Savage and Large1966; Schwarz, Reference Schwarz1970; Bürgin and Furrer, Reference Bürgin and Furrer1992; Lombardo and Tintori, Reference Lombardo and Tintori2005; Fig. 8). The labial teeth are small but distinct in B. aldingeri and B. americana n. sp., whereas in stratigraphically younger taxa they are either very small or absent (Fig. 8), with the exception of the problematic B.? costata (Bürgin and Furrer, Reference Bürgin and Furrer1993). Ørvig (Reference Ørvig1978), confirming observations by previous authors (e.g., Stensiö, Reference Stensiö1919), showed that the odontodes on the external side of the jaw bones become larger and tooth-like in vicinity to the marginal teeth, exhibiting both an acrodin cap and a pulp cavity. It is conceivable that the labial teeth developed from such odontodes.
The present paper provides additional evidence that Birgeria encompasses both species with three rows of teeth and species with two rows of teeth along the oral margins of the maxilla and dentary. Based on the current state of knowledge, it appears that birgeriids with three well-developed tooth rows as well as widely spaced intermediate teeth with varying height are restricted to the Early Triassic. On the other hand, species with only two principal rows of teeth and small, close-set, equal-sized intermediate teeth occur throughout the Triassic. Whether the reduction of the intermediate and labial tooth rows on the maxilla and dentary represents an evolutionary trend in Birgeria requires further comparative studies.
Compared to the intermediate teeth, the size distribution pattern of the lingual teeth is seemingly more conservative between species. The same size distribution pattern is found in B. americana n. sp., B. stensioei, and B. acuminata (Aldinger, Reference Aldinger1931; Boni, Reference Boni1937; Schwarz, Reference Schwarz1970; Romano and Brinkmann, Reference Romano and Brinkmann2009; Figs. 3, 4). In both B. stensioei and B. acuminata, the lingual teeth in the caudal part of the dentary are curved towards the anterior (Boni, Reference Boni1937; Bürgin and Furrer, Reference Bürgin and Furrer1992; Romano and Brinkmann, Reference Romano and Brinkmann2009; Fig. 8), which is not the case in B. americana n. sp., however. Nielsen (Reference Nielsen1949) noted that the lingual teeth of B. stensioei are more widely spaced than in B. groenlandica. Although this is true for some specimens of B. stensioei (e.g., the lectotype), the teeth are more densly distributed in other specimens (Aldinger, Reference Aldinger1931; Schwarz, Reference Schwarz1970; Fig. 8). Tooth loss cannot account for such regular distribution patterns, but more studies are necessary to better assess the taxonomic value of tooth spacing.
Previous workers (Stensiö, Reference Stensiö1932; Lehman, Reference Lehman1952; Schwarz, Reference Schwarz1970) suggested interspecific variation in the ornamentation of the acrodin cap, ranging from completely smooth, to only basally striated, to fully striated. Nevertheless, the taxonomic value of tooth ornamentation is doubtful, as this character also shows intraspecific variability. For instance, in B. stensioei the acrodin cap is fully striated in PIMUZ T 4780 (Schwarz, Reference Schwarz1970), but only basally striated in PIMUZ T 1 (Fig. 8). Nielsen (Reference Nielsen1949) also mentions variability concerning this character in B. groenlandica. Crown ornamentation is also subjected to wear.
The dentitions of the rostropremaxilla, maxilla, and dentary are supplemented by at least one row of macroscopic teeth on the prearticular, coronoid, ectopterygoid, and dermopalatine, respectively, and myriads of minute teeth also cover the lingual surfaces of the prearticular, ectopterygoid, entopterygoid, parasphenoid, and the bones of the branchial arches (Stensiö, Reference Stensiö1921; Nielsen, Reference Nielsen1949; Lehman, Reference Lehman1952; Bürgin and Furrer, Reference Bürgin and Furrer1992; Romano and Brinkmann, Reference Romano and Brinkmann2009; this study). All species are characterized by a strong dentition, which together with the weakly developed bones of the operculogular series, suggest that Birgeria was a ram feeder (contra Lombardo and Tintori, Reference Lombardo and Tintori2005; Tintori et al., Reference Tintori, Hitij, Jiang, Lombardo and Sun2014a), meaning that prey was chased and bitten rather than engulfed through current action (Schaeffer and Rosen, Reference Schaeffer and Rosen1961). The coronoid process on the mandible—developed in convergence to holosteans—reduced torque on the jaw joint (Schaeffer and Rosen, Reference Schaeffer and Rosen1961).
Birgeria is often allied with saurichthyids and the Acipenseriformes (sturgeons and paddlefish), even though they share only a few characters (e.g., reduced squamation, posterior elongation of the parasphenoid; Bemis et al., Reference Bemis, Findeis and Grande1997), some of which are also present in other actinopterygians. The suggested close affiliation goes mainly back to the ‘Stockholm school’ (Schultze, Reference Schultze2009), whose influential works repeatedly highlighted similarities between Birgeria and Acipenseriformes, some of which were later called into question (e.g., nerve sac groups, Ørvig, Reference Ørvig1978). Jessen (Reference Jessen1972) also doubted a close affinity due to differences in the pectoral girdle skeleton, and according to Coates’ (Reference Coates1999) cladistic analyses, Birgeria is resolved as closely related to Acipenser only if endocranial characters are omitted. A close relationship among Birgeria, Saurichthys, and Acipenseriformes was, nonetheless, recovered in the cladistic analysis of Gardiner et al. (Reference Gardiner, Schaeffer and Masserie2005).
Nielsen (Reference Nielsen1949), among others, compared the peculiar ray-like elements posteroventral to the operculum of B. groenlandica with the lobate suboperculum of Polyodon. However, as pointed out by Romano and Brinkmann (Reference Romano and Brinkmann2009), only the anteriormost subopercular ray of Nielsen (Reference Nielsen1949) borders on the operculum, and the same condition is also seen in B. nielseni, B. stensioei, and B. americana n. sp. (Beltan, Reference Beltan1980; Romano and Brinkmann, Reference Romano and Brinkmann2009; Fig. 3). Although Nielsen (Reference Nielsen1949) stated that the rays are proximally fused in B. groenlandica, like in Polyodon (e.g., Bemis et al., Reference Bemis, Findeis and Grande1997), fusion is not evident in our material. The present study supports the view that the ‘suboperculum’ of Nielsen (Reference Nielsen1949) is a composite element and that it is not homologous with the suboperculum of the American paddlefish. The homology of the slender suboperculum of Birgeria with the suboperculum of other actinopterygians requires further study; a homology with the ‘accessory operculum’ of early ray-fins (e.g., Cheirolepis) would also be possible.
Saurichthys Agassiz, Reference Agassiz1834
Saurichthys is known from Triassic sites around the world, both marine and freshwater, and with over forty named species (Kogan and Romano, Reference Kogan and Romano2016a and references therein), it is much more speciose than Birgeria. After its first appearance in the latest Permian, Saurichthys rapidly reached global distribution and high species richness during the Early–Middle Triassic, but later became less diverse and geographically more restricted (Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012). Within the United States, Saurichthys has previously been described from the early late Smithian Anasibirites beds west of Georgetown, Bear Lake County, Idaho (Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; a second skull, PIMUZ A/I 4621, was found in 2013, and a cranial fragment, NMMNH P-77359, in 2015 by JJ). Saurichthys was also described from the Middle Triassic of Pershing County, Nevada (Sander et al., Reference Sander, Rieppel and Bucher1994; Rieppel et al., Reference Rieppel, Kindlimann and Bucher1996).
Three-dimensionally preserved skulls or skull fragments of Saurichthys (preserved as either body fossils or external molds) are frequently found in strata of Early Triassic age (Stensiö, Reference Stensiö1925; Lehman, Reference Lehman1952; Schaeffer and Mangus, Reference Schaeffer and Mangus1976; Beltan and Janvier, Reference Beltan and Janvier1978; Minikh, Reference Minikh1981, Reference Minikh1982; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Kogan and Romano, Reference Kogan and Romano2016a; this study) or Middle Triassic age (Frech, 1903–Reference Frech1908; Hennig, Reference Hennig1909; Beltan et al., Reference Beltan, Janvier, Monod and Westphal1979; Rieppel, Reference Rieppel1985; Wu et al., Reference Wu, Sun, Hao, Jiang and Sun2015), but are seemingly rare in younger deposits. Although saurichthyid crania contain some diagnostic features (e.g., Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Werneburg et al., Reference Werneburg, Kogan and Sell2014), species are predominantly differentiated by postcranial characters (e.g., squamation or fin segmentation pattern, morphology of the axial skeleton; e.g., Rieppel, Reference Rieppel1985; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Tintori, Reference Tintori2013; Tintori et al., Reference Tintori, Huang, Jiang, Sun, Motani and Chen2014b; Maxwell et al., Reference Maxwell, Romano, Wu and Furrer2015; Kogan and Romano, Reference Kogan and Romano2016a, Reference Kogan and Romanob). Moreover, comparisons of dermal skull bone patterns between species are complicated due to the fusion of bones during ontogeny. However, PIMUZ A/I 4397 from the latest Smithian of Palomino Ridge shows some features useful for comparison, such as the elongate rostrum, the anterior extension of the lachrymal, or the distinct lateral flange of the dermopterotic.
In addition to Saurichthys sp. from Palomino Ridge, a dorsoventrally and anteroposteriorly expanded lateral flange on the dermopterotic is found in S. toxolepis Mutter, Cartanyà, and Basaraba, Reference Mutter, Cartanyà and Basaraba2008 from the Early Triassic of Canada, S. obrutchevi Minikh, Reference Minikh1981 from the Early Triassic of Russia, and some specimens ascribed to S. wimani Woodward, Reference Woodward1912, S. ornatus Stensiö, Reference Stensiö1925, and S. elongatus Stensiö, Reference Stensiö1925 from the Smithian of Spitsbergen. The same condition is possibly also seen in S. cf. elongatus from the early late Smithian of Idaho (Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012). In contrast, the lateral flange of the dermopterotic is more reduced in other Early Triassic species, such as S. madagascariensis Piveteau, 1945, S. cf. ornatus from Greenland, and S. wimani (Stensiö, Reference Stensiö1925; Piveteau, 1944–Reference Piveteau1945; Lehman, Reference Lehman1952; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Kogan and Romano, Reference Kogan and Romano2016a).
In PIMUZ A/I 4397, S. cf. elongatus, S. ornatus, and S. toxolepis the lachrymal reaches anteriorly beyond the level of the posterior external naris, whereas in S. cf. ornatus and S. madagascariensis the lachrymal extends less far rostrally (e.g., Stensiö, Reference Stensiö1925; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Kogan and Romano, Reference Kogan and Romano2016a). The significance of this character requires further study.
The rostrum is often incompletely preserved in Saurichthys. In addition to PIMUZ A/I 4397, largely complete, elongate rostra are preserved, for instance, in specimens ascribed to S. toxolepis, S. elongatus, and S. curionii (Stensiö, Reference Stensiö1925; Rieppel, Reference Rieppel1985; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008), as well as in an undescribed specimen from Idaho (PIMUZ A/I 4621).
Early Triassic vertebrate occurrences in the western USA
Localities yielding Early Triassic fishes are known from Greenland, Canada, and the USA (Schaeffer and Mangus, Reference Schaeffer and Mangus1976; Wilson and Bruner, Reference Wilson and Bruner2004; Brinkmann et al., Reference Brinkmann, Romano, Bucher, Ware and Jenks2010). Early Triassic paleoichthyological sites are absent in South America (López-Arbarello, Reference López-Arbarello2004; Brinkmann et al., Reference Brinkmann, Romano, Bucher, Ware and Jenks2010) and, to our knowledge, there are no such localities in Central America and the Caribbean Islands. Consequently, our knowledge of Early Triassic fishes from the former western Pangean margin relies mainly on sites that were then located at mid or high latitudes in the northern hemisphere (i.e., localities in present-day northern North America and Arctic Eurasia).
Marine Early Triassic fishes from Greenland are derived from several horizons, all of earliest Triassic age (Griesbachian–?early Dienerian; Perch-Nielsen et al., Reference Perch-Nielsen, Birkenmajer, Birkelund and Aellen1974). They have been extensively researched especially during the twentieth century (e.g., Stensiö, Reference Stensiö1932; Nielsen, Reference Nielsen1949; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Kogan, Reference Kogan2011). From Canada, Early Triassic fishes have been reported from sites in western Alberta (Neuman, Reference Neuman2015 and references therein), Ellesmere Island (Nunavut; Schaeffer and Mangus, Reference Schaeffer and Mangus1976), and Wapiti Lake area, British Columbia. Fishes from the latter locality in particular have been the focus of several recent studies (e.g., Mutter and Neuman, Reference Mutter and Neuman2006; Mutter et al., Reference Mutter, Cartanyà and Basaraba2008; Wendruff and Wilson, Reference Wendruff and Wilson2012, Reference Wendruff and Wilson2013). The western Canadian fish fossils come from various layers, but age constraints are limited (e.g., Mutter and Neuman, Reference Mutter and Neuman2006; Neuman, Reference Neuman2015). In addition to the aforementioned Canadian sites, Wignall and Newton (Reference Wignall and Newton2003) reported the occurrence of well-preserved remains of cf. Bobasatrania in the upper Grayling Formation (Dienerian?) of Ursula Creek, northwest of Wapiti Lake.
In contrast to Canada and Greenland, only a few, short papers were dedicated to the fishes from the Early Triassic of the United States. Exposures of Lower Triassic, marine rocks, which are found in Alaska, Montana, Wyoming, Idaho, Utah, Nevada, and California, have yielded a plethora of marine invertebrate fossils, including the earliest metazoan reefs of the Mesozoic (e.g., Silberling and Tozer, Reference Silberling and Tozer1968; Nichols and Silberling, Reference Nichols and Silberling1979; Brayard et al., Reference Brayard, Vennin, Olivier, Bylund, Jenks, Stephen, Bucher, Hofmann, Goudemand and Escarguel2011, Reference Brayard, Bylund, Jenks, Stephen, Olivier, Escarguel, Fara and Vennin2013, Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017; Hofmann et al., Reference Hofmann, Hautmann and Bucher2013a, Reference Hofmann, Hautmann, Wasmer and Bucherb). Several formations produce Early Triassic vertebrate remains, but these occurrences have often only been casually mentioned (e.g., Oversby, Reference Oversby1972; Silberling, Reference Silberling1973; Schaeffer and Mangus, Reference Schaeffer and Mangus1976; Poole and Wardlaw, Reference Poole and Wardlaw1978). On the other hand, the marine and freshwater fishes from the Middle Triassic of the USA have received slightly more research interest (e.g., Wemple, Reference Wemple1906; Wells, Reference Wells1947; Schaeffer and Gregory, Reference Schaeffer and Gregory1961; Sander et al., Reference Sander, Rieppel and Bucher1994; Rieppel et al., Reference Rieppel, Kindlimann and Bucher1996; Cuny et al., Reference Cuny, Rieppel and Sander2001).
One of the longest known collecting areas for Early Triassic marine vertebrate fossils is southeast Idaho, yielding ichthyoliths (Evans, Reference Evans1904; Goddard, Reference Goddard1907; Mutter and Rieber, Reference Mutter and Rieber2005; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Brayard et al., Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017), but also articulated fishes (Tanner, Reference Tanner1936; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012), and some ichthyopterygian reptile remains (Massare and Callaway, Reference Massare and Callaway1994; Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014). In addition to these published specimens, new vertebrate material (isolated bones or articulated partial/complete skeletons) was recently discovered at several sites in Idaho, for instance in the middle Smithian ‘Meekoceras beds’ (personal observation, CR, JJ, KGB, HB, 2013–2016), the late Smithian Anasibirites beds (Saurichthys: PIMUZ A/I 4621, NMMNH P-77359; several unlabelled specimens of Actinopterygii indet.), and the early Spathian Tirolites beds (personal communication, A. Brayard, 2016; personal observation, JJ, KGB, HB, 2013–2016).
Very little is known about marine vertebrate occurrences in Lower Triassic formations in the other western states. Aside from the Smithian and Spathian fish material from Nevada described herein, some ichthyopterygian remains from Spathian layers were documented by Mazin and Bucher (Reference Mazin and Bucher1987) and Kelley et al. (Reference Kelley, Motani, Embree and Orchard2016). Isolated vertebrate remains have also been found in the Smithian ‘Meekoceras beds’ at Crittenden Springs as well as in Spathian strata at several sites in Nevada (personal observation, JJ, RJ, TMS, KGB, HB, 2010–2016). Articulated osteichthyan fossils of Dienerian age have recently been discovered in the Candelaria Hills in southwest Nevada (e.g., Brinkmann et al., Reference Brinkmann, Romano, Bucher, Ware and Jenks2010; Romano et al., in preparation). From Wyoming, Case (Reference Case1936) described a sauropterygian reptile of Spathian age (Lovelace and Doebbert, Reference Lovelace and Doebbert2015; Kelley et al., Reference Kelley, Motani, Embree and Orchard2016).
Most vertebrates from the Early Triassic of the Western United States are Smithian or Spathian in age, whereas Griesbachian and Dienerian fossils are less well known. The material from Nevada presented herein is derived from equatorial shelfal series deposited on the North American Craton. Material from sites located farther to the East (Utah, Idaho) was deposited in a low-latitude, epicontinental sea (western USA basin; Brayard et al., Reference Brayard, Bylund, Jenks, Stephen, Olivier, Escarguel, Fara and Vennin2013). The depth and areal extent of this Early Triassic sea were controlled by both regional tectonics and eustatic sea level changes (e.g., Paull and Paull, Reference Paull and Paull1993; Brayard et al., Reference Brayard, Bylund, Jenks, Stephen, Olivier, Escarguel, Fara and Vennin2013).
In conclusion, several localities in the western USA yield Early Triassic vertebrate remains, but for the most part these fossils received little interest by researchers. The specimens described herein, in combination with other published and unpublished material from the Smithian of the western USA, contradict Sun et al.’s (2012) notion of a near absence of vertebrates within low latitudes due to ‘lethally hot’ temperatures during this interval. This alleged ‘equatorial vertebrate eclipse’ largely ignored the contradictory data available from China (e.g., Tong et al., Reference Tong, Zhou, Erwin, Zuo and Zhao2006), and was also based on a wrong conodont age (Goudemand et al., Reference Goudemand, Romano, Brayard, Hochuli and Bucher2013). Sun et al. (Reference Sun, Joachimski, Wignall, Yan, Chen, Jiang, Wang and Lai2012) further inferred too high sea surface temperatures (~38°C, possibly over 40°C), because the presence of fishes in the USA and China suggests that a 36°C physiological limit was not crossed (Motani and Wainwright, Reference Motani and Wainwright2015).
Spathian Osteichthyes
Of interest is the presence of vertebrate fossils in Spathian strata in the western USA. The Spathian had a duration of ~3 myr, therefore longer than the first three stages of the Early Triassic combined (~2 myr; Ovtcharova et al., Reference Ovtcharova, Bucher, Schaltegger, Galfetti, Brayard and Guex2006, Reference Ovtcharova, Goudemand, Hammer, Guodun, Cordey, Galfetti, Schaltegger and Bucher2015; Galfetti et al., Reference Galfetti, Bucher, Ovtcharova, Schaltegger, Brayard, Brühwiler, Goudemand, Weissert, Hochuli, Cordey and Guodun2007a; Fig. 8). However, only a few, well-dated Spathian sites yield articulated osteichthyan fossils (Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a).
Apart from the new material from Crittenden Springs (NMMNH P-77357, PIMUZ A/I 4641), articulated actinopterygian remains (a perleidid and a saurichthyid) have been described from Spathian deposits of Anhui Province, China (Sun et al., Reference Sun, Tintori, Jiang and Motani2013; Tintori et al., Reference Tintori, Huang, Jiang, Sun, Motani and Chen2014b). In addition, largely complete marine osteichthyan fossils have also been documented from beds close to the Smithian-Spathian boundary at localities in Anhui, Hubei and Jiangsu provinces, China (e.g., Tong et al., Reference Tong, Zhou, Erwin, Zuo and Zhao2006 and references therein). A saurichthyid (Saurichthys elongatus?) with a short postorbital skull portion was found slightly above the Smithian ‘Fischniveau’ of Spitsbergen, thus being of possible Spathian age (Stensiö, Reference Stensiö1925; Kogan and Romano, Reference Kogan and Romano2016b). Two saurichthyids and a colobodontid were found in the Gogolin beds of Upper Silesia, Poland, whose age is close to the Spathian-Anisian boundary (e.g., Eck, Reference Eck1865; Frech, 1903–Reference Frech1908; Nawrocki and Szulc, Reference Nawrocki and Szulc2000; Szulc and Becker, Reference Szulc and Becker2007). Aldinger (Reference Aldinger1931) mentioned undescribed actinistian material from Gogolin. Marine and freshwater osteichthyan remains of presumable Spathian age have been described from localities in Canada, Russia, Kazakhstan, northern China, and South Africa (e.g., Jubb and Gardiner, Reference Jubb and Gardiner1975; Minikh, Reference Minikh1981; Mutter and Neuman, Reference Mutter and Neuman2006), although often with limited biochronostratigraphic constraints. Besides, there are several Spathian occurrences of isolated fish remains (e.g., Stensiö, Reference Stensiö1921; Mutter and Rieber, Reference Mutter and Rieber2005; Kogan and Romano, Reference Kogan and Romano2016b; Brayard et al., Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017), but such material often provides little taxonomic information.
Griesbachian–Smithian fishes differ notably from post-Spathian ones. Earliest Triassic sites are characterized by cosmopolitan taxa, whereas Middle Triassic assemblages comprise many endemic taxa, with a distinct neopterygian component (Tintori et al., Reference Tintori, Hitij, Jiang, Lombardo and Sun2014a; Romano et al., Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016a). This suggests an ichthyofaunal turnover either during the Spathian or in conjunction with the end-Smithian event. This extinction event decimated nektopelagic clades such as ammonoids and conodonts (Galfetti et al., Reference Galfetti, Hochuli, Brayard, Bucher, Weissert and Vigran2007b; Orchard, Reference Orchard2007) and saw the replacement of trematosauroid ‘amphibians’ by reptiles among the marine tetrapod apex predators (Scheyer et al., Reference Scheyer, Romano, Jenks and Bucher2014). However, the impacts of the end-Smithian event on fishes remain elusive, particularly with regard to the timing of the radiation of the ‘Triassic Middle Fish Fauna’ of Tintori et al. (Reference Tintori, Hitij, Jiang, Lombardo and Sun2014a). The sparse Spathian actinopterygians known thus far show more affinities with Middle Triassic taxa than with Griesbachian–Smithian ones (Sun et al., Reference Sun, Tintori, Jiang and Motani2013; Tintori et al., Reference Tintori, Huang, Jiang, Sun, Motani and Chen2014b). There is a need for more research on Spathian Osteichthyes, emphasizing the importance of the discovery of articulated osteichthyans in the Spathian of Nevada and Idaho (Brayard et al., Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017; this study).
Conclusions
The presented material from three new sites in Elko County (Nevada, USA) notably improves the fossil record of Early Triassic fishes from the western USA. Although Early Triassic fish remains occur in several places in the United States, most of them have not been studied. We introduce a new Spathian fish occurrence. Spathian Osteichthyans are preserved in several sites in the western USA and their study may provide vital clues concerning the post-Paleozoic evolution of fishes.
The Smithian material studied here includes two large-sized individuals of the predatory actinopterygian Birgeria, providing the first fossil evidence that birgeriids expanded their distribution into the western USA Sea, even during the Smithian thermal maximum (Goudemand et al., Reference Goudemand, Romano, Brayard, Hochuli and Bucher2013; Romano et al., Reference Romano, Goudemand, Vennemann, Ware, Schneebeli-Hermann, Hochuli, Brühwiler, Brinkmann and Bucher2013). One specimen can be ascribed to a new species, Birgeria americana n. sp., which is characterized by three rows of teeth on the maxilla and dentary, and a less reduced operculogular series compared to other taxa. We further describe material of the ambush predator Saurichthys that, together with other late Smithian occurrences in Idaho (PIMUZ A/I 3900, PIMUZ A/I 4621, NMMNH P-77359), suggests that this taxon was relatively common in the western USA basin. The presented fishes from low paleolatitudes, along with other Smithian vertebrate occurrences in the United States and South China (Tong et al., Reference Tong, Zhou, Erwin, Zuo and Zhao2006), invalidate the alleged ‘equatorial vertebrate eclipse’ of Sun et al. (Reference Sun, Joachimski, Wignall, Yan, Chen, Jiang, Wang and Lai2012). These low-latitude occurrences imply that a 36°C temperature tolerance was not permanently exceeded during the Smithian thermal maximum (Motani and Wainwright, Reference Motani and Wainwright2015). The presence of the actinopterygian top predators Birgeria and Saurichthys also contradicts anew the claim of missing apex predators and truncated food chains during the Early Triassic made by Chen and Benton (Reference Chen and Benton2012).
Acknowledgments
We appreciate information from and insightful discussions with T. Argyriou, M. Leu, D. Ware, T. Brühwiler, M. Brosse, C. Klug, M. Hautmann, W. Brinkmann (all PIMUZ), I. Kogan (Technische Universität Bergakademie Freiberg, Germany), P. Skrzycki and R. Skrzycka (Krakow, Poland), A. Brayard (Université de Bourgogne, Dijon, France), R. Hofmann (Museum für Naturkunde Berlin), and A. Trintori (MPUM). E.E. Maxwell (Staatliches Museum für Naturkunde Stuttgart, Germany) and the editor are thanked for comments that improved this paper. I. Kogan and A. Tintori are also thanked for photographs. M. Leu, C. Kolb, and F. Blattmann (all PIMUZ) are thanked for lab support. CR thanks M. Hebeisen, R. Roth, and T. Argyriou for advice during preparation of fossils, and M. Véran, G. Clément (both MNHN.F), and J.O. Ebbestad (PMU) for help during collection visits. Fossils were collected under BLM permit number N-92672 and we thank B. Hockett (Bureau of Land Management Nevada) for swift administrative processing. We deeply acknowledge past and present support by the Swiss National Science Foundation (project numbers 120311/135075 and 144462 to W. Brinkmann, 160055 to HB, and 149506 to TMS).