The adult CHD population is markedly increasing in the last decades. These adults have a 15–50 times higher risk of infective endocarditis that of the general population, due to the substrate of prosthetic materials and residual lesions usually present, corresponding up to 18% of the total adult infective endocarditis cases. Reference Verheugt, Uiterwaal and van der Velde1–Reference Cahill, Jewell and Denne3
These facts change the epidemiological profile of infective endocarditis in the developed world, with CHD now predominating in young adults infective endocarditis cases and not rheumatic valve disease. Reference Di Filippo4
Although infective endocarditis diagnosis and its sequelae are reported to account for 4–5% of in-hospital admissions of patients with CHD, accurate lesion-specific risk estimates of infective endocarditis are lacking. Reference Di Filippo4,Reference Bauer, Helm and Diller5
Also, no randomised study has been conducted yet to elucidate the efficacy and usefulness of infective endocarditis prophylaxis and more recent European guidelines limit antibiotic prophylaxis to the highest-risk patients undergoing the highest-risk procedures. Reference Habib, Plonska-Gosciniak and Price6 While prosthetic material implanted during repair and palliation constitute additional infective endocarditis targets that may be of increasing importance, several studies showed a non-negligible risk in mild complexity disease, as non-repaired bicuspid aortic valves and ventricular septal defects. Reference Verheugt, Uiterwaal and van der Velde1,Reference Mylotte, Rushani and Therrien2,Reference Dodo and Child7–Reference Siu and Silversides10
In a disease that still presents a significant mortality risk (4–24%) in a predominantly young population, target patients who would maximally benefit from preventive measures or increased medical surveillance is essential for lowering their life-long risk of developing IE. Reference Cahill, Jewell and Denne3,Reference Fortún, Centella and Martín-Dávila11–Reference Hays13
We sought to analyse the clinical course and mortality risk factors of infective endocarditis in the adult population with CHD followed in our tertiary centre.
Material and methods
We retrospectively reviewed all cases of proven and probable infective endocarditis in our adult CHD database (approximately 3000 patients) between 1970 and August, 2021, including patients with and without prior percutaneous intervention or surgery.
The study population was divided according to previous intervention (none, percutaneous and/or surgical), CHD complexity (mild, moderate or severe, according to the Bethesda classification) and main diagnosis or pathophysiological status: tetralogy of Fallot, transposition of the great arteries, single ventricle physiology, systemic pulmonary shunts (at atrial and/or ventricular or arterial left), left ventricular outflow tract disease (bicuspid aortic valve, aortic and subaortic stenosis), aortic coarctation and right ventricular outflow tract disease (pulmonary stenosis or atresia).
Diagnostic criteria for infective endocarditis were based on the modified Duke’s criteria, applied retrospectively to the entire population. Hospital records were examined and data collected on the demographic characteristics, site of infection, outcome, complications, and clinical and echocardiographic features.
Post-operative infective endocarditis was defined as onset within 6 months of intervention. All cases of post-operative infective endocarditis were considered to be nosocomial infective endocarditis. In addition, nosocomial infective endocarditis was defined as an infection occurring <72 hours after admission or acquired in association with a significant invasive procedure performed during hospitalisation up to 8 weeks before the onset of symptoms. The remaining cases of infective endocarditis were considered to be community-acquired.
Regarding the complications, embolism included both major arterial and pulmonary embolism and heart failure was defined as a condition needing heart failure therapy, namely diuretics. Surgical intervention was defined as cardiovascular surgery to treat infective endocarditis.
The statistical analysis was performed using SPSS Statistics version 22 (IBM SPSS, Chicago, IL). Data for categorical variables are reported as frequency and percentage (%) and continuous variables are expressed as mean ± standard deviation. Continuous variables that were not normally distributed are reported as median and range (minimum and maximum). Predictors of complications, surgical treatment and mortality were assessed using regression analysis. For all analyses, a value of p < 0.05 was considered significant.
Results
During a mean follow-up of 15.8 ± 10.9 years, 96 patients had 105 infective endocarditis episodes. The majority were male (57%), a minority had infective endocarditis at paediatric age (17%) and seven patients had recurrent episodes. The patients’ demographics are exposed in Table 1. Half of the patients had a previous cardiac surgery (corrective or palliative) and three patients had infective endocarditis after percutaneous pulmonary valve replacement (Fig 1).

Figure 1. Those with previous surgical and/or percutaneous intervention before Infective Endocarditis episode (N = 105). ASD – Atrial septal defect; VSD – Ventricular septal defect.
Table 1. Patient demographics
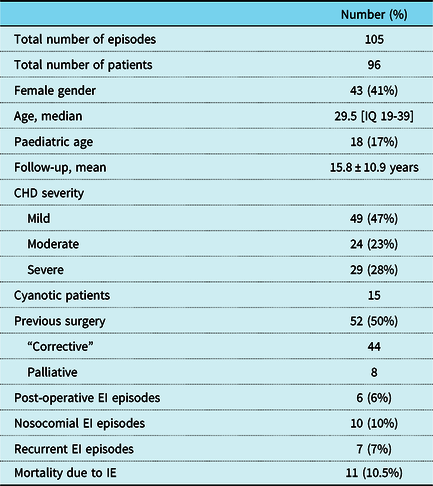
EI – Infective Endocarditis.
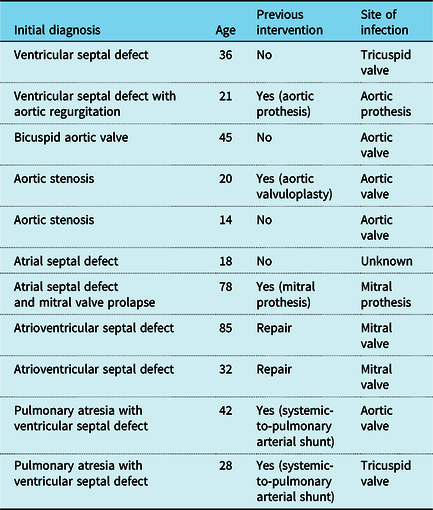
The most frequent diagnoses found were: ventricular septal defect, left outflow tract obstruction lesions, namely bicuspid aortic valve, Tetralogy of Fallot and complex lesions as pulmonary atresia with ventricular septal defect (Fig 2). Concerning the total population of CHD adults followed in our outpatient clinic, we observe the following prevalence of infective endocarditis: 8% in bicuspid aortic valve, 5% ventricular septal defect, 4.5% complex defects, 4.2% Tetralogy of Fallot, 3.4% Transposition of the Great Arteries, 1.9% aortic coarctation, and <1% of other defects.

Figure 2. Total number of cases of Infective Endocarditis and number of those with previous surgery for each congenital heart disease diagnosis. ASD – Atrial septal defect; AVCD – Atrioventricular canal defect; BAV – Bicuspid Aortic Valve; CoA – Aortic Coarctation; MVP – Mitral valve prolapse; PDA – Patent ductus arteriosus; PA – Pulmonary atresia; PS – Pulmonary Stenosis; SubAS – subaortic stenosis; TGA – Transposition of the Great Arteries; TOF – Tetralogy of Fallot; UV – Univentricular Heart; VSD – Ventricular septal defect.
It was possible to analyse the echocardiographic studies (transthoracic and transesophagical) performed in 90 infective endocarditis episodes. The site of infection (vegetation, abscess, and destroyed heart structure) was identified in 82 episodes (91%), namely in aortic valve (n = 27), tricuspid valve (n = 15), mitral valve (n = 13), aortic prosthesis (n = 6), pulmonary valve (n = 5), pulmonary prosthesis (n = 4), ventricular septal defect (n = 3), pacemaker leads (n = 3), mitral prosthesis (n = 2), right ventricle-pulmonary artery conduct (n = 2), and aortic coarctation (n = 1). In addition, four patients had an aortic abscess and two an aortic pseudoaneurysm.
A pathogen was isolated in 65 infective endocarditis cases, being streptococci (n = 29) and staphylococci (n = 23) the predominant pathogens (Table 2). Eighteen patients had systemic embolisation, the majority in the central nervous system (n = 7), followed by splenic (n = 6), pulmonary (n = 5), renal (n = 1), hepatic (n = 1), and spine (n = 1). Heart failure complicated the course of infective endocarditis in 25 cases (24%), namely in all the cases with right ventricle-pulmonary artery conduct endocarditis, 38% of mitral valve involvement, 33% aortic valve, 27% tricuspid valve, 25% pulmonary prothesis, and in 20% of pulmonary valve endocarditis.
Table 2. Microbiology of infective endocarditis.

MRSA – Methicillin-resistant Staphylococcus aureus; MSSA – Methicillin-susceptible Staphylococcus aureus.
In terms of treatment, surgical management was necessary in 40% of cases (n = 42) in acute phase, 31% of these with prior surgery. We did not find a significant relation between acute and prior surgery, although a surgical treatment for infective endocarditis was significantly less performed in cyanotic patients (13% versus 45%, p = 0.023).
Eleven patients died (11%) during infective endocarditis episode. Mortality rates by CHD severity were: 12% in mild, 9% in moderate, and 7% in severe CHD (Table 3). Mortality was associated with congestive heart failure at presentation (p < 0.001; OR 13.5) and with conservative management (p = 0.003; OR 5.06).
Table 3. Characteristics of the patients who died during the episode of infective endocarditis.
Discussion
In this cohort of patients with adult CHD treated in a tertiary adult CHD centre, infective endocarditis is still associated with significant morbidity and mortality. Surgery was often necessary (40%) and the in-hospital mortality was 10.5%, associated with heart failure and a non-surgical approach.
In our study, the lesions most frequently affected by endocarditis were ventricular septal defect and bicuspid aortic valve, followed by tetralogy of Fallot and complex defects as pulmonary atresia and univentricular hearts. These results were consistent with a higher prevalence of infective endocarditis in the same defects in the total population of patients followed in our centre’s outpatient clinic.
Risk in CHD has been traditionally classified into three groups, being high-speed shunts and non-operated native aortic valve disease considered a moderate risk group, opposing to high-risk cyanotic heart disease, patients with previous infective endocarditis, valve prostheses, heart disease operated on with residual lesions, and during the 6 postoperative months. Reference Di Filippo14
Other authors had previous demonstrated the increase risk of infective endocarditis in non-repair simple lesions in left ventricular outflow tract and ventricular septal defect, although infective endocarditis prophylaxis in these conditions is no longer recommend. Reference Habib, Plonska-Gosciniak and Price6,Reference Li and Somerville15–Reference Niwa, Nakazawa, Tateno, Yoshinaga and Terai17 In a nationwide NHS study, the authors also found higher incidence of infective endocarditis in those with acyanotic congenital valve anomalies than those with cyanotic CHD and a similar risk compare to other “high-risk” conditions. Reference Thornhill, Jones and Prendergast18
For an adult CHD patient with unrepaired ventricular septal defect, is estimated that the lifetime risk for infective endocarditis at age 30 years is ∼10% and by the end of life is 12%, Reference Knirsch and Nadal16 while in bicuspid aortic valve more recent data estimates an infective endocarditis risk of 0.3–2% per year. Reference Siu and Silversides10 Shear stress of the endothelium caused by turbulent flow can alter cell shape and cytoskeletal organisation, and increases leukocytes and bacteria adhesion to the endothelium. Reference Knirsch and Nadal16 Even more, it is thought that the high velocity of the stream immediately beyond the orifice in a high-speed shunt lesion, as a ventricular septal defect, is associated with a marked drop in lateral pressure with subsequent reduction in perfusion of the intima of this segment, with increasing risk for infective endocarditis during episodes of bacteraemia. Reference Dodo and Child7 This suggests these lesions carry innate risk or are present in patients with conditions that place them at particularly high risk (for instance, their lifestyle).
However, motivated by lack of robust data of the effectiveness of antibiotic prophylaxis, potential hazards of antibiotic use (anaphylaxis and antibiotic resistance) and recognition of the importance of bacteraemias from routine daily activities in causing infective endocarditis, European guidelines now limit antibiotic prophylaxis to the highest-risk patients undergoing the highest-risk procedures and emphasise the role of primary prevention. Reference Habib, Plonska-Gosciniak and Price6,Reference Mulder19
While right-side CHD and atrial septal defects are at the lowest infective endocarditis risk, the hazard of endocarditis in patients with other simple lesions considered as moderate risk and left out of the European prophylaxis recommendations could be underestimated. In fact, in our cohort and in the study of Moore et al., the highest mortality rate was seen in simple lesions, which can be related, in part, to a higher delay in diagnosis. Reference Moore, Cao, Kotchetkova and Celermajer20 The Japanese Circulation Society infective endocarditis guidelines recognises this problem and still recommends antibiotic prophylaxis in moderate-risk groups (class of recommendation IIa) since they have a high incidence of infective endocarditis in national surveys in Japan, although with documented lower morbidity and mortality comparing to high-risk groups. Reference Nakatani, Ohara and Ashihara21
On the other hand, the high prevalence of infective endocarditis noted in patients after repair of tetralogy of Fallot and pulmonary atresia are probable related with residual lesions, valve-containing prosthetics, conduits and subsequent interventions, namely transcatheter pulmonary valve replacement that has recognised increased risk of infective endocarditis. Reference Kuijpers, Koolbergen and Groenink8,Reference McElhinney, Sondergaard and Armstrong22,Reference Egbe, Vallabhajosyula, Akintoye and Connolly23
Although safety, efficacy, and cost-effectiveness data are lacking regarding antibiotic prophylaxis, it is likely that there are patients that may benefit from prophylaxis, particularly those at high-risk of adverse outcomes, Reference Mylotte, Rushani and Therrien2 what can include ventricular septal defect and bicuspid aortic valve lesions. Put it all together, there is an urgent need for models that can better predict the risk of developing IE and its complications in individual patients with adult CHD. Reference Montanaro, Dimopoulos and Shore24
A study of Bauer et al. showed also important knowledge gaps regarding infective endocarditis and antibiotic prophylaxis in adult CHD patients which highlight the importance of discussion these subjects during regular clinical contacts. Reference Bauer, Helm and Diller5 Patients with ventricular septal defect, bicuspid aortic valve and other well-known high-risk lesions should be targeted for tailored intensified medical surveillance and educational counselling on proper daily dental and skin care, and regarding signs or symptoms of IE. Reference Verheugt, Uiterwaal and van der Velde1
Concerning the microbiological characteristics of infective endocarditis, although the incidence of staphylococcal disease and health-care associated infection are increasing, in our cohort streptococcus is still the leading causative agent in CHD patients, as it was showed in a United Kingdom study. Reference Cahill, Jewell and Denne3 In 20% of cases, blood culture reports were not available. In the remaining cases, blood cultures were only positive in 79%, which suggests the early identification and collection of cultural exams prior to the onset of antibiotics is still a current problem, along with difficulty of isolating unusual organisms.
On the other hand, echocardiography is important in identifying the site of infection and related complications. Even though we reported a good diagnostic capacity of ultrasonographic methods, previous reports showed lower accuracy due to poor echo windows and frequent infections outside the heart, as on shunts, collaterals and conduits, that are difficult to demonstrate by transthoracic or transesophageal echocardiography. Reference Li and Somerville15,Reference Niwa, Nakazawa, Tateno, Yoshinaga and Terai17 Other imaging methods as 18F-FDG-PET/CT could be a useful diagnostic tool in the complex group of adult patients with CHD. Reference Pizzi, Dos-Subirà and Roque25
Regarding infective endocarditis treatment, no specific recommendations has been made for surgical indication in CHD, but it is usually required in over one-third of cases. Reference Tutarel, Alonso-Gonzalez and Montanaro12,Reference Murakami, Niwa, Yoshinaga and Nakazawa26 ‘Removing’ the cardiac lesion could be indicated as soon as infective endocarditis is cured, because of possible recurrence after the first episode. Reference Di Filippo14 However, and according to what we observed (less surgical approach in cyanotic patients), surgical repair is not an option in complex heart disease, and these patients remain lifelong exposed to infective endocarditis risk.
Limitations
This was a retrospective study from a single centre with limited sample size and with a significant proportion of missing data, mostly in the first years of follow-up that did not have computerised data. The conduction of the study at a tertiary congenital centre may have also resulted in selection or referral bias.
Conclusion
In an adult CHD cohort, infective endocarditis was more frequent in patients with non-corrected native lesions, particularly those with ventricular septal defect and bicuspid aortic valve, which contradicts the current European guidelines that excludes them from prophylaxis. Elevated risk of infective endocarditis in patients with CHD is therefore mediated by not only corrective surgery, prostheses and/or conduits implantations, but also by the uncorrected shunts or stenotic lesions present. Surgical treatment is often necessary and mortality remains substantial, however lower than described in general population, and was associated with heart failure and a non-surgical approach. Prevention of this serious complication should be one of the major tasks in the care of adults with CHD.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.








