Developmental psychopathology theories emphasize the importance of examining brain function to explain individual differences in the development of psychopathology (Cicchetti & Blender, Reference Cicchetti and Blender2006). Despite emerging evidence linking brain development to adverse experiences (i.e., deviations from the species-expectant experiences, Sapolsky, Reference Sapolsky2021) in the family environment, such as low socioeconomic status (SES; Farah, Reference Farah2017) and maltreatment (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021), little is known about the role of brain development in the long-term pathways connecting the family environment to later psychopathology. In the current longitudinal investigation, we examined whether neurocognitive functioning during adolescence specifically, functional brain activation underlying cognitive control explains the prospective link between adverse experiences in the family environment (i.e., child maltreatment, socioeconomic disadvantage) and psychopathology in young adulthood (i.e., internalizing and externalizing symptomatology).
By age 18, more than half of adults in the United States have experienced at least one type of adversity (Kessler et al., Reference Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky, Aguilar-Gaxiola, Alhamzawi, Alonso, Angermeyer, Benjet, Bromet, Chatterji, de Girolamo, Demyttenaere, Fayyad, Florescu, Gal, Gureje, Haro, Hu, Karam, Kawakami, Lee, Lépine, Ormel, Posada-Villa, Sagar, Tsang, Üstün, Vassilev, Viana and Williams2010; Merrick et al., Reference Merrick, Ford, Ports and Guinn2018). Individuals with adverse experiences are more prone to develop psychopathology (Hughes et al., Reference Hughes, Bellis, Hardcastle, Sethi, Butchart, Mikton, Jones and Dunne2017; Norman et al., Reference Norman, Byambaa, De, Butchart, Scott, Vos and Tomlinson2012). Yet, our understanding remains vastly insufficient regarding how adverse experiences may alter neurobiological trajectories that confer vulnerability to psychopathology. Here, we present new longitudinal data to investigate how two prominent types of adverse experiences in the family environment i.e., child maltreatment and socioeconomic disadvantage shape neurocognitive development across adolescence. Adolescence is a crucial developmental period to investigate cognitive control, as the prefrontal cortex, involved in high-level cognitive functioning such as cognitive control, is still developing. Further, we investigate whether altered neurocognitive functioning following adverse experiences predicts later psychopathology during young adulthood.
Developmental psychopathology models of the effects of adverse experiences
In studying the effects of adverse experiences, we follow a developmental psychopathology framework, which underscores the value of incorporating neurobiological mechanisms through multiple levels of analysis in research on psychopathology (Cicchetti & Blender, Reference Cicchetti and Blender2006). Of particular importance, Cicchetti (Reference Cicchetti2016) proposed that behavioral and biological factors each make unique contributions to resilience against psychopathology. Resilience is viewed as a dynamic developmental process encompassing positive adaptation despite exposure to significant adversity (Luthar & Cicchetti, Reference Luthar and Cicchetti2000). From the resilience viewpoint, brain plasticity throughout the lifespan can be expected because the brain is a mutable, self-organizing system, guided by self-regulatory mechanisms (Cicchetti & Tucker, Reference Cicchetti and Tucker1994).
There are several predominant perspectives in the current literature that address how adversity affects the developing brain. First, the cumulative stress approach is rooted in stress physiology and emphasizes the similarities of childhood adversity effects. This perspective argues that physiological stress responses in the brain are not specific to particular adverse experiences (e.g., poverty, abuse, neglect) but instead generalize to broad adversity effects, suggesting a common stress-related mechanism across early experiences (Pollak & Wolfe, Reference Pollak and Wolfe2020; Sapolsky, Reference Sapolsky2017). In practice, this approach emphasizes the high prevalence of co-occurring adversity types and focuses on the total number of adversities rather than each specific adversity separately (Hughes et al., Reference Hughes, Bellis, Hardcastle, Sethi, Butchart, Mikton, Jones and Dunne2017; Smith & Pollak, Reference Smith and Pollak2020).
Second, the dimensional model of adversity and psychopathology argues that threat and deprivation are distinct central dimensions of adversity that have unique influences on neurobiological development (Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). According to this model, deprivation refers to experiences that are expected but do not occur, encompassing omissions in caregiving, such as child neglect (Rogosch & Cicchetti, Reference Rogosch and Cicchetti1994), as well as poverty. In contrast, threat pertains to experiences that occur in unconventional or harmful ways, encompassing commissions in caregiving, such as child abuse (Rogosch & Cicchetti, Reference Rogosch and Cicchetti1994), as well as community violence.
More recently, research on adversity effects has paid increasing attention to robust, unique effects driven by interpersonal types of adversity compared to other non-interpersonal types of adversity (DeJoseph et al., Reference DeJoseph, Sifre, Raver, Blair and Berry2021; Dennison et al., Reference Dennison, Rosen, Sambrook, Jenness, Sheridan and McLaughlin2019; Font & Maguire-Jack, Reference Font and Maguire-Jack2020; Lawson et al., Reference Lawson, Camins, Wisse, Wu, Duda, Cook, Gee and Farah2017). The powerful impact of interpersonal adversity that impedes healthy development is expected, considering the critical role of social relationships in the survival and healthy development of human beings. For example, Vannucci et al. (Reference Vannucci, Fields, Hansen, Katz, Kerwin, Tachida, Martin and Tottenham2023) used a meta-analytic approach to examine the effects of early adversity on brain structures among adversity-exposed youths from birth to 18 years. Differential effects between interpersonal adversity (e.g., family-based maltreatment) and socioeconomic disadvantage (e.g., poverty) emerged, suggesting that child maltreatment has distinct effects from covarying factors such as socioeconomic disadvantage because it involves close interpersonal relationships. As such, child maltreatment is further distinguished from socioeconomic disadvantage by its ecological proximity to the child, with maltreatment directly shaping the child’s immediate environment, whereas economic disadvantage represents a more distal context.
A prior study compared the cumulative effects of maltreatment versus the dimension-specific effects of maltreatment (abuse representing threat and neglect representing deprivation) relevant to adolescent neurocognitive functioning (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021). The current investigation seeks to elucidate the differential effects of child maltreatment (representing adverse experiences with interpersonal nature and close proximity) versus socioeconomic disadvantage (representing adverse experiences with non-interpersonal nature in a more distal ecological context) on neurocognitive functioning.
The effects of maltreatment on cognitive control
Maltreatment effects on brain development are well documented (Cicchetti & Toth, Reference Cicchetti, Toth and Cicchetti2016). Latent vulnerability theory (McCrory et al., Reference McCrory, Gerin and Viding2017) offers a useful theoretical framework to explain the effects of maltreatment on the developing brain. It proposes that neurocognitive functioning is calibrated to neglectful or abusive environments and the resulting altered neurocognitive functioning serves as a latent mechanism that confers vulnerability to psychopathology. One important stipulation of this theory is that neurocognitive adaptations to neglectful or abusive environments occur to protect or benefit the individual within the maladaptive context. However, such adaptations come with a long-term cost because they make it more difficult to negotiate the demands of normative non-abusive environments (McCrory et al., Reference McCrory, Gerin and Viding2017). This idea is consistent with the stress-acceleration hypothesis (Callaghan & Tottenham, Reference Callaghan and Tottenham2016) and empirical evidence on a link between early adverse experiences and more rapid brain maturation.
Neural processes affected in individuals with a history of childhood maltreatment are predominantly in frontolimbic networks including the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, hippocampus, and amygdala (Gee, Reference Gee2021). A few functional neuroimaging studies have examined effects specifically on the cognitive control system using inhibitory control tasks. Findings indicate that youths who experienced neglect and/or abuse in early life demonstrated heightened activation in the prefrontal regions that have been linked to inhibitory control and conflict/error processing (Bruce et al., Reference Bruce, Fisher, Graham, Moore Iii, Peake and Mannering2013; Jenness et al., Reference Jenness, Peverill, Miller, Heleniak, Robertson, Sambrook, Sheridan and Mclaughlin2020; Lim et al., Reference Lim, Hart, Mehta, Simmons, Mirza and Rubia2015; Mueller et al., Reference Mueller, Maheu, Dozier, Peloso, Mandell, Leibenluft, Pine and Ernst2010). Additionally, a recent meta-analysis reported that maltreatment experiences of threat and deprivation were both found to be associated with reduced executive functioning (Johnson et al, Reference Johnson, Policelli, Li, Dharamsi, Hu, Sheridan, McLaughlin and Wade2021). However, there is little work examining maltreatment effects on brain activation during cognitive control. In our data, we previously reported that a history of abuse was related to accelerated neurodevelopment of the cognitive control system during middle adolescence (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021), which is in line with the stress-acceleration hypothesis and empirical work suggesting stress-related accelerated maturation of emotion brain systems during adolescence (Callaghan & Tottenham, Reference Callaghan and Tottenham2016). Still, the long-term consequences of such stress-accelerated maturation are unknown (Mclaughlin et al., Reference McLaughlin, Weissman and Bitrán2019). The present study clarifies the sequelae of stress-acceleration effects of maltreatment on the adolescent brain, and whether those variations predict later psychopathology in young adulthood.
The effects of socioeconomic disadvantage on cognitive control
Developmental neuroscience research shows that neurodevelopment is influenced by family SES, with low SES or the lack of available material and social resources serving as a prominent source of stress (Farah, Reference Farah2017). It has been proposed that low SES invokes stress-related scarcity (i.e., a sense of having more needs than resources) that in turn increases focus on immediate pressing needs instead of other goals and long-term consequences, and that this can result in impaired cognitive control (Mullainathan & Shafir, Reference Mullainathan and Shafir2013). Although neuroimaging studies have shown that SES plays an important role in the development of cognitive control-related brain regions (see Rakesh & Whittle, Reference Rakesh and Whittle2021 and Rakesh et al., Reference Rakesh, Whittle, Sheridan and McLaughlin2023 for reviews), most prior neuroimaging studies are primarily focused on brain structure, with few examining brain functioning during cognitive control tasks. One task-based functional imaging study suggested that pre-adolescents with lower SES exhibited less efficient cognitive control processing indicated by increased activation in frontoparietal regions (Spielberg et al., Reference Spielberg, Miller, Heller and Banich2015). Additionally, data from our lab suggest that lower SES during early adolescence predicted weaker dorsal anterior cingulate cortex-insula connectivity assessed during a cognitive control task across adolescence (Lindenmuth et al., Reference Lindenmuth, Chen, Lee, Brieant, Lee, Noble, Casas and Kim-Spoon2024). These results indicate less efficient cognitive control development among adolescents from low SES families. Given that the dorsal anterior cingulate cortex plays a crucial role in conflict monitoring and self-regulatory processes, the findings highlight the long-term detrimental impact of socioeconomic disadvantage on neurocognitive development during adolescence.
Similarly, studies examining associations between SES (and related constructs such as neighborhood safety) and measures of brain structure and resting-state functional activation have documented that material deprivation and low SES are consistently associated with accelerated cortical thinning in frontoparietal regions, faster developmental trajectories of gray matter structure, and less efficient activity in executive function networks (see Pollak & Wolfe, Reference Pollak and Wolfe2020; Rakesh & Whittle, Reference Rakesh and Whittle2021 for reviews). However, most past studies are limited due to their cross-sectional designs, leaving an important gap in knowledge regarding longitudinal effects of SES on developmental changes in brain regions related to cognitive control.
To date, there has been no direct investigation comparing the effects of maltreatment and socioeconomic disadvantage on adolescent neurodevelopment related to cognitive control. However, there is preliminary evidence that alludes to differential effects of maltreatment versus socioeconomic disadvantage on neurocognitive functioning more broadly. For example, in a sample of adolescents, lower parent education (a key indicator of SES) but not abuse experience was associated with less efficient recruitment of the prefrontal cortex during a task assessing working memory, a part of executive functioning closely related to cognitive control (Sheridan et al., Reference Sheridan, Peverill, Finn and McLaughlin2017). Further, in a sample of children and adolescents, those who experienced material deprivation (i.e., food insecurity), but not emotional deprivation or trauma exposure, exhibited significant reductions in frontostriatal white matter integrity and showed lower performance in reward processing (Dennison et al., Reference Dennison, Rosen, Sambrook, Jenness, Sheridan and McLaughlin2019). Taken together, current literature indicates that adverse experiences such as child maltreatment and socioeconomic disadvantage contribute to neurocognitive development, which may be a particularly important mechanism linking adverse experiences to psychopathology development.
Role of cognitive control in the development of psychopathology
Cognitive control impairment is observed in many forms of psychopathology. Cognitive control variations in those with psychopathology are evident in the frontal-cingulate-parietal-insular (i.e., “multiple demand”) network (Duncan, Reference Duncan2010). This network forms a common functional substrate undergirding successful adaptation both to diverse cognitive processing demands as well as adaptive cognitive functioning (see McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017 for a review). McTeague et al., also highlighted disruption of a cognitive control network evident transdiagnostically, including those that are encompassed by broader dimensions such as externalizing and internalizing syndromes. Evidence suggests an important role of cognitive control in the development of both internalizing and externalizing symptomatology in adolescence (Brieant et al., Reference Brieant, King-Casas and Kim-Spoon2022; Quach et al., Reference Quach, Tervo-Clemmens, Foran, Calabro, Chung, Clark and Luna2020).
From a neuroscience point of view, the prefrontal cortex is instrumental to cognitive control, a higher-order cognitive ability involving the flexible regulation of behavior to override an inappropriate response (Crone & Steinbeis, Reference Crone and Steinbeis2017; Luna, Reference Luna2009). Current neurobiological theories suggest that the prefrontal cortex continues to develop across adolescence and into the mid-to-late 20s (Casey, Reference Casey2019). This developmental pattern is reflected in improvements in cognitive control abilities. Specifically, studies using growth curve modeling report a linear increase in cognitive control abilities throughout adolescence, paralleled by lower activation in the prefrontal cortex during cognitive control tasks (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Elder, Lee, Deater-Deckard and King-Casas2021; Ordaz et al., Reference Ordaz, Foran, Velanova and Luna2013). This gradual and prolonged developmental process presents a long period of time for environmental factors to influence prefrontal cortex development (Sapolsky, Reference Sapolsky2017). Thus, cognitive control and prefrontal cortical functioning may be particularly salient pathways through which adversity has its effects during adolescence and young adulthood.
The present study
Although child maltreatment and socioeconomic disadvantage tend to co-occur, experiences of these two types of adversity appear to be distinct enough to differentially influence neurodevelopment (see Vannucci et al., Reference Vannucci, Fields, Hansen, Katz, Kerwin, Tachida, Martin and Tottenham2023). To date, no prospective longitudinal study has simultaneously examined how maltreatment and socioeconomic disadvantage may be differentially related to brain development throughout adolescence, let alone how any such changes pertain to subsequent emergence of psychopathology in the transition to adulthood. To address these key limitations, the current longitudinal study utilized a well-characterized sample with a wide range of SES and maltreatment experiences to examine how adversity alters brain functioning underlying cognitive control in adolescence to predict psychopathology in young adulthood. We performed path analyses using structural equation modeling to test longitudinal mediation models in which two prominent types of adverse experiences in the family environment (maltreatment and socioeconomic disadvantage) are linked with cognitive control development during adolescence, which in turn contributes to later psychopathology (internalizing and externalizing symptomatology) during young adulthood.
Method
Participants
Participants were 167 adolescents (52.7% male) from a longitudinal study who were recruited from rural, suburban and urban communities in southeastern/Appalachian states in the U.S. Adolescents were aged 13 to 14 (M = 14.07, SD = 0.54) when they first participated (“age 14” hereafter). About 78% of adolescents identified as White, 14% as Black or African-American, 6% as more than one race, 1% as American Indian or Alaska Native, and 1% as Asian. At age 14, the median household income of the sample ranged from $35,000–$49,999. Based on an income-to-needs (ITN) ratio (the level of household income divided by the poverty threshold for family size), 25% of the sample were “poor” (ITN < 1), 22% were considered “near poor” (ITN < 2), and 52% “non-poor” (ITN ≥ 2).
Exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for > 10 minutes, orthodontia impairing image acquisition, and contraindications to magnetic resonance imaging. At the study’s outset, during 2014, 157 adolescents were recruited and additional 10 adolescents were recruited in 2015 for a final sample of 167. The current analyses used cognitive control data collected at ages 14 (n = 157) and 17 (n = 150), as well as psychopathology data collected at ages 21 and 22 (n = 129). SES data were collected at age 14, and data on maltreatment during ages 1–13 were collected at ages 21 and 22 (n = 138). Multivariate GLM analyses indicated that there were no significant differences in any predicted variables (i.e., cognitive control and psychopathology) based on demographic variables (adolescent sex, race, ITN), or participation rate (all ps > .099), thus they were not included in the model as covariates.
Procedures
Participants were recruited by advertisement methods including flyers, recruitment letters, and email. Adolescents and their primary caregivers (“parents” hereafter) visited the laboratory to complete behavioral measures and MRI scans and were compensated for their participation. All adolescent participants provided written assent and parents provided written consent. All procedures were approved by the University’s Institutional Review Board.
Measures
Maltreatment
The Maltreatment and Abuse Chronology of Exposure questionnaire (Teicher & Parigger, Reference Teicher and Parigger2015) was used to assess the severity of exposure to different types of maltreatment from age 1 to age 13. At ages 18 and 19, adolescents reported retrospectively on whether they experienced events related to abuse and neglect during each year of childhood, committed by caregiver figures with the exception of sexual abuse for which perpetrators included caregiver figures, adults not living in the house, and peers. We created a composite maltreatment variable (using the maximum scores reported across ages 18 and 19) by averaging six subscales: sexual abuse, physical abuse, verbal abuse, non-verbal abuse, emotional neglect, and physical neglect. These subscale scores were scaled using an algorithm provided by Teicher and Parigger (Reference Teicher and Parigger2015), with higher scores indicating higher maltreatment. Previous research has demonstrated good to excellent test-retest reliability for all six maltreatment subtypes (Teicher & Parigger, Reference Teicher and Parigger2015).
Socioeconomic status (SES)
Parents reported on their and their spouse’s years of schooling, their total family income ($0/month, less than $1,000/month, $1,000–$2,999/month, $3,000–$4,999, $5,000–$7,499, $7,500–$9,999, $10,000–$14,999, $15,000–$19,999, $20,000–$24,999, $25,000–$34,999, $35,000–$49,999, $50,000–$74,999, $75,000–$99,999, $100,000–$199,999, or $200,000 or more), and the total number of individuals in the household. This information was used to calculate the family income-to-needs (ITN) ratio. Family SES at age 14 was computed by averaging the parents’ years of education (averaged between two parents whenever applicable) and the family ITN ratio.
Internalizing and externalizing symptomatology
The Adult Self-Report (ASR; Achenbach & Rescorla, Reference Achenbach and Rescorla2003) was used to assess internalizing and externalizing symptomatology at age 21 and age 22. Participants responded to questions regarding internalizing symptomatology (i.e., withdrawn, anxious/depressed, and somatic complaints) and externalizing symptomatology (i.e., aggression, rule-breaking and intrusive behaviors). Participants responded on a 3-point scale ranging from 0 = not true to 2 = very true or often true. Mean internalizing and externalizing symptomatology scores were computed by averaging participants’ T-scores from age 21 and age 22, with higher scores indicating higher symptomatology. Reliability (averaged across ages 21–22) in the current sample was α = .94 for internalizing symptomatology and α = .86 for externalizing symptomatology.
Cognitive control
Task and behavioral cognitive control
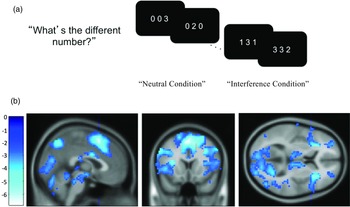
The Multi-Source Interference Task (MSIT; Bush et al., Reference Bush, Shin, Holmes, Rosen and Vogt2003) requires detecting and responding to conflicts associated with cognitive interference and was used to measure neural and behavioral cognitive control at age 14 and age 17. Adolescents completed the task while undergoing a functional MRI scan. The MSIT presents three numbers and requires participants to respond with the number that is different from the other two. The target’s identity was congruent with the target’s relative position in the neutral condition, whereas the target’s identity did not match its relative position in the interference condition (see Fig. 1a). There are 96 neutral conditions and 96 interference conditions. For behavioral cognitive control, the intraindividual standard deviation (ISD; MacDonald et al., Reference MacDonald, Karlsson, Rieckmann, Nyberg and Backman2012) of each participant’s reaction time for correct responses in the interference conditions was used, with higher scores indicating worse cognitive control.

Figure 1. Schematic display of the multi-source interference task (MSIT) and activation maps showing significant changes in activation for the interference-neutral contrast. ( a) Adolescents were instructed to identify the different digit while ignoring its position. (b) Map showing a significant negative linear relationship between the time points and the interference effect on BOLD using the Sandwich Estimator Toolbox. Displayed using voxel-wise false discovery rate corrected threshold of p < .05 and gray matter mask.
fMRI data acquisition and analysis
The MRI data were obtained using a 3T Siemens Trio scanner equipped with a standard 12-channel head matrix coil. Functional MRI data were collected using an echo-planar imaging sequence with the following parameters: FoV = 220x220 mm, slice thickness = 4 mm, 34 axial slices, flip angle = 90 degrees, TR = 2 s, TE = 30 ms, voxel size = 3.4x3.4x4 mm, 64x64 grid, and slices hyper-angulated at 30 degrees from the anterior-posterior commissure. High-resolution structural images were acquired using a magnetization prepared rapid acquisition gradient echo sequence, with the following parameters: field of view (FoV) = 245x245 mm, repetition time (TR) = 1200 ms, echo time (TE) = 2.66 ms, and 192 slices at a spatial resolution of 1x1x1 mm. The imaging data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). To account for head motion, functional scans underwent rigid body realignment with six motion parameters. The mean functional image was then coregistered to the anatomical image, normalized to the MNI template, and smoothed using a 6 mm full-width-half-maximum Gaussian filter. Low-frequency signals were removed using a high-pass filter with a cutoff of 0.006 Hz (168 s) to capture the expected signal (Henson, Reference Henson, Penny, Friston, Ashburner, Keibel and Nichols2007).
Neural cognitive control
Analyzing preprocessed MRI data, interference and neutral blocks were modeled in the General Linear Model (GLM) using boxcars convolved with the canonical haemodynamic response function, with six motion regressors included. This was done in SPM8 at a first-level analysis. Additionally, framewise displacement (FD) was calculated from the translational realignment parameters (Power et al., Reference Power, Barnes, Snyder, Schlaggar and Petersen2012; Siegel et al., Reference Siegel, Power, Dubis, Vogel, Church, Schlaggar and Petersen2014). Volumes with FD > 0.9 mm were censored by adding a volume-specific regressor for each scrubbed volume in the GLM, utilizing a frame censoring approach that proved beneficial for repeated measures data analysis. For each GLM, an interference greater than neutral contrast map was generated by subtracting the neutral beta map from the positive beta map. These contrast maps were then entered into four second-level GLMs in SPM8 at each time point, using root mean frame displacement as a regressor non-interest. First-level contrast maps were entered into a longitudinal group-level model using the Sandwich Estimator Toolbox version 2.1.0 (Guillaume et al., Reference Guillaume, Hua, Thompson, Waldorp and Nichols2014), with root mean frame displacement as a no-interest regressor to account for age-related changes in within-scanner head motion (Satterthwaite et al., Reference Satterthwaite, Wolf, Loughead, Ruparel, Elliott, Hakonarson, Gur and Gur2012). Regions of interest were defined around peaks of time point related change in BOLD responses using cluster-derived masks with a cluster defining voxel-wise false discovery rate corrected threshold of p < 1e-5 and using a gray matter mask.
As we reported in Kim-Spoon et al., (Reference Kim-Spoon, Herd, Brieant, Elder, Lee, Deater-Deckard and King-Casas2021), we found significant linear decreases from age 14 to age 17 in the interference effect on BOLD responses in frontoparietal cognitive control regions identified by the MSIT. The finding indicated that the magnitude of frontoparietal activation during cognitive control decreased as adolescents’ brains matured with age. The SwE derived map of time-related changes in BOLD was used to identify these seven clusters of interest for ROI analyses, including bilateral insula, bilateral middle frontal gyrus, left pre-supplementary motor area, left inferior parietal lobule, and right precuneus (see Fig. 1b; for coordinates for peak regions within each time point, see Supplementary Tables S1–2). To test our hypothesized mediation models, we used “frontoparietal” latent factor scores at age 14 and age 17 that were derived from the longitudinal confirmatory factor analysis of those seven ROIs (see Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Elder, Lee, Deater-Deckard and King-Casas2021 for details).
Data analysis plan
We estimated the hypothesized longitudinal mediation models using Mplus version 8.1 (Muthén & Muthén, Reference Muthén and Muthén1998–2017). Full information maximum likelihood was used to use all participants’ available data regardless of the patterns of missing data (Arbuckle, Reference Arbuckle, Marcoulides and Schumacker1996), given its superiority to listwise deletion or other ad hoc methods (Schafer & Graham, Reference Schafer and Graham2002). Cognitive control at age 14 and age 17 were included as serial mediators that link the predictors of maltreatment (age 1–13) and SES (age 14) and the outcomes of internalizing symptomatology and externalizing symptomatology at ages 21–22. Two models were tested separately for neural cognitive control and behavioral cognitive control. Indirect effects were estimated via maximum likelihood estimation with bootstrap confidence intervals (CIs), using 10,000 bootstrapping samples (Preacher & Hayes, Reference Preacher and Hayes2008). Confidence intervals not including 0 indicate statistically significant indirect paths. All estimated models were fully saturated (i.e., all possible paths estimated; RMSEA = .00, CFI = 1.00, χ 2 = 0, df = 0, p = 0).
Results
Descriptive statistics and bivariate correlations between the main study variables are presented in Table 1.
Table 1. Descriptive statistics and correlations of SES, maltreatment, cognitive control, and internalizing and externalizing symptomatology

Note. SES = socioeconomic status. Internalizing and externalizing symptomatology scores are mean scores across ages 21 − 22. *p < .05; **p < .01; ***p < .001.
Neural cognitive control
Figure 2 presents summarized model results with standardized estimates for the path analysis model with neural cognitive control as a mediator. Higher maltreatment at age 1–13 (b = 1.29, SE = 0.58, p = .027) and higher frontoparietal activation (i.e., lower cognitive control) at age 14 (b = 3.10, SE = 1.23, p = .012) predicted higher internalizing symptomatology at age 21–22. Further, higher maltreatment at age 1–13 predicted higher frontoparietal activation (i.e. lower cognitive control) at age 14 (b = 0.11, SE = 0.05, p = .022). Those with higher frontoparietal activation at age 14 showed higher frontoparietal activation at age 17 (b = 0.33, SE = 0.06, p < .001). Lastly, there was a significant, negative effect of frontoparietal activation at age 17 (after controlling for frontoparietal activation at age 14) on internalizing symptomatology at age 21–22 (b = −3.74, SE = 1.59, p = .019), indicating that smaller changes in neural cognitive control from age 14 to age 17 predicted higher internalizing symptomatology at age 21-22. Similarly, there was a significant direct effect of maltreatment from age 1–13 on externalizing symptomatology at age 21–22 (b = 1.03, SE = 0.50, p = .038) and a significant direct effect of frontoparietal activation at age 14 on externalizing symptomatology at age 21–22 (b = 2.33, SE = 1.02, p = .023). The results suggest that higher maltreatment in childhood and lower neural cognitive control in early adolescence predicted higher externalizing symptomatology in young adulthood.

Figure 2. Path analysis model of longitudinal associations among SES, maltreatment, neural cognitive control, and internalizing and externalizing symptomatology. All estimates are standardized, significant paths are in boldface. SES = socioeconomic status. *p < .05; **p < .01; ***p < .001.
With respect to indirect effects, there were significant indirect effects of maltreatment at age 1-13 on both internalizing symptomatology at age 21–22 (95% CI [0.05, 0.85]) and externalizing symptomatology at age 21–22 (95% CI [0.03, 0.65]) mediated through neural cognitive control at age 14. As such, higher levels of maltreatment in childhood predicted worse neural cognitive control in early adolescence, which, in turn, predicted higher levels of externalizing and internalizing symptomatology in young adulthood. Importantly, there was a significant indirect effect of maltreatment at age 1–13 on internalizing symptomatology at age 21–22 mediated through neural cognitive control at age 14 and neural cognitive control at age 17 (95% CI [−0.37, −0.02]). Specifically, higher maltreatment at age 1–13 predicted higher frontoparietal activation at age 14, which was positively associated with frontoparietal activation at age 17, which in turn predicted lower levels of internalizing symptomatology at age 21–22.
Behavioral cognitive control
Figure 3 presents summarized model fitting results with standardized estimates for the path analysis model with behavioral cognitive control as a mediator. There was a significant direct effect of SES on behavioral cognitive control at age 14 (b = −0.01, SE = 0.00, p = .006), and of behavioral cognitive control at age 14 on behavioral cognitive control at age 17 (b = 0.44, SE = 0.09, p < .001). These results suggest that lower SES predicted higher ISD scores, which indicates lower behavioral cognitive control. Additionally, there were significant direct effects of maltreatment at age 1–13 on internalizing symptomatology (b = 0.15, SE = 0.06, p = .019) and externalizing symptomatology (b = 0.13, SE = 0.06, p = .025) at age 21–22. The indirect effect of SES on behavioral cognitive control at age 17 via behavioral cognitive control at age 14 was significant (95% CI [−0.01, −0.001]). Higher SES at age 14 was associated with better behavioral cognitive control at age 14, which was associated with better behavioral cognitive control at age 17. There were no significant effects of behavioral cognitive control at age 14 or age 17 on internalizing symptomatology or externalizing symptomatology at age 21–22.

Figure 3. Path analysis model of longitudinal associations among SES, maltreatment, behavioral cognitive control, and internalizing and externalizing symptomatology. All estimates are standardized, significant paths are in boldface. SES = socioeconomic status. *p < .05; **p < .01; ***p < .001.
Discussion
We examined whether two prominent types of adversity in the family environment maltreatment and socioeconomic disadvantage are differentially associated with neurodevelopmental functioning of cognitive control in adolescence to predict later psychopathology outcomes in young adulthood. Our data suggest that although adverse experiences of maltreatment and socioeconomic disadvantage may engender similar developmental consequences such as altered neurocognitive functioning (i.e., equifinality, Cicchetti, Reference Cicchetti, Lamb, Freund and Lerner2010), the extent and nature of their effects on neurocognitive functioning are different, which can bring forth different types of psychopathology (i.e., multifinality, Cicchetti, Reference Cicchetti, Lamb, Freund and Lerner2010). In our models, maltreatment, but not socioeconomic disadvantage, was a significant longitudinal predictor for psychopathology. In addition to the direct effects of maltreatment in childhood on internalizing and externalizing symptomatology in young adulthood, maltreatment had significant indirect effects through the level of cognitive control neural activation in early adolescence. Further, maltreatment had significant indirect effects on internalizing symptomatology through changes in cognitive control neural activation from early to late adolescence. In contrast, for cognitive control behavioral performance, socioeconomic disadvantage assessed in early adolescence was a significant longitudinal predictor for cognitive control in late adolescence, mediated through behavioral cognitive control in early adolescence.
In the current sample, frontoparietal activation decreased as adolescents’ behavioral cognitive control improved from ages 14 to 17 years. The observed decreases in frontoparietal activation are consistent with prior research demonstrating age-related decreases in brain activation during cognitive control, reflecting more optimal neural processing with development (Crone & Steinbeis, Reference Crone and Steinbeis2017; Luna et al., Reference Luna, Padmanabhan and O’Hearn2010). When examining the effects of maltreatment and socioeconomic disadvantage on psychopathology through neural cognitive control, maltreatment (but not socioeconomic disadvantage) was associated with less optimal neural processing of cognitive control in early adolescence, which in turn predicted higher internalizing and externalizing symptomatology in young adulthood. This result suggests that the detrimental effects of maltreatment during childhood may result in the delayed development of neural cognitive control in early adolescence. It extends findings from prior cross-sectional findings of maltreatment and prefrontal activation during cognitive control based on small samples of individuals with severe maltreatment (e.g., Bruce et al., Reference Bruce, Fisher, Graham, Moore Iii, Peake and Mannering2013; Lim et al., Reference Lim, Hart, Mehta, Simmons, Mirza and Rubia2015; Mueller et al., Reference Mueller, Maheu, Dozier, Peloso, Mandell, Leibenluft, Pine and Ernst2010) by illustrating that neural cognitive control in early adolescence plays an important role as a latent vulnerability factor heralding the development of psychopathology over time.
We also found long-term effects of maltreatment on psychopathology mediated through longitudinal changes in neural cognitive control. Specifically, there was a significant indirect pathway from maltreatment in childhood (ages 1-13) to greater decreases in frontoparietal activation during cognitive control from early to late adolescence (from age 14 to age 17), which, in turn, were associated with lower internalizing symptomatology in young adulthood (ages 21-22). This finding complements our previous study using growth curve modeling with the same sample, reporting that cumulative maltreatment (ages 1-17) predicted lower frontoparietal activation during cognitive control in late adolescence (age 17) as well as steeper decreases in frontoparietal activation during cognitive control across age 14 through 17 (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021). Furthermore, our finding aligns with results from a study involving 9–12-year-olds in the Adolescent Brain Cognitive Development sample. Although that study examined resting-state connectivity (not brain functioning during a cognitive control task), more negative life events were associated with more “mature” patterns of change in frontolimbic connectivity, which in turn were associated with lower internalizing symptomatology but not externalizing symptomatology (Brieant et al., Reference Brieant, Sisk and Gee2021).
As such, the current and prior relevant findings suggest maltreatment-related accelerated maturation of brain regions involved in cognitive control, thus supporting the stress-acceleration hypothesis (Callaghan & Tottenham, Reference Callaghan and Tottenham2016). Similarly, results from a meta-analysis showed patterns of age differences in the volume of frontolimbic regions indicative of stress acceleration due to interpersonal adversity, broadly aligning with our findings regarding frontoparietal activation. However, the developmental patterns of structural acceleration in this meta-analysis were evident in early years but not in adolescence (Vannucci et al., Reference Vannucci, Fields, Hansen, Katz, Kerwin, Tachida, Martin and Tottenham2023). Thus, interpersonal adversity-related stress acceleration may be observed structurally during the early years but may not manifest functionally until later in development during adolescence. It is also possible that neural acceleration following interpersonal adversity is region-specific (e.g., Vannucci et al., Reference Vannucci, Fields, Hansen, Katz, Kerwin, Tachida, Martin and Tottenham2023), with frontolimbic and frontoparietal regions exhibiting distinctive patterns and timing of adversity effects. Taken together, the current and prior relevant findings highlight the importance of considering the age of the participants, developmental timing of the adversity, and brain regions, in parsing the longitudinal effects of adversity on brain structures and functions.
It is important to simultaneously consider how early adolescent brain activation and within-person changes in activation from early to late adolescence may contribute to later psychopathology, conjointly and across time. We found that maltreatment at age 1–13 was related to higher frontoparietal activation at age 14, which may imply delayed neurodevelopment. This initial stress-related delay observed in early adolescence served as a vulnerability factor linking childhood maltreatment to young adult internalizing and externalizing symptomatology. As such, our data present longitudinal evidence supporting the latent vulnerability theory (McCrory & Viding, Reference McCrory and Viding2015) by demonstrating that changes in neurocognitive functioning, reflecting altered calibration to adverse environments, serve as vulnerability to mental health problems. However, examination of longitudinal change in neurocognitive functioning revealed additional important developmental insights. Findings revealed that accelerated neurodevelopment during adolescence served as a mediator linking childhood maltreatment to young adult psychopathology. Notably, in our data, accelerated cognitive control improvement (i.e., greater decreases in frontoparietal activation from age 14 to 17) predicted lower internalizing symptomatology. This finding suggests adolescence as a developmental window during which neural plasticity can reshape adaptive systems. It is also consistent with the observation that cognitive control skills appear to be a malleable mediator of adverse childhood experiences, responsive to preventive interventions (Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021).
The indirect link we identified between maltreatment and lower internalizing symptomatology, mediated by the faster improvement of neural cognitive control, may indicate experience-dependent neural plasticity in the development of cognitive control-related brain functions following maltreatment. As proposed by Cicchetti (Reference Cicchetti, Lamb, Freund and Lerner2010), the brain, a dynamic and self-organizing system, may attempt to compensate for the negative influences of maltreatment. At the same time, maltreated adolescents may seek out new experiences in areas where they possess strengths. In particular, during adolescence, social contexts outside the home (such as school, peer, and religious and social clubs) become increasingly influential relative to childhood, likely opening up opportunities for compensatory development. Given that experience-dependent neural plasticity is a central feature of the human brain (Cicchetti, Reference Cicchetti2016; Nelson & Gabard-Durnam, Reference Nelson and Gabard-Durnam2020), early neurobiological alterations related to maltreatment should not be considered deterministic of vulnerability to psychopathology. Instead, elucidating how the brains of youths who have experienced maltreatment develop and function resiliently can inform design and implementation of multilevel prevention and intervention approaches to redirect future behaviors and brain functioning in more positive, adaptive pathways (Cicchetti & Blender, Reference Cicchetti and Blender2006; Davidson & McEwen, Reference Davidson and McEwen2012; Luthar & Cicchetti, Reference Luthar and Cicchetti2000).
With respect to the effects of socioeconomic disadvantage, a significant indirect effect indicated that lower SES predicted lower behavioral cognitive control (indicated by higher variability in reaction time to interference) in early adolescence, which in turn was related to lower behavioral cognitive control in late adolescence. This long-term effect of socioeconomic disadvantage on cognitive control performance is consistent with a meta-analysis revealing a significant association between deprivation experiences (broadly defined as the absence of expected environmental input, including physical and emotional neglect) and poor inhibitory control performance among children and adolescents (Johnson et al., Reference Johnson, Policelli, Li, Dharamsi, Hu, Sheridan, McLaughlin and Wade2021). However, we did not find significant effects of socioeconomic disadvantage on neural activation of cognitive control, after controlling for the effects of maltreatment. A recent theoretical model posits that chronic environmental stressors such as lower SES are likely to be associated with accelerated neurodevelopment, whereas higher SES environments may be associated with decelerated neurodevelopment (Tooley et al., Reference Tooley, Bassett and Mackey2021). Accordingly, the prolonged period of plasticity in higher SES and lower stress environments may support the development of more efficient cortical networks in adulthood, whereas shortened time windows of plasticity in lower SES contexts may limit the window for optimizing cortical network segregation and efficiency. Inconsistent with this model, however, a recent review of structural development suggests that lower childhood SES is associated with delayed (as opposed to accelerated) patterns of brain structure maturation (Rakesh et al., Reference Rakesh, Whittle, Sheridan and McLaughlin2023). Future research examining the effects of socioeconomic disadvantage on the development of cognitive control-related brain functions is warranted, particularly exploring possible variations of socioeconomic disadvantage effects across developmental periods and imaging modalities.
By examining the relative effects of maltreatment and socioeconomic disadvantage, we obtained a more nuanced understanding of ways that neural versus behavioral cognitive control development is affected by different types of adversity. Our data provide support for the latent vulnerability model (McCrory & Viding, Reference McCrory and Viding2015) suggesting that early interpersonal adversity (maltreatment) predicted altered cognitive control neural functioning in adolescence, which in turn predicted later psychopathology in young adulthood. In contrast, socioeconomic disadvantage (low SES) predicted delayed cognitive control, as manifested in behavioral performance over time, from early to late adolescence. Our finding indicates more prominent effects of maltreatment over socioeconomic disadvantage in predicting psychopathology as long-term consequences of adversity, mediated through neurocognitive functioning. Relatedly, a study that tested distinctive effects between childhood maltreatment and SES on brain structure reported significant effects of childhood maltreatment, but not SES, on hippocampal volumes among young adults (Lawson et al., Reference Lawson, Camins, Wisse, Wu, Duda, Cook, Gee and Farah2017). These findings align with behavioral research suggesting that the effects of child maltreatment are distinct from those of poverty, and child maltreatment represent a more severe risk factor for adult adverse outcomes than poverty (Font & Maguire-Jack, Reference Font and Maguire-Jack2020).
The contributions of the current study should be considered in light of several limitations. First, although this study used prospective longitudinal data, the detected significant effects should not be interpreted as causal because of the correlational nature of the data. Second, maltreatment was assessed using retrospective reports at ages 18 and 19, which were close enough to childhood to provide as reliable recall as possible but could have been affected by recall bias. However, studies indicate that the concern about the unreliability of retrospective reports may be exaggerated. For example, there is no clear link between current psychiatric or mood status and less valid recall of early experiences (Brewin et al., Reference Brewin, Andrews and Gotlib1993). Further, there is evidence that retrospective self-reports of maltreatment are verifiable (Chu et al., Reference Chu, Frey, Ganzel and Matthews1999) and are related to poor behavior outcomes regardless of concordance with official records (Negriff et al., Reference Negriff, Schneiderman and Trickett2017). Third, SES was measured at age 14 as data during ages 1–13 were not available. Given declining economic mobility (i.e., the ability of families to move up or down the income ladder over time) in the US in recent decades (Chetty et al., Reference Chetty, Grusky, Hell, Hendren, Manduca and Narang2016), we believe that SES measured at age 14 would serve as a representative proxy for the history of family SES that adolescents have experienced in their lifespan. Nevertheless, we recommend that future research, especially that focuses on differential effects according to the timing of adversity, should employ SES measures assessed during the developmental periods of interest. Finally, cognitive control brain regions, particularly the frontal cortex, are the last to mature, and by definition it is the brain region least constrained by genes and most sculpted by experience (Sapolsky, Reference Sapolsky2017). Nevertheless, future research examining genetic risk or resilience factors that may interact with SES and maltreatment to contribute to neurocognitive functioning will enhance our ability to identify young people who are vulnerable or resilient to the detrimental effects of adverse experiences.
Future directions and conclusion
Reviewing the current state of research on the effects of adversity on the brain and psychopathology, a critical area for advancing the field, involves elucidating within-person developmental processes that demonstrate impairment and compensatory development following adversity. Despite increasing studies on the effects of adversity on the brain, empirical evidence on brain development (especially long-term, beyond two time points) is extremely rare. Consequently, published empirical studies and meta-analyses are almost exclusively based on cross-sectional samples of differing ages, where observations rely on age-related differences instead of within-person developmental changes in the brain. Further, interpretation of findings regarding accelerated versus delayed brain development in most prior studies is speculative (Rakesh & Whittle, Reference Rakesh and Whittle2021). This is primarily due to the absence of brain data on the within-person level and subsequent psychopathology. Hence, using longitudinal samples is critical to understanding true developmental changes in the brain and better delineating developmental sequelae of adversity. For example, in the current study, had we measured neurocognitive functioning only at age 14 or at age 17, we would have missed the role of neurocognitive development in predicting later psychopathology. We further emphasize that careful consideration of time sequencing using prospective longitudinal data is critical for modeling developmental cascades and attempting to explain mechanisms of change.
It remains to be discovered whether the neurobiological aberrations displayed by young people who have experienced adversity are reversible or not (Cicchetti, Reference Cicchetti2016). Given that the brain is a dynamic, self-organizing system that is mutable (Cicchetti, Reference Cicchetti2016) and that the prefrontal cortex is the most experience-dependent region of the brain (Sapolsky, Reference Sapolsky2017), we can expect that neural plasticity can occur to various degrees across the lifespan. Our longitudinal findings on the indirect effects of maltreatment through changes in brain functioning provide preliminary evidence illustrating that although cognitive control neural functioning is delayed in early adolescence following childhood maltreatment, there may be adaptation (i.e., “catching up,” as reflected by steeper declines in activation) during middle to late adolescence. One important direction of future research is to identify promotive factors facilitating neural plasticity of cognitive control, as well as reversibility from impaired cognitive control following adversity. In particular, research enhancing our understanding of socioenvironmental contexts (e.g., supportive social interactions; Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021) holds considerable promise for prevention and intervention efforts to improve the healthy development among young people faced with adversity.
The data presented here underscore the critical role of interpersonal adversity in the family environment. One fruitful direction of future research would be to examine how the effects of interpersonal adversity outside the family environment, such as peer victimization, may be similar or different from those within the family environment, such as maltreatment. According to an organizational perspective on developmental psychopathology (Cicchetti, Reference Cicchetti2016), children exposed to interpersonal adversity in the family environment are expected to be more vulnerable to experiencing interpersonal adversity outside the family environment. With respect to behavioral adaptation, negative relational patterns acquired in a maltreating home—due in part to negative expectations of others and low trust in others would impede the ability to establish positive peer relations. With respect to neurobiological adaptation, maltreated children’s stress response to the one type of adversity (e.g., maltreatment) may prepare them to face the repetition of similar types of interpersonal adversity-related stressors later in their life (e.g., peer victimization) (Sapolsky, Reference Sapolsky2021). As per the latent vulnerability hypothesis (McCrory et al., Reference McCrory, Gerin and Viding2017), however, such adaptations are anticipated to pose challenges for young people in navigating the demands of normative non-abusive social relationships.
From a sampling viewpoint, more research is needed on the effects of maltreatment on brain development, utilizing community samples of young people, given that the majority of research in the current literature is based on adult psychiatric samples (e.g., hospitalized patients, clinical samples going through interventions). This is a problem because many of these individuals suffer from comorbid mental health disorders and substance use disorders, making it difficult to distinguish the effects of maltreatment from the consequences of mental health disorders or neurotoxic effects of substances (Cicchetti, Reference Cicchetti, Lamb, Freund and Lerner2010). We note that attempting to control for these confounding factors statistically (e.g., controlling for the effects of covariates) cannot address potential bias in findings stemming from the sampling issue. From a methodological viewpoint, the majority of prior studies on adversity and brain development have focused on brain structure. More research using functional MRI (particularly task-based functional MRI) is needed to move beyond the descriptive anatomical level of structural MRI and obtain clearer insights into brain functioning underlying specific psychological phenotypes (e.g., cognitive control) that are influenced by adversity.
Using multiple levels of analyses and the incorporation of a neurobiological framework into developmental psychopathology research (Cicchetti & Blender, Reference Cicchetti and Blender2006; Cicchetti, Reference Cicchetti, Lamb, Freund and Lerner2010), we showed cognitive control brain functioning serving as contributors to the etiology (i.e., prospective proximal predictor of psychopathology), course (i.e., mediating process linking adversity and psychopathology), and sequelae of maladaptation and resilience (i.e., impaired functioning and compensatory development following adversity). Our prospective longitudinal analyses provide crucial insights into the effects of adversity on neurocognitive development, as well as the role of neurocognitive functioning in the development of psychopathology. When compromised by childhood adverse experiences such as maltreatment, neurocognitive functioning can serve as a latent vulnerability for psychopathology. Research on neural plasticity during adolescent development sheds light on the brain’s potential as a target for preventive interventions aimed at promoting resilient functioning among young people faced with adversity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579424000531.
Acknowledgements
This work was supported by a grant from the National Institute of Drug Abuse (R01 DA036017 to Jungmeen Kim-Spoon and Brooks Casas). We thank the former and current JK Lifespan Development Lab members for their help with data collection. We are grateful to adolescents and parents who participated in our study.
Competing interests
None.