Late 2019, a pandemic characterised by the rise of a novel severe acute respiratory syndrome coronavirus, namely SARS-COV-2, has spread globally not sparing a continent nor a particular age group. Reference Atzrodt, Maknojia and McCarthy1,Reference Wu, Chen and Cai2 Specifically, the associated respiratory tract infection with that virus was known more widely as COVID-19. The disease appeared to have a spectrum of severity, being critical or even lethal for the eldest and the immunocompromised. Overall, it exhibits decreasing severity when going down the age groups. Reference Wu, Chen and Cai2–Reference Merli, Perricone and Lauterio4 In effect, infections by the SARS-COV-2 were scarce in children and adolescents, whose majority did not require medical intervention. Reference Bhopal, Bagaria, Olabi and Bhopal5,Reference Feldstein, Rose and Horwitz6
In these younger age groups, the reported incidence of viral infection was the lowest, generally representing only a few percent of the total infections. Reference Zare-Zardini, Soltaninejad, Ferdosian, Hamidieh and Memarpoor-Yazdi7,Reference She, Liu and Liu8 The present literature shows little to no severity in children aged 15 years and below. Similarly, death in this age group represented an insignificant fraction of the total death caused by SARS-COV-2. Reference Bhopal, Bagaria, Olabi and Bhopal5,Reference Zare-Zardini, Soltaninejad, Ferdosian, Hamidieh and Memarpoor-Yazdi7,Reference Whittaker, Bamford and Kenny9 Although COVID-19 only followed a mild course of illness in children and adolescents, in the first quarter of 2020, many instances of cardiac and gastrointestinal complications in children were reported in addition to the respiratory ones. Patients admitted to the hospitals showed symptoms of multiple organ inflammations. Reference Radia, Williams and Agrawal3,Reference Whittaker, Bamford and Kenny9–Reference Siebach, Piedimonte and Ley11
The clinical manifestations, intriguingly mimicking that of Kawasaki disease, involve a severe immune-mediated response characterised by fever, tissue damage, and multiple organ failure. Reference Holstein10,Reference Verdoni, Mazza and Gervasoni12–Reference Pavlyshyn, Slyva, Dyvonyak and Horishna14 This condition known as the multisystem inflammatory syndrome in children (MIS-C) was first detected in late April 2020 and is known to occur several weeks after infection by SARS-COV-2. Reference Holstein10,Reference Siebach, Piedimonte and Ley11,Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15 This specific contiguity has hinted about the role of the virus as the infection agent at cause of MIS-C, possibly correlated with the response of the adaptive immune system. Reference Holstein10,Reference Verdoni, Mazza and Gervasoni12,Reference Levin16 In fact, most of the patients with MIS-C tested positive for either SARS-COV-2 antibodies, for the virus itself through reverse transcription-polymerase chain reaction (RT-PCR) testing or had a relative positive for the virus at the time of hospitalisation. Reference Feldstein, Rose and Horwitz6,Reference Holstein10,Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15,Reference Levin16 The large overlap between the clinical manifestations of Kawasaki disease and MIS-C whether they are cardiac or gastrointestinal may show that both conditions are part of one large disease spectrum, rather than two distinct entities. Reference Holstein10,Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15,Reference Levin16 Overall, MIS-C clinically expresses itself through the involvement and inflammation of more than one system, including cardiac, neurological, gastrointestinal, pulmonary, and haematologic. Reference Radia, Williams and Agrawal3,Reference Holstein10,Reference Verdoni, Mazza and Gervasoni12 This article investigates the epidemiology, pathophysiology, and clinical manifestations of MIS-C. In addition, it examines the employed treatment weapons and the overall prognosis.
Epidemiology
Children and young adults are much less susceptible to SARS-COV-2 infection and constitute only between 1 and 2% of the cases. Reference Swann, Holden and Turtle17 Clinical symptoms are absent or mild in most of these young patients. Reference Swann, Holden and Turtle17,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 In April 2020, however, the Pediatric Intensive Care Society of the United Kingdom alerted the international medical community about the emergence of a severe form of toxic shock syndrome in an increasing number of children following SARS-COV-2 infection. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 Within a few weeks, children and adolescents presenting with a multisystem inflammatory disease were reported worldwide, more frequently in regions with greater incidence of infections. Reference Verdoni, Mazza and Gervasoni12 The term MIS-C was used by the WHO to label this severe disease which was thought to be very similar to Kawasaki disease. Reference Swann, Holden and Turtle17 Later, each of the Centers for Disease Control and Prevention (CDC) and WHO has issued a case definition for MIS-C (Table 1). The reported incidence of MIS-C was diversely appreciated, ranging from 2 per 100.000 COVID-19 cases in the US study, Reference Feldstein, Rose and Horwitz6 to 0.8/1000 in the UK series, Reference Swann, Holden and Turtle17 and 1/1000 during the Bergamo, Italy outbreak. Reference Verdoni, Mazza and Gervasoni12
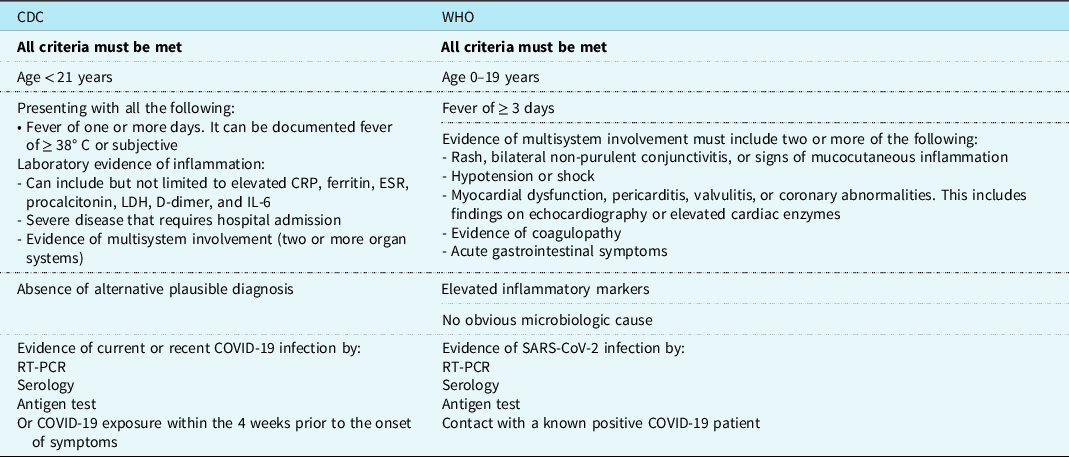
CDC = Center for Disease Control and Prevention; CRP = C-Reactive Protein; ESR = Erythrocyte Sedimentation rate; LDH = lactate dehydrogenase; RT-PCR = Reverse Transcription- Polymerase Chain Reaction; WHO = World Health Organization.
1 Organization WH. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Published 2020. Updated May 15, 2020. Accessed November 1, 2022.
2 Prevention CfdCa. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). CDC. https://www.cdc.gov/mis/mis-c/hcp/index.html#:∼:text=Patients%20with%20MIS%2DC%20usually,cases%2C%20with%20hypotension%20and%20shock. Published 2021. Updated May 20, 2021. Accessed November 1, 2022.
Children presented with MIS-C between 6 and 51 days following infection with SARS-COV-2, the median being 36 days in the French study Reference Toubiana, Poirault and Corsia19 and 25 days in a US series including 26 states. Reference Feldstein, Rose and Horwitz6 The initial COVID-19 infection was symptomatic in 27 to 43% of the cases, reported as an acute febrile or respiratory illness. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19 Between 20 and 50% of the patients reported contact with a COVID-19-positive family member. Reference Feldstein, Rose and Horwitz6,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19
Patients ranged between 6 and 20 years of age, with a median age of above 5 years in most cases. Reference Feldstein, Rose and Horwitz6,Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15,Reference Swann, Holden and Turtle17,Reference Toubiana, Poirault and Corsia19 Male preponderance was reported by all authors, with a male-to-female ratio of 2–3:1. Reference Feldstein, Rose and Horwitz6,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19 However, one unique study from the United Kingdom found no association between MIS-C and patient’s gender. Reference Swann, Holden and Turtle17 Between 57 and 66% of MIS-C patients were of Black/African or Hispanic/Latino ethnicity; Reference Feldstein, Rose and Horwitz6,Reference Swann, Holden and Turtle17–Reference Toubiana, Poirault and Corsia19 these same ethnic origins were associated with higher instances of hospitalisation, severe disease, and death. Reference Swann, Holden and Turtle17,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18
Pathophysiology
MIS-C is a newly described rare entity that consists of a severe inflammation targeting more than one organ system, characterised by a fever, multi-organ dysfunction and markedly elevated inflammatory markers. Reference Feldstein, Rose and Horwitz6,Reference Consiglio, Cotugno and Sardh20 This entity has been compared throughout the literature to Kawasaki disease, or even to an “incomplete Kawasaki disease” in practically all reports, as they both present with a wide range of symptoms related to inflammation and overlapping clinical features, without specific and reliable diagnostic tests. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Consiglio, Cotugno and Sardh20 In fact, 50% of MIS-C patients will present the complete Kawasaki disease symptom and criteria spectrum. Reference Verdoni, Mazza and Gervasoni12,Reference Toubiana, Poirault and Corsia19 In addition, Kawasaki disease was described 46 years ago, providing a well-established framework for this puzzling and similar new disease called MIS-C. Reference Kawasaki, Kosaki, Okawa, Shigematsu and Yanagawa21 Thus, it is important to describe what is currently known about the pathophysiology of both these diseases and highlight the specific features of each. Figure 1 summarises the main features of each condition.

Figure 1. Major differences between KD and MIS-C. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Gatterre, Oualha and Dupic68–Reference Burns and Glodé70 KD: Kawasaki disease.
Kawasaki disease is usually described as a medium-calibre vessel vasculitis targeting children below 5 years of age in most cases. Reference Verdoni, Mazza and Gervasoni12 The cause of Kawasaki disease is still not identified and is thought to be an autoimmune reaction, generated by an unknown pathogen in children with genetic predisposition. Reference Verdoni, Mazza and Gervasoni12,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Consiglio, Cotugno and Sardh20 In 5% of the cases, patients may develop shock and haemodynamic instability in the early phase of the disease and demonstrate a clinical picture mimicking macrophage activation syndrome. Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15 The underlying pathophysiology in MIS-C is relatively similar to that of Kawasaki disease and is increasingly suspected to also be an unregulated immune reaction triggered by the viral infection with SARS-COV-2. Reference Verdoni, Mazza and Gervasoni12,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19 However, the inflammatory reaction in MIS-C is much more intense, displaying shock in 50% of the patients, macrophage activation syndrome in 20–30%, Reference Verdoni, Mazza and Gervasoni12,Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15 and more than 90% of the patients exhibit a steep elevation of more than four markers of inflammation. Reference Feldstein, Rose and Horwitz6,Reference Consiglio, Cotugno and Sardh20 This unusual “hyperinflammatory” syndrome in MIS-C is thought to be secondary to a sudden cytokine storm and not to a direct viral-induced cell injury. Reference Toubiana, Poirault and Corsia19,Reference Consiglio, Cotugno and Sardh20 This assumption is supported by the absence of viral particles in nasopharyngeal specimen in around 30% of patients with MIS-C, and the presence of SARS-CoV-2 antibodies (IgG3, IgM, and IgA) in 80–100% of the cases. Reference Feldstein, Rose and Horwitz6,Reference Verdoni, Mazza and Gervasoni12,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18–Reference Consiglio, Cotugno and Sardh20 The cytokine storm is primarily mediated by interleukin 6 (IL-6) and IL-8. Reference Verdoni, Mazza and Gervasoni12,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18–Reference Consiglio, Cotugno and Sardh20 IL-6 was increased in virtually all patients with MIS-C and is used as a specific diagnostic test. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19 Particularly, IL-6 is increased by SARS-CoV-2 and other viruses through the amplification of IL-6 mRNA transcription, or by stabilisation of IL-6 mRNA. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18
In contrast to MIS-C, IL-1 and IL-17A are the primary cytokines driving inflammation in children with Kawasaki disease; in MIS-C, IL-17A levels are significantly lower when compared to children with Kawasaki disease, Reference Consiglio, Cotugno and Sardh20 and IL-1 levels are usually around the normal range. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19 An imbalance in both directions, between IL-17A-producing T-lymphocytes and regulatory T-lymphocytes, may be the underlying mechanism behind IL-17A levels, both in Kawasaki disease and MIS-C. Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15,Reference Consiglio, Cotugno and Sardh20
Along with the established generalised inflammatory reaction, the possibility and extent of additional direct viral cell injury is debated. In Kawasaki disease, this possibility has been disregarded during the last 2 decades. IgA-producing plasma cells and neutrophils infiltrate the arteries in Kawasaki disease and are detected within the walls of the arteries; the resulting arteritis destroys the arterial connective tissue, causing aneurysmal dilatation of the artery wall. Reference Consiglio, Cotugno and Sardh20
In contrast to Kawasaki disease, patients with COVID-19 and in one child with MIS-C, significant loads of viral particles were found within the endothelial cells of various organs; this may explain the clinical symptoms related to impaired microcirculation in severe COVID-19 illness and may also suggest a direct virus-mediated injury. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Consiglio, Cotugno and Sardh20 However, these children respond well to anti-inflammatory agents and immuno-regulatory therapies, which supports the first hypothesis of autoimmune disease regarding MIS-C. Reference Consiglio, Cotugno and Sardh20
The overlap in the pathophysiology of Kawasaki disease, COVID-19, and MIS-C is best illustrated by the research undertaken around the pathogens that triggers Kawasaki disease. A viral trigger had always been considered, including coronavirus subtypes. Reference Toubiana, Poirault and Corsia19 These studies are particularly interesting today, following the appearance of SARS-COV-2 and MIS-C. However, conflicting results were reported. Concerning the Human Coronavirus isolated in New Haven (HCoV-NH), it was detected in 8 of 11 children with Kawasaki disease in the New Haven study, Reference Takashi Ebihara, Ma, Ishiguro and Kikuta22 but in none of 19 Kawasaki disease patients in Japan. Reference Takashi Ebihara, Ma, Ishiguro and Kikuta22,Reference Turnier, Anderson, Heizer, Jone, Glode and Dominguez23 In another Japanese series, a KD-like disease was associated with another subtype of coronavirus by detecting higher antibodies directed against the HCoV-229E. Reference Verdoni, Mazza and Gervasoni12 Thus, coronaviruses will remain prime suspects for Kawasaki disease and MIS-C triggering, as SARS-COV-2 has been confirming its potential to initiate such intense inflammatory and immune reactions during the past 3 years, presenting with clinical features overlapping with Kawasaki disease. Reference Verdoni, Mazza and Gervasoni12
Genetic susceptibility is undoubtedly another important predisposing factor for both MIS-C and Kawasaki disease and is illustrated by race and gender susceptibilities. Kawasaki disease incidence is 12 times higher in Japan than in the United States. Reference Toubiana, Poirault and Corsia19 In contrast, MIS-C was found to be significantly more prevalent in African/American and Hispanic/Latino patients, with a significant increase in severe illness and mortality. Reference Swann, Holden and Turtle17,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 Both MIS-C and Kawasaki disease are more preponderant in male gender, as more than 60% of MIS-C patients are males in most series. Reference Feldstein, Rose and Horwitz6,Reference Verdoni, Mazza and Gervasoni12,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Toubiana, Poirault and Corsia19
Further, there is a striking difference in the age groups between Kawasaki disease and MIS-C. While Kawasaki disease is generally diagnosed in children below 5 years of age, the median age of patients with MIS-C is 7.5 years, ranging from 6 to 20 years in most studies. Reference Verdoni, Mazza and Gervasoni12 The reason why younger children seem to be protected against MIS-C may be a “cross-reactive” immunity: These young patients could be protected because they may have been already in contact with another coronavirus during one of their frequent respiratory tract infections. Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15 Another explanation involves a common amino-acid sequence between SARS-CoV-2 and measles and rubella viruses; thus, younger children can be protected through the measles, mumps, and rubella (MMR) vaccine. Reference Sidiq, Sabir, Ali and Kodzius24 It is also possible that the decreased efficiency of the cell surface enzyme angiotensin-converting enzyme 2 (ACE-2) confers additional protection for children between 4 and 9 years. In fact, ACE-2 is a receptor that promotes cellular infection with SARS-COV-2. Children in this age range exhibit lower gene expression of ACE-2 in the upper respiratory tract epithelium. Reference Bunyavanich, Do and Vicencio25 Finally, a last possible reason for children belonging to a very young age group to be “immune” to MIS-C is the immature immune system, leaving them unable of developing a cytokine storm, presented earlier as one of the physiological factors of MIS-C. Reference Lingappan, Karmouty-Quintana, Davies, Akkanti and Harting26
Clinical manifestations of MIS-C
The MIS-C displays a wide range of clinical features and severities (illustrated in Fig. 2). It touches several organ-systems on a full scale of presentations. Children might present in a shock and end-organ damage or might exhibit a minor illness. This is reflected by the vast difference in the initial presentation reported by the varying studies. Table 2 displays the presenting symptoms of children diagnosed with MIS-C along with their frequency, as reported by various investigations.

Figure 2. Clinical features of MIS-C.
Table 2. Clinical features of MIS-C documented by varying investigators

ARDS = acute respiratory distress syndrome; AKI = acute kidney injury; EF = ejection fraction; GI = gastrointestinal; LV = left ventricle.
1 Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of United States of America children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. Jama. 2021;325(11):1074-1087.
2 Shobhavat L, Solomon R, Rao S, et al. Multisystem inflammatory syndrome in children: Clinical features and management—Intensive care experience from a pediatric public hospital in Western India. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2020;24(11):1089.
3 Sethy G, Mishra B, Jain MK, et al. Clinical profile and immediate outcome of multisystem inflammatory syndrome in children associated with COVID-19: A multicentric study. J Glob Infect Dis. 2021;13(4):159.
4 Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. New England Journal of Medicine. 2020;383(4):347-358.
5 Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in US children and adolescents. New England Journal of Medicine. 2020;383(4):334-346.
6 Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. The Lancet Child & Adolescent Health. 2020;4(9):669-677.
7 Riollano-Cruz M, Akkoyun E, Briceno-Brito E, et al. Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience. Journal of Medical Virology. 2021;93(1):424-433.
8 Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. The Journal of clinical investigation. 2020;130(11):5942-5950.
9 Hameed S, Elbaaly H, Reid CE, et al. Spectrum of imaging findings at chest radiography, US, CT, and MRI in multisystem inflammatory syndrome in children associated with COVID-19. Radiology. 2021;298(1):E1.
10 Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology. 2020;72(5):1522-1527.
11 Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single centre experience of 44 cases. Gastroenterology. 2020;159(4):1571-1574. e1572.
12 Ciftdogan DY, Keles YE, Karbuz A, et al. Multisystem inflammatory syndrome in children associated with COVID-19 in 101 cases from Turkey (Turk-MISC study). J Paediatr Child Health. 2022;58(6):1069.
The gastrointestinal tract appears to be considerably impacted by the hyperinflammatory process of MIS-C. This is mirrored by the high occurrence of such symptoms, reported in 47–100% of children. Diffuse abdominal discomfort, diarrhoea, and vomiting are the most frequently reported symptoms, affecting up to 87% of patients. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 Paralytic ileus and ascites have also been reported. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 Notably, growing evidence depicts a subset of patients presenting with acute surgical abdomen. Though, most of these cases are in fact non-surgical and are attributed to varying other reasons such as mesenteric lymphadenitis and terminal ileitis. Nevertheless, due to the alarming initial presentation and the intense pain experienced by children, surgical interventions and exploratory laparotomies were carried in some instances. They were later discovered to be unnecessary. Reference Rouva, Vergadi and Galanakis27,Reference Belhadjer, Méot and Bajolle28 A typical presentation would be intense diffuse abdominal pain that is most severe at the right lower quadrant, which would raise high suspicion of appendicitis. However, further laboratory evaluation and imaging would reject this diagnosis. In fact, abdominal imaging has exposed a remarkable variety of findings. Air fluid level, free fluid in the abdominal cavity, ileitis, colitis, bowel wall thickening, lymphadenopathy, and mesenteric fat stranding, especially in the right iliac fossa similar to those seen in inflammatory bowel disease, are all described. Reference Sahn, Eze and Edelman29–Reference Lee, Day-Lewis and Henderson31 In addition, hepatosplenomegaly, peri-portal and pericholecystic oedema, enlargement of intra-hepatic bile ducts, and gall bladder wall thickening have also been detected. Reference Sethuraman, Kannikeswaran and Ang32,Reference Palabiyik, Akcay, Sevketoglu, Hatipoglu, Sari and Inci33 Despite most cases being non-surgical, complications requiring surgical intervention might occur in MIS-C patients. Evidence of venous microthrombi and necrotic lymphadenitis has been discovered. Reference Sahn, Eze and Edelman29 Intra-surgical pathologies revealed intestinal necrosis that required segmental resection. Reference Khesrani, Sadar, Dahdouh, Ladjadj and Bouguermouh34 Indeed, one study tried to assess the difference in laboratory values between patients who required surgical intervention and those who presented with acute non-surgical abdomen, in an attempt to minimise unnecessary invasive interventions. Investigators found that laboratory values, inflammatory markers, and biochemical levels were not significantly different among the two groups. Reference Rouva, Vergadi and Galanakis27 To complicate things even more, increasing evidence is suggesting a correlation between MIS-C and appendicitis in children. Reference Anderson, Campbell and Durowoju35 Therefore, a proper history, physical examination, and imaging are necessary for the assessment of abdominal pain in these patients.
Congruently, the involvement of skin and mucus membrane is frequently common. Extremity swelling and erythema, skin rash, mucositis, conjunctivitis, peri-orbital oedema, strawberry tongue, and palmar erythema are prominent features. Reference Shobhavat, Solomon and Rao36–Reference Dufort, Koumans and Chow38 In one case series, the duration of mucocutaneous symptoms ranged between 0 and 11 days. Reference Bhopal, Bagaria, Olabi and Bhopal5 Skin rashes were also variable, including morbilliform, maculopapular, urticarial, and petechial rashes. Reference Feldstein, Rose and Horwitz6
Neurologic manifestations in MIS-C are largely variable in terms of incidence, severity, and symptoms. Involvement of the neurologic system is not uncommon, reported in almost half of the patients in one study. Reference Sethy, Mishra and Jain37 Children might present in seizure, altered level of consciousness, headache, irritability, or confusion. Reference Lee, Day-Lewis and Henderson31,Reference Sethuraman, Kannikeswaran and Ang32,Reference Dufort, Koumans and Chow38 Severe neurologic manifestations have been reported such as cerebral infarcts, encephalopathy, status epilepticus, Guillain Barre syndrome or its variant, and even acute fulminant cerebral oedema. Such complications are estimated to occur at a rate of 12% Reference Palabiyik, Akcay, Sevketoglu, Hatipoglu, Sari and Inci33,Reference Shobhavat, Solomon and Rao36 . Meningitis was also reported, affecting around 12–56% of children. Reference Lee, Day-Lewis and Henderson31,Reference Dufort, Koumans and Chow38 Reference Kaushik, Aydin and Derespina41 Brain oedema, diffuse signal changes, acute disseminated encephalomyelitis-like lesions, laminar necrosis, necrotising encephalomyelitis, and atrophy were revealed on MRI. Reference Sethy, Mishra and Jain37
Cardiac involvement and myocardial insult are frequent and critical. In severe cases, children can present with cardiogenic shock, myocardial dysfunction, hypotension, myocarditis, and pericarditis. Reference Grimaud, Starck and Levy39 Varying degrees of depressed left ventricular function is reported in almost all the studies and in up to 80% of patients in some investigations. Reference Feldstein, Rose and Horwitz6 Severe depression, defined as ejection fraction of < 30%, is estimated to complicate around 20–30% of cases. Reference Lingappan, Karmouty-Quintana, Davies, Akkanti and Harting26,Reference Belhadjer, Méot and Bajolle28 In a case series of 186 patients, almost half required vasoactive agents on presentation. Reference Lee, Day-Lewis and Henderson31 Intriguingly, in some studies, the left ventricular dysfunction was not present at the time of admission but developed during hospitalisation. Reference Atzrodt, Maknojia and McCarthy1 Some patients even required extracorporeal membrane oxygenation. Reference Davies, Evans and Kanthimathinathan40 Fortunately, affected children are expected to have rapid myocardial recovery. For instance, 100 and 95% of cases described in two separate studies had recovery of the left ventricle documented before hospital discharge. Reference Belhadjer, Méot and Bajolle28,Reference Kaushik, Aydin and Derespina41 Besides left ventricular depression, arrythmias, chest pain, coronary artery dilation, or aneurysm, pericardial effusions and hypotension are relatively common at presentation. Coronary artery involvement is highly evident. Dilatation and aneurysms have been reported in up to 93% of presenting cases. Reference Toubiana, Poirault and Corsia19,Reference Davies, Evans and Kanthimathinathan40,Reference Ramcharan, Nolan and Lai42 Furthermore, cardiac involvement is marked on laboratory evaluation by elevation in serum cardiac enzymes, troponin, and pro-BNP. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Shobhavat, Solomon and Rao36 Variable Electrocardiogram (EKG) findings including atrioventricular block, bradycardia, tachycardia, ST changes, T-wave changes, ventricular arrythmias, and elongation of QT interval were also reported. Reference Radia, Williams and Agrawal3 Thus, when MISC is suspected, it is of high importance to obtain serum cardiac enzymes, echocardiogram, EKG, and sometimes advanced imaging such as cardiac MRI or CT might be needed.
Luckily, a minority of children present with acute kidney injury. Reference Riollano-Cruz, Akkoyun and Briceno-Brito18 Nevertheless, some studies reported acute kidney failure in up to 70% of patients. Reference Grimaud, Starck and Levy39 However, all of these cases were temporary. Reference Grimaud, Starck and Levy39 Respiratory symptoms are also infrequent and complicate around 9% of presentations. Reference Zou, Lu and Liu43 Lung pathologies are not usually reported. On imaging, the most common finding is pleural effusions. Reference Anderson, Campbell and Durowoju35 Opacities and peribranchial cuffing are also frequently seen. Reference Matucci-Cerinic, Caorsi, Consolaro, Rosina, Civino and Ravelli15,Reference Lee, Day-Lewis and Henderson31 Rare cases of diffuse ground glass opacities on chest imaging might be encountered. Reference Belhadjer, Méot and Bajolle28,Reference Bayramoglu, Canıpek and Comert44 Besides, about 9% of patients might require respiratory support, with peak worsening of respiratory status at around 4 days from the onset of symptoms. Reference Davies, Evans and Kanthimathinathan40,Reference Feldstein, Tenforde and Friedman45
From a hematological perspective, laboratory testing is consistently significant for elevated inflammatory markers such as C-Reactive protein (CRP), ferritin, erythrocyte sedimentation rate (ESR), IL-6, and procalcitonin. Reference Feldstein, Rose and Horwitz6,Reference Sethy, Mishra and Jain37,Reference Zou, Lu and Liu43 At the same time, hyponatremia, thrombocytopenia, lymphopenia, elevated liver enzymes, anaemia, and hypoalbuminemia are also seen. Reference Feldstein, Rose and Horwitz6,Reference Sethy, Mishra and Jain37,Reference Kaushik, Aydin and Derespina41,Reference Zou, Lu and Liu43 High levels of fibrinogen and D-dimers are also found, reflecting the hypercoagulable state of MIS-C. Reference Zou, Lu and Liu43,Reference Harwood, Allin and Jones46 Rare occurrence of aplastic anaemia was reported. Reference Shobhavat, Solomon and Rao36 Incidence of deep vein thrombosis and pulmonary embolism have been also described by some studies, although rare in the paediatric population compared to adults with multi-system inflammatory syndrome Reference Davies, Evans and Kanthimathinathan40 . Hyperglycemia might also complicate the course of illness. It can be severe enough to require transient insulin therapy. Reference Shobhavat, Solomon and Rao36
Therapeutic modalities in MISC
The management of MIS-C is not clearly defined until this moment; however, several international health organisations have developed guidelines to approach patients with MIS-C. In addition, multiple pharmacologic therapies have been proposed and used in the management of paediatric MIS-C. In general, a multidisciplinary approach that includes infectious diseases, cardiology, neurology, haematology, and intensive care teams is recruited. Each hospital would develop a specific protocol that would be updated on regular basis. Table 3 represents the guidelines for managing MIS-C patients, developed by the various organisations.
Table 3. MIS-C management guidelines

KDSS = Kawasaki disease Shock Syndrome; MAS = Macrophage Activation Syndrome.
1 Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated With SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis & Rheumatology. 2020;72(11):1791-1805.
2 Pediatrics AAo. Multisystem Inflammatory Syndrome in Children (MIS-C) Interim Guidance. American Academy of Pediatrics. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/. Published 2022. Updated October 6, 2022. Accessed October 22, 2022.
3 Berard RA TH, Scuccimarri R, Haddad E, Morin MP, Chan KJ, Dahdah NS, McCrindle BW, Price VE, Yeung RSM, Laxer RM,. Paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (spring 2021 update). Canadian Pediatric Society. https://cps.ca/en/documents/position/pims#Figure%201. Published 2021. Updated May 2, 2021. Accessed October 23, 2022.
4 Organization WH. Living Guidance for Clinical Management of COVID-19. WHO. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Published 2021. Updated November 23, 2021. Accessed November 1, 2022.
5 Health RCoPaC. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians. Royal College of Pediatrics and Child Health. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance. Published 2020. Accessed October 23, 2022.
6 Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. The Lancet Child & Adolescent Health. 2021;5(2):133-141.
Supportive care should be initiated rapidly at presentation. After providing the proper haemodynamic support, treatment regimen is decided on based on the clinical picture and laboratory evaluation. A significant subset of patients require admission to the ICU. Reference Mahmoud, El-Kalliny, Kotby, El-Ganzoury, Fouda and Ibrahim47 The treatment aims to reduce inflammation in affected organs, prevent permanent damage, and provide prophylaxis against thromboembolic events. Reference McArdle, Vito and Patel48 Figure 3 depicts the treatment pathway of patients presenting with suspicion of MIS-C. The supportive care needed incorporates hydration, oxygen therapy, and blood pressure regulation. Reference Zhang, Xu and Du49 The severity of MIS-C presentation dictates how aggressive the supportive care should be. Children presenting in shock require support with inotropic agents such as epinephrine, norepinephrine, or dobutamine to restore blood pressure and cardiac contractility. Reference Radia, Williams and Agrawal3 Besides, a subset of patients with severe disease requires mechanical ventilation. Reference Ahmed, Advani and Moreira50 Extracorporeal membrane oxygenation may also be required in a small number of additional patients. Reference Feldstein, Rose and Horwitz6
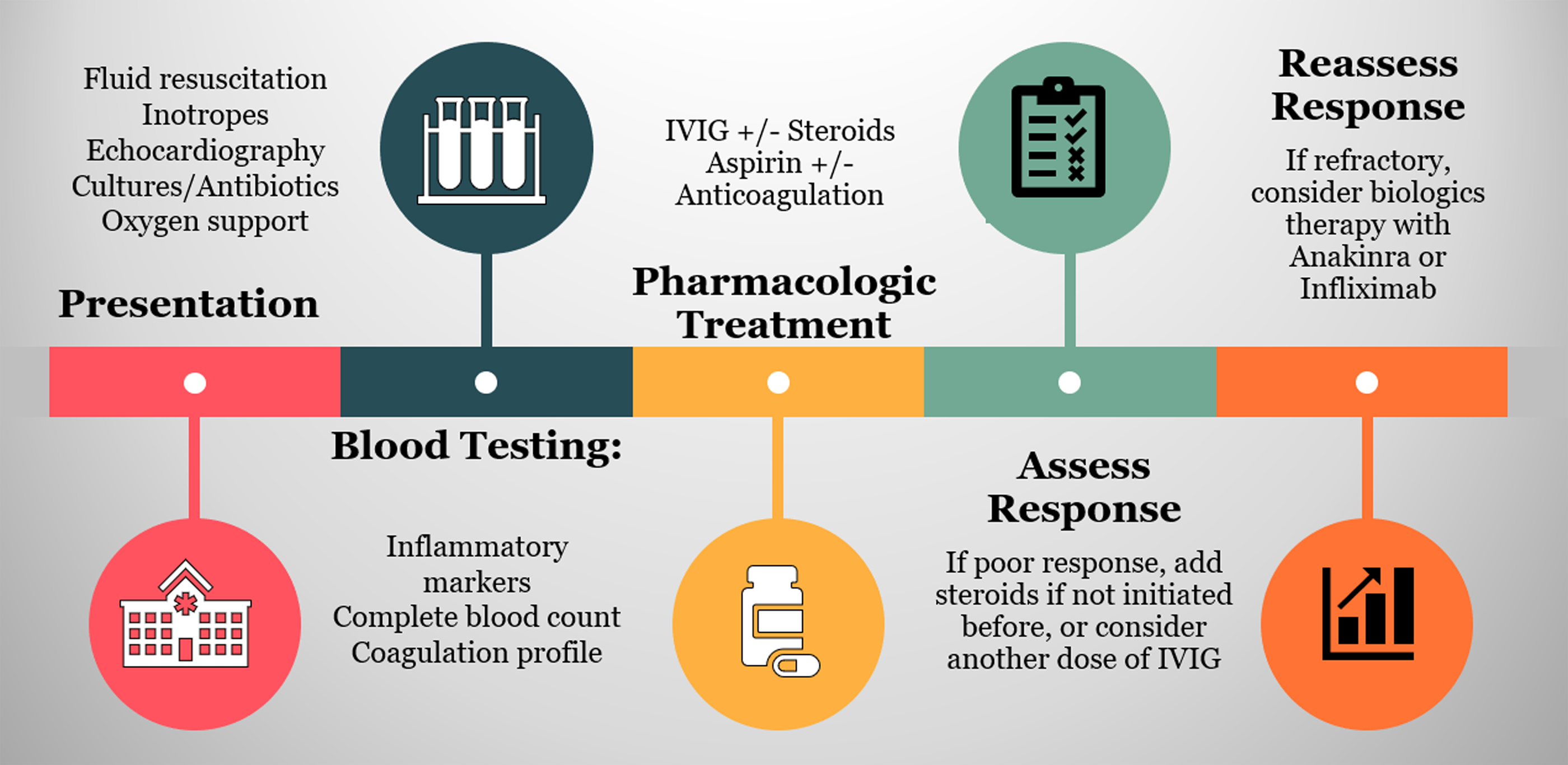
Figure 3. Treatment timeline for children with suspicion of MIS-C. Iinitial step involves stabilisation through providing supportive care, collecting cultures to assess for any microbial aetiology, and initiating proper antibiotics. It also should include initial echocardiography to evaluate the cardiac function. This is followed by laboratory evaluation. Inflammatory markers, cardiac indicators, and coagulation profile should be assessed. Once the diagnosis is confirmed, the initial treatment starts with IVIG 1-2 mg/kg with or without concomitant steroids therapy. The addition of aspirin and anticoagulation agents should be considered. The initial response is evaluated through clinical improvement and inflammatory markers. A rapid response is usually observed within days form treatment initiation. In refractory cases, a dose of steroids should be considered if not previously given, or a second dose is recommended. If the patient’s condition did not improve despite using second-line therapy, the use biologics agents is recommended, including anakinra and infliximab.
Pharmacologically, treatment might include coverages with broad-spectrum antibiotics for suspected bacterial aetiology. Some healthcare centres support the use of broad-spectrum antibiotics in all patients with suspected or confirmed MIS-C. Reference Toczyłowski, Łasecka-Zadrożna and Pałyga-Bysiecka51 Empiric treatment is usually continued until all cultures exclude bacterial infection. The most frequently utilised antibiotics in MIS-C include coverage for gram positive, gram negative, and anerobic organisms. Reference Hennon, Penque and Abdul-Aziz52 The use of broad-spectrum antibiotics was also endorsed by the Canadian Pediatric Society and by the American Academy of Pediatrics in cases of severe disease. Reference Berard R.A., Scuccimarri and Haddad53,Reference AAo54
As described above, patients with MIS-C are at a high risk of developing thrombotic complications such as thromboembolisms or apical LV thrombi. Reference Soma, Shust and Ratner55 Hence, aspirin and/or low-molecular-weight heparin are usually recommended and included in the treatment regimen. Reference Goldenberg, Sochet and Albisetti56,Reference Patel57 Low-dose aspirin is advised to counteract the increased risk of blood clots and to provide protective effect in children with coronary artery involvement, thrombocytosis, or Kawasaki disease features. A single daily dose of 3–5 mg/kg/day of aspirin is usually prescribed. Reference Jonat, Gorelik and Boneparth58
Furthermore, because of its similar presentation to Kawasaki disease and toxic shock syndrome, Reference Sperotto, Friedman, Son, VanderPluym, Newburger and Dionne59,Reference Sharma, Ganigara and Galeotti60 practitioners have chosen immunomodulatory agents that have already shown beneficial results in the treatment of these diseases. Reference Soma, Shust and Ratner55 Therefore, treatment of children with MIS-C include both intravenous immunoglobulin (IVIG) and anti-inflammatory drugs such as glucocorticoids. Reference Patel57 First-line treatment in most healthcare centres is IVIG, with a dose of 1–2 g/kg/day depending on severity. The timing of steroids initiation differs among the practitioners. Typically, 2mg/kg/day of methylprednisolone is used. Some studies showed no significant difference in clinical mortality or prognosis between the IVIG and steroid dual therapy and monotherapy with either one of the two. Reference McArdle, Vito and Patel48 A study conducted on 614 children who met the WHO criteria for MISC, 99 received glucocorticoids alone while 208 received the dual therapy of IVIG and glucocorticoids. The results showed that the adjusted odds ratio for the decrease in disease severity was similar in both groups. Indeed, 93 versus 90% of children receiving glucocorticoids alone versus IVIG + glucocorticoids, respectively, showed a decrease in the severity of their disease. Reference McArdle, Vito and Patel48 However, other studies show that patients receiving the combination therapy were less likely to experience recurrent fever, had a reduced need for haemodynamic support, and were less likely to have left ventricular dysfunction. Reference Ouldali, Toubiana and Antona61 In addition, a retrospective cohort was performed in France to investigate the role of IVIG and steroids. It included 111 children who met the WHO criteria of MISC from whom 34 received the dual therapy of IVIG and steroids, in that case methylprednisolone, while 72 received the IVIG monotherapy. The authors measured the rate of treatment failure in that sample defined as the persistence of fever for 2 days after initiation of therapy or the redundancy of fever within 7 days. Nine per cent of children receiving the dual therapy showed a pattern of failure in the treatment received, while 51% of children on IVIG alone showed the same pattern. Moreover, only 5% of children experienced acute left ventricular dysfunction and the need of haemodynamic support in the dual-therapy group, versus 24% in the IVIG alone group. Reference Ouldali, Toubiana and Antona61 This conclusion was in line with the results of one systematic review and meta-analysis. Authors concluded that children who received dual therapy have significantly lower risks of treatment failure and lower need for adjunct immunomodulator therapy than children receiving IVIG alone. In fact, out of the 756 total children included in the different studies, 44% of children receiving IVIG alone showed a positive result for treatment failure while only 31% of children receiving both IVIG and steroids exhibited treatment failure. These results were statistically significant. Reference Rauniyar, Mishra and Kharel62
In addition to that, Anakinra (IL1 receptor antagonist) and infliximab (TNF -α receptor antagonist) are used in the treatment of refractory MIS-C in order to further reduce the patients’ hyperinflammatory state. Reference Patel57 Patients are considered refractory if they do not show improvement during the first 24 hours of administration of treatment. Studies also showed that the administration of anakinra as an adjunct therapy to IVIG or before invasive mechanical ventilation is also beneficial. Reference Mahmoud, El-Kalliny, Kotby, El-Ganzoury, Fouda and Ibrahim47 It is also worth mentioning that infliximab is not recommended for use in all the patients and should be only limited to those who did not respond to prior treatments or have other comorbidities like Crohn’s disease. Reference Dolinger, Person and Smith63,Reference Henderson, Canna and Friedman64
Finally, despite the severe and critical presentations of MIS-C, children exhibit excellent prognosis. In fact, clinical improvement is noted within few days of treatment initiation. Reference Feldstein, Rose and Horwitz6,Reference Toubiana, Poirault and Corsia19 Rapid cardiac improvement and significant drop in inflammatory markers are usually observed following treatment. Reference Grimaud, Starck and Levy39,Reference Zou, Lu and Liu43 Studies showed that by 6 months, most of the patients treated with the appropriate regimen had normal and clear cardiac MRI imaging, Reference Giacalone, Scheier and Shavit65,Reference Giacalone, Scheier and Shavit65 and no signs of myocardial oedema or fibrosis were seen with normal left ventricular functioning. Reference Capone, Misra and Ganigara66 The mortality rate is low, estimated at around 1–3%. Reference Feldstein, Rose and Horwitz6,Reference Riollano-Cruz, Akkoyun and Briceno-Brito18,Reference Dufort, Koumans and Chow38
Conclusion
MIS-C is an intriguing new phenomenon observed in the past 2 years and is attributed to COVID-19 infection. It shares overlapping features with Kawasaki disease. The hyperinflammatory storm influences multiple-organ systems and can mark end-organ damage. A clear pathogenesis of this newly arising condition is yet to be confirmed. Although with the appropriate and timely initiation of treatment children are exhibiting excellent prognosis, many questions are yet to be addressed.
Acknowledgement
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
Authors have nothing to disclose with regard to commercial support or conflict of interest.









