The prevalence of type 2 diabetes has dramatically increased over the last several decades throughout the world(Reference Wild, Roglic and Green1). This has resulted in considerable increases in morbidity and mortality related to type 2 diabetes, as well as a large economic cost for caring for those with the disease, creating an increasingly burdensome global health problem(2). Consequently, there is an urgent need for interventions that prevent or delay the development of type 2 diabetes and ameliorate its complications. Although there are a number of modifiable risk factors for type 2 diabetes, diet is clearly of paramount importance(Reference van Dam3). However, which specific dietary components may be effective in reducing the incidence and progression of type 2 diabetes remains incompletely understood.
Several prospective cohort studies have shown a strong inverse relationship between the consumption of whole grain foods and the risk of developing type 2 diabetes(Reference Meyer, Kushi and Jacobs4–Reference Montonen, Knekt and Jarvinen6). There is also evidence that increased whole grain consumption improves blood glucose control and reduces insulin resistance and related diabetes risk factors, including oxidative stress(Reference Jang, Lee and Kim7, Reference Pereira, Jacobs and Pins8). Although whole grain intake did not demonstrate a beneficial effect on insulin sensitivity in healthy subjects(Reference Andersson, Tengblad and Karlstrom9, Reference Tighe, Duthie and Vaughan10), Pereira et al. (Reference Pereira, Jacobs and Pins8) showed that insulin resistance was significantly improved with the consumption of whole grain foods in overweight hyperinsulinaemic subjects. Thus, increased consumption of whole grain foods may be a useful dietary approach to lowering the risk of developing diabetes.
The mechanism by which whole grains might convey such a benefit is not well understood. Possible mechanisms include a reduced postprandial plasma glucose response and a reduction in oxidative stress. Viscous dietary fibres have repeatedly been shown to lower the postprandial glucose response, an effect that may decrease the risk for diabetes(Reference Hallfrisch, Facn and Behall11). Several studies have demonstrated that viscous fibre such as β-glucans, which are present in high concentrations in oats and barley, improves glucose control in type 2 diabetes(Reference Pick, Hawrysh and Gee12, Reference Jenkins, Jenkins and Zdravkovic13). Viscous fibre may lower postprandial glycaemia by delaying gastric empting(Reference Torsdottir, Alpsten and Andersson14, Reference Leclere, Champ and Boillot15) or by slowing glucose absorption in the small intestine(Reference Rainbird, Low and Zebrowska16). Reducing oxidative stress by dietary antioxidants may also contribute to a reduction in the incidence of type 2 diabetes. Several prospective cohort studies suggest an inverse relationship between dietary antioxidant intake and the incidence of type 2 diabetes(Reference Mayer-Davis, Costacou and King17, Reference Montonen, Knekt and Jarvinen18). Whole grains, particularly bran fractions, are concentrated sources of antioxidant compounds. Compared with their refined counterparts, whole grains have two- to three-fold greater antioxidant capacity(Reference Halvorsen, Holte and Myhrstad19). In a clinical trial by Jang et al. (Reference Jang, Lee and Kim7), the consumption of a whole grain diet for 16 weeks significantly reduced markers of oxidative stress, plasma malondialdehyde and urinary 8-isoprostane, in subjects with coronary artery disease. Consumption of a diet containing whole grains favourably altered markers of antioxidant defence(Reference Bruce, Spiller and Klevay20) and reduced the level of markers of lipid peroxidation(Reference Jang, Lee and Kim7), suggesting a decrease in oxidative stress.
The objective of the present study was to evaluate the effect of the consumption of different whole grains on diabetic control and diabetic progression in an animal model of type 2 diabetes, the Goto–Kakisaki (GK) rat. GK rats exhibit moderate but stable hyperglycaemia, insulin resistance and impaired glucose tolerance, which appears at 3–4 weeks of age(Reference Movassat, Bailbe and Lubrano-Berthelier21). The whole grain flours used, wheat, barley, oats or maize, were chosen because they varied in the two dietary factors that relate to the possible mechanisms by which whole grains may reduce the risk of developing type 2 diabetes, viscous fibre (as β-glucans) and the antioxidant capacity, thus providing the possibility of elucidating the characteristic(s) of whole grain that may protect against type 2 diabetes. To this end, glucose control, insulin resistance, β-cell dysfunction and oxidative stress were measured at an early and later stage of diabetes in this well-established animal model of type 2 diabetes.
Materials and methods
Animals and diets
Male GK rats (7–8-weeks old) weighing 151–200 g were purchased from Taconic Farms, Inc. (Hudson, NY, USA). The animals were individually housed in wire-bottomed stainless steel cages in an air-conditioned room (22 ± 2°C, 55 ± % relative humidity) with a 12 h light–12 h dark cycle. All experimental procedures were approved by the University of Minnesota Committee on Animal Care.
Two factors, the content of β-glucan and the antioxidant capacity, were used to choose the appropriate species of whole grains for the present study to allow a 2 × 2 factorial design. The selected whole grains were wheat, barley, oats and maize. As shown in Table 1, barley and oats had equivalent β-glucan content but barley had more than double the antioxidant activity. Maize had relatively high antioxidant activity with very little β-glucan. Wheat had both low β-glucan content and antioxidant capacity. Barley and oats were classified as having a high level of β-glucan and wheat and maize were classified as having a low level of β-glucan. Barley and maize were classified as having a high level of antioxidant capacity and oats and wheat were classified as having a low level of antioxidant capacity.
Table 1 β-Glucan concentration and antioxidant activity of whole grains

* Antioxidant activity was expressed as μmol Trolox equivalents/100 g sample.
Whole grain flours were obtained from a local milling company (Whole Grain Milling Company, Welcome, MN, USA) and stored at − 20°C. A proximate analysis was conducted by a commercial laboratory (Medallion Laboratories, Minneapolis, MN, USA) to obtain the protein, carbohydrate, lipid, total dietary fibre and ash content of the whole grain flours. The β-glucan concentration and antioxidant activity of the flours are shown in Table 1. The β-glucan content was determined using an enzymatic spectrophotometric method (β-Glucan Assay Kit; Megazyme, Wicklow, Ireland). Antioxidant capacity was measured by the 2,2-diphenyl-1-picrylhydrazyl assay, as described by Miller et al. (Reference Miller, Rigelhof and Marquart22). A modification of the American Institute of Nutrition 93G purified diet was used as the basal diet. Whole grain diets contained 65 % of wheat, barley, oats or maize flour. To allow comparison with human diets, this quantity of flour provided 14·8 g β-glucan/10 000 kJ for the barley and oat flour-based diets, 0·3 g β-glucan/10 000 kJ for the maize flour-based diet, and 2·2 g β-glucan/10 000 kJ for the wheat flour-based diet. Based on a proximate analysis of the flours, the macronutrient content (carbohydrate, protein, fat and total dietary fibre) of the whole grain diets were closely matched to that of the basal diet. The composition of the diets is shown in Table 2. The diets were prepared every month and stored at − 20°C.
Table 2 Composition of the diets

* AIN-93G mineral mix.
† AIN-93G vitamin mix.
Experimental design
The animals were adapted to the basal diet for 3 d. A total of ten rats were randomly assigned to either the basal diet or one of the four whole grain diets. Diets were fed ad libitum for 5 months with free access to water. Body weight and food intake were measured bi-weekly and monthly, respectively. After 2 months of feeding, blood was collected from the retro-orbital sinus after a 12 h fast. A 24 h fasting urine collection was made at 2 and 5 months of the feeding period, beginning after a 12 h fast to eliminate lipid oxidation products emanating from the diets. At the end of the dietary treatment, the rats fasted for 12 h and were anaesthetised with isoflurane and blood was collected by cardiac puncture into EDTA-containing syringes. The kidneys and epididymal fat pads were removed and weighed. The pancreas was removed, weighed and embedded in paraffin for immunohistochemistry.
Biochemical analysis
The percentage of glycated Hb (GHb) in whole blood was analysed by an affinity chromatographic method (Helena Glyco-Tek Affinity Column Method; Helena Laboratories, Beaumont, TX, USA). GHb represents all glycated fractions of Hb, whereas HbA1c represents HbA with glucose attached to the NH2-terminal valines of the β-chain. Although these two fractions are measured using different methods, clinically they are equivalent(Reference Goldstein, Little and Lorenz23). Fasting plasma glucose and NEFA concentrations were measured with an enzymatic colorimetric method using commercial kits (Autokit Glucose and HR Series NEFA-HR (2), respectively; WAKO Chemicals, Richmond, VA, USA). The concentrations of fasting plasma insulin and C-peptide were measured by RIA specific for rat insulin and C-peptide (Linco Research, St Charles, MO, USA). Urinary concentration of urinary thiobarbituric acid-reactive substances (TBARS) was measured as described by Mihara & Uchiyama(Reference Mihara and Uchiyama24) with minor modifications. Urine samples (100 μl) were mixed with 300 μl of 1 % (v/v) phosphoric acid and 200 μl of 0·6 % (w/v) thiobarbituric acid and heated at 100°C for 45 min. After cooling, 100 μl of n-butanol was added to each tube and mixed vigorously to extract the colour reactant. The butanol layer was separated by centrifugation at 750 g for 10 min, collected, and the absorbance measured at 535 nm. Malondialdehyde standards were prepared from malondialdehyde tetramethylacetal as described by Lee et al. (Reference Lee, Shoeman and Csallany25) and treated as were the urine samples. Urinary 8-iso-PGF2a (8-isoprostane) concentrations were measured using an ELISA kit (Northwest Life Science Specialties; LLC, Vancouver, WA, USA). The concentration of creatinine in urine and plasma was determined by the Jaffe reaction using a commercially available kit (R&D System, Minneapolis, MN, USA). Creatinine clearance (CCr) was calculated according to the following formula:
Calculation of insulin resistance
The homoeostasis model assessment (HOMA), which is based on fasting plasma insulin and glucose concentrations, was used to estimate insulin resistance(Reference Haffner, Kennedy and Gonzalez26). The formula is as follows:
Estimation of total β-cell mass
The paraffin-embedded pancreas was sectioned into 7 μm sections throughout its length. The section was counted when 25 % of the section was occupied with pancreatic tissue. Every 30th to 35th section was sampled on glass slides and was immunostained for insulin using guinea pig anti-insulin antibody (1:2000 dilution, a generous gift from Dr Robert L. Sorenson), followed by peroxidase-conjugated donkey anti-guinea pig IgG (1:500 dilution; Jackson Immunoreseach Laboratories, Inc., West Glove, PA, USA). The sections were developed with diaminobenzadine-containing nickel chloride, which yields a grey colour for β cells (DAB Substrate Kit; Vector Laboratories, Inc., Burlingame, CA, USA). The sections were counterstained with Van Gieson's picric acid–fuchsin stain. The relative β-cell mass was estimated by the method of point counting on insulin antibody-stained sections of the pancreas(Reference Gundersen, Bendtsen and Korbo27). A microscope (Olympus BX40; Melville, NY, USA) connected to a digital camera (Olympus DP11-N) and monitor (Sony PVM-14N5U; Tokyo, Japan) were used to obtain digital images at 100 × magnification. For each pancreas, 161–318 images were examined for the quantification of the β-cell volume. Adobe Photoshop 7.0 software was used to observe the images and superimpose counting grids over the same images. Using a point counting grid containing 192 fine points and one coarse point (encircled), the number of fine points hitting β-cells and the number of coarse points hitting pancreatic tissue was counted.
Total β-cell mass was calculated according to:
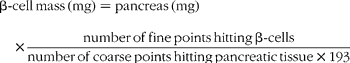
Statistical analysis
The results are expressed as means with their standard errors. Diet group differences were analysed by one-way ANOVA, followed by Duncan's test to inspect individual differences among the diet groups. Groups fed the whole grain flours were also analysed by two-way ANOVA with two levels of antioxidant capacity (low and high) and β-glucan content (low and high) as the main effects. The results from the plasma and urinary assays obtained from the two time points were analysed by three-way ANOVA with factors of antioxidant capacity (low and high), β-glucan content (low and high) and time (2 and 5 months). Pearson correlation analysis was used to assess the correlations between the measurements. Values for P < 0·05 were considered statistically significant. All statistical analyses were performed by using Statistical Analysis Systems statistical software package version 9.1 (SAS Institute, Cary, NC, USA).
Results
Food intake, body and tissue weight
Fig. 1 shows the changes in the body weight of GK rats fed either the basal or whole grain diets. Overall, the wheat group gained less weight than the other groups at each time point after 6 weeks of feeding. However, body weight gain averaged over the feeding trial did not differ among the diet groups. The rats fed the wheat diet consumed significantly less during the first month compared with rats in the basal group (P < 0·05). However, after 2 months, daily food consumption for the whole grain groups did not differ significantly from that of the basal group (data not shown).
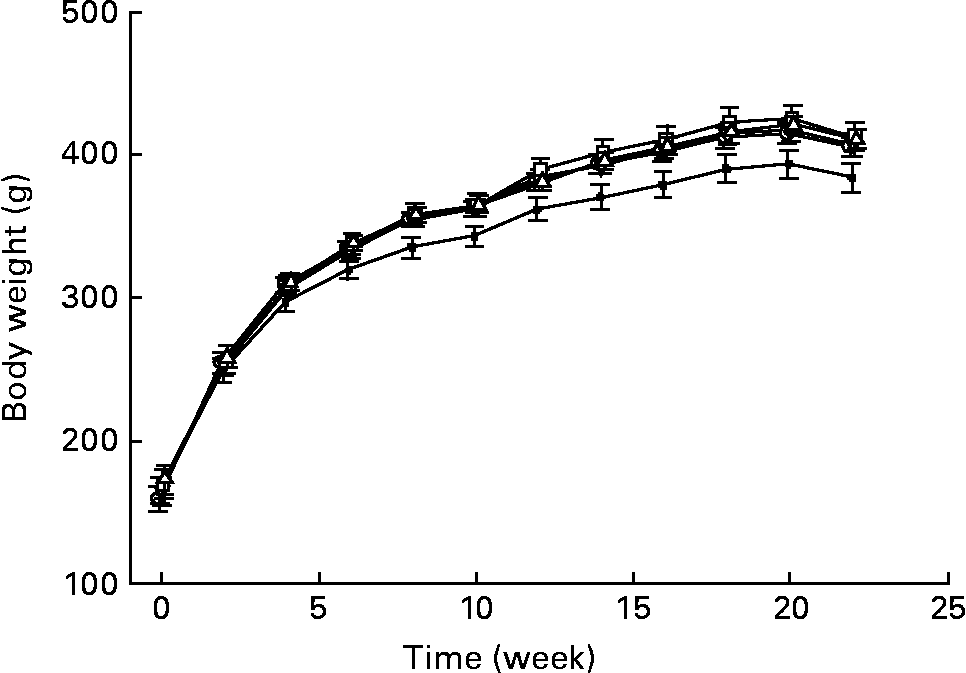
Fig. 1 Body weight changes of Goto–Kakisaki rats fed the basal (–○–), wheat (–●–), barley (–▽-), oat (-□-) or maize-based (–Δ–) diets for 22 weeks. Values are means, with their standard errors represented by vertical bars, n 9–10.
Neither the absolute nor the relative weights of the pancreas or epididymal fat pads differed among the diet groups (Table 3). Although the absolute kidney weights did not differ among the groups, the animals fed the wheat and oats diets had a greater relative kidney weight (g/100 g body weight) compared with the maize diet (P < 0·05). Two-way ANOVA revealed a significant main effect of antioxidant capacity on the relative kidney weight (P = 0·013), such that the groups fed diets with greater whole grain antioxidant capacity had lower relative kidney weight.
Table 3 Weights of pancreas, kidneys and epididymal fat pad tissues in Goto–Kakisaki rats at the end of 5 months feeding trial
(Mean values with their standard errors, n 9–10)

a,b Mean values with unlike superscript letters within a row were significantly different (P < 0·05).
Plasma glucose concentration
At 2 months, the rats fed the wheat, barley and oats diets had significantly lower fasting plasma glucose concentrations compared with the basal group (Table 4; P < 0·05). However, fasting plasma glucose concentrations in the oats group were significantly greater than all other groups at 5 months (P < 0·05). Fasting plasma glucose level significantly increased from 2 to 5 months (P < 0·001). There was an interaction effect of time × β-glucan on fasting plasma glucose (P = 0·027), such that animals fed diets with a high content of β-glucan had a lower fasting plasma glucose at 2 months but a greater concentration of plasma glucose at 5 months. The percentage of GHb was significantly greater in the wheat group at 2 and 5 months and the oats group at 5 months compared with the basal group (Table 4; P < 0·05). GHb also significantly increased from 2 to 5 months (P < 0·001). Three-way ANOVA indicated that antioxidant capacity had a significant main effect on GHb (P = 0·045), such that the animals fed whole grains with a high antioxidant capacity had a lower GHb. In addition, GHb at 5 months was correlated with relative kidney weight (r 0·546, P < 0·0001).
Table 4 Concentration of glycated Hb (GHb), glucose, insulin and C-peptide, and insulin resistance of Goto–Kakisaki rat fed either control or whole grain diets for 2 months and 5 months*
(Mean values with their standard errors, n 9–10)

HOMA-IR, homeostasis model of assessment-insulin resistance.
a,b Within a time period, mean values with unlike superscript letters within a row were significantly different (P < 0·05).
* Data obtained from fasted rats.
Plasma insulin and C-peptide concentration and insulin resistance
Fasting plasma insulin levels did not differ among the groups and significantly decreased from 2 to 5 months in all diet groups (Table 4; P = 0·004). Fasting plasma C-peptide concentrations were significantly greater in the barley and maize groups compared with the wheat group at 2 months (P < 0·05). Fasting plasma C-peptide levels decreased from 2 to 5 months in all groups except the wheat group (Table 4; P < 0·001). Three-way ANOVA indicated that antioxidant capacity had a significant main effect on C-peptide levels (P = 0·042), such that the diet groups with greater whole grain antioxidant capacity had greater fasting plasma C-peptide concentrations. In addition, there was a significant interaction between time and β-glucan content (P = 0·011), such that the diet groups with a high β-glucan content had a greater concentration of C-peptide at 2 months but a lesser concentration of plasma C-peptide at 5 months. Insulin resistance, determined by HOMA, was not significantly different among the groups at either 2 or 5 months (Table 4). However, there was a trend for a lower HOMA (less insulin resistance) with the barley and oats diets at 2 months (P = 0·06). Three-way ANOVA revealed a significant time effect on insulin resistance, such that resistance increased with time (P = 0·004). Fasting plasma glucose showed a significant inverse correlation to C-peptide concentration at 2 months (r − 0·33, P = 0·021); however, at 5 months this correlation was no longer evident (r − 0·04, P = 0·79).
Oxidative stress
When the concentration of urinary TBARS was expressed as μg/24 h, the level of TBARS was significantly increased in the oats group at 2 months and the barley group at 5 months, compared with the other diet groups and the maize group, respectively (Table 5; P < 0·05). Three-way ANOVA indicated significant main effects of antioxidant capacity (P = 0·039) and β-glucan content (P < 0·001), such that the amount of urinary TBARS was decreased with the higher level of antioxidant capacity and the lower level of β-glucan.
Table 5 Concentration of urinary thiobarbituric acid-reactive substances (TBARS) and 8-isoprostane of Goto–Kakisaki rat fed either control or whole grain diets for 2 and 5 months*
(Mean values with their standard errors, n 9–10)

a,b,c Within a time period, mean values with unlike superscript letters within a row were significantly different (P < 0·05).
* Data obtained from fasted rats.
The concentration of urinary 8-isoprostane, expressed as ng/24 h, is shown in Table 5. At 2 months of diet treatment, the oats groups had a significantly greater concentration of 8-isoprostane compared with the barley and wheat groups (P < 0·05). However, at 5 months, the maize diet had a significantly greater concentration of 8-isoprostane compared with the basal and wheat diets, and the oats group had a significantly greater level of 8-isoprostane than the basal group (P < 0·05). Three-way ANOVA revealed a significant interaction between antioxidant capacity and β-glucan content (P < 0·001), such that the diet groups with the higher level of antioxidant capacity had lower concentrations of 8-isoprostane when the level of β-glucan content was low but when the level of β-glucan content was high, the concentration of 8-isoprostane was increased with the high level of antioxidant capacity. In addition, at 5 months, urinary 8-isoprostane concentration (ng/24 h) was positively correlated with urinary TBARS (μg/24 h) (r 0·47, P < 0·009).
Plasma NEFA concentration and creatinine clearance
Plasma NEFA concentrations did not differ among diet treatments at 5 months (Table 6). CCr, an indicator of glomerular hyperfiltration, was not significantly different among the diet groups at 5 months (Table 6). Two-way ANOVA indicated a significant main effect of β-glucan level on CCr (P = 0·016), such that the diet groups with a high β-glucan content had greater CCr.
Table 6 The level of NEFA and creatinine clearance of Goto–Kakisaki rats fed either basal or whole grain diets at 5 months*
(Mean values with their standard errors, n 9–10)

* Data obtained from fasted rats.
Relative volume of β-cells
Fig. 2 shows representative pancreas sections stained for insulin with the point-counting grid overlaid. There were no significant differences in the mean total β-cell mass among the diet groups (data not shown).

Fig. 2 Insulin immunostained pancreatic tissue sections from Goto–Kakisaki rats with the point counting grid overlain.
Discussion
In order to identify which whole grains might be most effective in slowing the onset of type 2 diabetes, we investigated the effect of the consumption of four commonly consumed whole grains in diabetic control and progression in the GK rat. The GK rat is the most commonly used spontaneous animal model of type 2 diabetes mellitus, which was produced by repeated inbreeding of Wistar rats with mild glucose intolerance(Reference Goto, Kakizaki and Masaki28). GK rats exhibit hyperglycaemia, insulin resistance, impaired insulin secretion upon glucose stimulation and reduction of β-cell mass(Reference Movassat, Bailbe and Lubrano-Berthelier21, Reference Hughes, Suzuki and Goto29, Reference Movassat, Saulnier and Serradas30). However, unlike most type 2 diabetes patients, the GK rat does not develop obesity and dyslipidaemia(Reference Hussain31, Reference Sachidanandam, Elgebaly and Harris32). Therefore, the GK rat appears to be an appropriate animal model to study the effect of whole grain intake on the pathogenesis of type 2 diabetes in the absence of such confounding factors. To our knowledge, this is the first study to examine and compare the effect of consumption of individual whole grains on diabetic control in an animal model of non-obese type 2 diabetes.
In the present study, we focused on two characteristics of whole grains that might act to delay the progression of diabetes. β-Glucans, a viscous fibre present in oats and barley, are well known for reducing the postprandial blood glucose response. High antioxidant capacity, such as found in barley and to a lesser extent in maize, may reduce the oxidative stress associated with diabetes. These two factors, the content of β-glucan and the antioxidant capacity, were used to guide the choice of whole grains for the present study and as a way to explore the impact of whole grains on outcomes related to diabetes control and progression.
The results suggest that certain whole grains may have a modest beneficial effect early in the course of type 2 diabetes. Feeding of whole wheat, barley and oats for 2 months lowered fasting plasma glucose in GK rats relative to the grain-free basal diet. Further, we found evidence for an improvement in insulin resistance, as demonstrated by a strong trend for a lower HOMA, after the consumption of whole barley and oats for 2 months. These results are consistent with recent findings from clinical trials indicating that the consumption of whole grains may protect against type 2 diabetes by improving glucose control and reducing insulin resistance(Reference Jang, Lee and Kim7, Reference Pereira, Jacobs and Pins8). The whole grains used in those studies, however, were a mixture of grains, including wheat, oat, barley, rice and maize, thus making it impossible to determine the effect of individual whole grains on the development of type 2 diabetes. Nevertheless, in the present study, longer-term consumption of whole grains (5 months) did not demonstrate beneficial effects on glucose and insulin responses, insulin resistance, oxidative stress or on preserving β-cell mass.
Body weights among the dietary groups did not differ, with the exception of the wheat group, which had a significantly lower body weight than other groups during the last 4 months of feeding. A lower body weight might suggest a worsening of the diabetic state. However, measures of glucose control and insulin secretion did not differ between the wheat group and the other groups, suggesting that this was not the case. Thus, the reason for the lower body weight in the wheat group is unclear.
Increases in fasting plasma glucose and GHb level from month 2 to month 5 clearly indicated a progression of diabetes in the GK rats over the feeding period. After 2 months of feeding, the fasting plasma glucose concentration was significantly lower in the wheat, barley and oats groups compared with the basal group. In addition, fasting plasma glucose was decreased with the consumption of grains containing a high β-glucan content for 2 months whereas, surprisingly, the effect was reversed at the end of the feeding period. In type 2 diabetes, the reduction in postprandial glucose and insulin level by the consumption of β-glucan from oats and barley appears to be dose dependent(Reference Jenkins, Jenkins and Zdravkovic13, Reference Tappy, Gugolz and Wursch33). Thus, it may be that the dietary β-glucan concentration in the present study was sufficient to improve glucose response at the 2 month time period, early in the progression of diabetes, but not sufficient to exert the effect at the end of the study when glucose control had greatly deteriorated. Surprisingly, GHb concentration was significantly greater at both time points in the wheat and oats groups compared with the basal group. It is unclear why the pattern of fasting plasma glucose and GHb concentrations did not parallel each other. GHb has been used as a measure of long-term blood glucose(Reference Bunn, Gabbay and Gallop34, Reference Lapolla, Traldi and Fedele35). Formation of GHb is non-enzymatic and controlled by the concentrations of glucose and protein and the half-life of the glycated protein(Reference Lapolla, Traldi and Fedele35). In addition, a high degree of oxidative stress also appears to increase glycation of Hb(Reference Jain36, Reference Balamurugan, Selvaraj and Bobby37), and reducing oxidative stress with vitamin E was found to reduce glycation of Hb in glucose-treated erythrocytes(Reference Jain and Palmer38). The antioxidant capacity of the grains had a significant main effect on GHb, suggesting that the consumption of grains with high antioxidant capacity may have reduced glycation of Hb relative to those with lower antioxidant capacity. Thus, early in the development of diabetes, whole grains appear to improve glucose control, but this improvement is not maintained as glucose control worsens with the progression of diabetes.
The decreased concentrations of plasma insulin and C-peptide from month 2 to month 5 indicate that insulin secretion was progressively impaired during the feeding period. Insulin and C-peptide are secreted in equimolar concentrations from pancreatic β-cells, but C-peptide has a longer half-life than insulin(Reference Horwitz, Starr and Mako39) and thus has been used as a marker for insulin secretion. In GK rats, impaired insulin secretion in response to glucose occurs in isolated islets and perfused pancreas from 8 weeks of age(Reference Hughes, Suzuki and Goto29, Reference Kimura, Toyota and Kakizaki40, Reference Ohneda, Johnson and Inman41). Thus, the greater fasting plasma C-peptide concentrations in the barley and maize groups compared with that of the wheat group at 2 months suggest a slower development of diabetes in young GK rats with a consumption of the grains with higher antioxidant capacity. Yet, groups fed grains with high β-glucan content had the paradoxical effect of having greater C-peptide concentrations after 2 months of diet treatment, but lower concentrations after 5 months, as indicated by an interaction of the main effects of time and β-glucan. However, as C-peptide concentrations at 5 months in the whole grain groups did not differ from the basal group, it is likely that by 5 months the progression of diabetes overwhelmed the modest benefits provided by the whole grains at 2 months.
The HOMA values at 2 months showed a trend towards improvement in insulin resistance with the consumption of whole barley and whole oats diets (P = 0·06 v. basal for both). It has been suggested that a correlation between insulin secretion and insulin resistance may be weakened or lost in overt diabetes when insulin secretion is impaired(Reference Mari, Ahren and Pacini42). Therefore, at 5 months of diet treatment, the use of HOMA for calculating insulin resistance likely is not valid since the disease in GK rats most probably had progressed from insulin resistance to overt diabetes.
Urinary TBARS (μg/24 h) was decreased with the higher level of antioxidant capacity whereas the content of β-glucan was positively associated with urinary TBARS excretion. Since TBARS is a less specific marker of lipid peroxidation, the role of whole grain intake on oxidative stress in GK rats was also determined by measuring the more specific marker, urinary 8-isoprostane(Reference Milne, Musiek and Morrow43). Using this marker, a significant interaction between antioxidant capacity and β-glucan content was found, such that the higher level of antioxidant activity decreased the excretion of urinary 8-isoprostane only when the β-glucan content is low. Urinary TBARS and 8-isoprostane were positively correlated only at the 5 month time period (r 0·47, P < 0·009). A possible explanation of the lack of effect of the whole grains on oxidative stress would include poor bioavailability of the antioxidants from the whole grain. Whole grains, particularly within the bran fraction, are a rich source of phenolic compounds, which are potent antioxidants(Reference Adom and Liu44–Reference Liyana-Pathirana and Shahidi46). Since most phenolics from plant sources are bound to cell wall polymers, the physiological impact of grain phenolics depends on their bioavailability. It has been shown that the absorption of ferulic acid, a major phenolic compound in grain, is limited due to poor bioavailability of insoluble bound phenolics(Reference Adam, Crespy and Levrat-Verny47).
It is well established that total β-cell mass in GK rats is decreased by more than 50 % in the late fetal age and remains reduced into adulthood, relative to age-matched Wistar rats(Reference Movassat, Saulnier and Serradas30, Reference Miralles and Portha48). This β-cell deficit has been shown to result from impaired β-cell neogenesis(Reference Movassat, Calderari and Fernandez49). The depletion of β-cells accelerates with age and with the consumption of a high- sucrose diet, which induces severe hyperglycaemia and apoptosis of β-cells in GK rats(Reference Koyama, Wada and Sakuraba50). Chronic exposure of β-cells to either hyperglycaemia or elevated concentrations of NEFA induces β-cell dysfunction and β-cell death, giving rise to the term ‘glucolipotoxicity’(Reference Harmon, Gleason and Tanaka51, Reference Prentki, Joly and El-Assaad52). In the present study, the total β-cell mass was not altered with the consumption of whole grains compared with the basal diet, which is consistent with the general lack of difference among diet groups in plasma glucose and NEFA concentrations at the end of the feeding period.
In the earliest stage of diabetic nephropathy (hypertrophy-hyperfunction), glomerular filtration and kidney size are increased(Reference Mogensen and Andersen53). Increased glomerular hyperfiltration has been shown to be correlated with kidney enlargement(Reference Garcia Puig, Mateos Anton and Grande54). In GK rats, prolonged hyperglycaemia has been associated with increased kidney size at 6 months of age(Reference Nobrega, Fleming and Roman55), consistent with studies showing a strong relationship between the degree of kidney enlargement and the concentration of blood glucose(Reference Seyer-Hansen56, Reference Gallaher, Olson and Larntz57). In the present study, relative kidney weight (g/100 g body weight) was significantly decreased by the whole maize diet compared with the whole wheat and oats diets. We also found a statistically significant correlation between relative kidney weight and percentage of GHb measured at the end of the feeding trial. A significant main effect of antioxidant activity on both the relative kidney weight and GHb was found, such that greater antioxidant capacity was associated with a reduction in relative kidney weight and GHb. We have previously shown a reduction in kidney weight and plasma glucose concentration by antioxidant treatment in streptozotocin-induced diabetic rats(Reference Kim, Gallaher and Csallany58). Thus, grains with high antioxidant capacity may be beneficial in reducing kidney enlargement, an early stage in the development of diabetic nephropathy, relative to those with low antioxidant capacity.
In the present study, changes in renal hyperfiltration were evaluated by the measurement of CCr, which did not differ significantly among the diet groups at 5 months. However, the consumption of whole grains with a high level of β-glucan had slightly but significantly greater CCr compared with grains with low β-glucan, suggesting greater hyperfiltration with the consumption of β-glucan-rich grains. Given that reports of changes in CCr in GK rats, compared with age-matched Wistar rats, are inconsistent, having been reported as elevated(Reference Kim, Sohn and Kim59) or unchanged(Reference Sato, Komatsu and Kurumatani60), it is uncertain whether β-glucan-rich grains may influence the development of diabetic nephropathy. The role of antioxidant activity and β-glucan from whole grains in altering the risk of diabetic renal disease warrants further investigation.
In conclusion, the consumption of whole grains overall did not demonstrate beneficial effects on glucose control, insulin resistance or on markers of oxidative stress in GK rats after 5 months of feeding. However, the finding of lower fasting plasma glucose in the wheat, barley and oats fed groups after 2 months of feeding raises the possibility of a modest beneficial effect early in the course of diabetes. Consumption of whole barley and oats might also be advantageous in slightly delaying the progression of type 2 diabetes by improving insulin resistance in the early stage of the disease. Cereals with high β-glucan content may also limit diabetes development at an early stage by improving short-term glucose control and insulin secretion. The consumption of whole grains with a high antioxidant capacity may also exert a beneficial effect on the development of type 2 diabetes through the reduction in relative kidney weight, GHb and oxidative stress. However, whether these effects result from high β-glucan content or antioxidant capacity or whether some other component of the whole grain is responsible for the above-mentioned effects will require further investigation.
Acknowledgements
The present study was supported by General Mills Bell Institute of Health and Nutrition (Minneapolis, MN, USA) and the Minnesota Agricultural Experimental Station. None of authors have any conflicts of interest. M. Y., A. S. C., and D. D. G. designed the research, M. Y. conducted the research, M. Y. and D. D. G. analysed the data and wrote the paper. D. D. G. had primary responsibility for the final content. We thank Cindy Gallaher for excellent technical assistance. We also thank Dr Robert L. Sorenson for the kind gift of the insulin antibody and for expert guidance on the quantification of β-cell mass. The authors gratefully acknowledge Dr Cyprian Weaver and John M. Basgen for providing excellent guidance on insulin immunohistochemistry and the point counting method, respectively.










