There is some experimental evidence that anthocyanins may exhibit skin-protective properties against UV-induced oxidative stress and inflammation(Reference Tarozzi, Marchesi and Hrelia1, Reference Cimino, Ambra and Canali2). Anthocyanins, such as cyanidin, occur abundantly in various fruits and vegetables as colouring pigments(Reference Wu, Beecher and Holden3). Different mechanisms by which anthocyanins exert chemopreventive effects in the skin are postulated(Reference Afaq, Malik and Syed4). Apart from their radical-scavenging properties, one potential mechanism may be the induction of the antioxidant and the detoxifying response mediated by the redox-regulated transcription factor Nrf2(Reference Shih, Yeh and Yen5). Hwang et al. (Reference Hwang, Choi and Yun6) observed that anthocyanins from purple sweet potato extract, which is rich in cyanidin and peonidin acyl glucosides(Reference Qiu, Luo and Yao7), may induce Nrf2-dependent gene expression in dimethylnitrosamine-induced injured hepatocytes in vivo. However, to the best of our knowledge, Nrf2 activation by cyanidin has not yet been systematically studied in skin cells.
Nrf2 is a basic leucine zipper transcription factor that upon activation translocates into the nucleus, where it binds to the antioxidant response element (ARE)(Reference Lee and Surh8). The ARE is located in the promoter region of many antioxidant and phase II genes such as γ-glutamylcysteine synthetase (γGCS), NAD(P)H:quinone oxidoreductase 1 (NQO1) and haem oxygenase-1 (HO-1)(Reference Venugopal and Jaiswal9–Reference Wasserman and Fahl11). Isothiocyanates, especially sulforaphane (SFN), have been shown to induce Nrf2 gene expression in cultured fibroblasts and keratinocytes(Reference Wagner, Ernst and Iori12, Reference Ernst, Wagner and Schuemann13). SFN derives from the glucosinolate glucoraphanin present in cruciferous vegetables such as broccoli and red cabbage(Reference Steinbrecher and Linseisen14).
Interestingly, SFN and flavonoids such as quercetin and epigallocatechin-3-gallate were shown to exert synergistic gene-regulatory effects in cancer cell lines(Reference Srivastava, Tang and Zhu15, Reference Nair, Hebbar and Shen16). However, the interaction between SFN and cyanidin in terms of Nrf2 signalling has not yet been systematically investigated in the skin. We therefore studied Nrf2 and its target gene expression in HaCaT keratinocytes following incubation with SFN, a potent inductor of Nrf2, in the absence and presence of cyanidin.
Materials and methods
Cyanidin chloride (Extrasynthese, Genay, France) was freshly dissolved in dimethylsulfoxide (Carl Roth, Karlsruhe, Germany) before each experiment. SFN (Sigma-Aldrich Company, Munich, Germany), dissolved in dimethylsulfoxide, was stored at − 80°C until usage.
Cell culture
HaCaT human keratinocytes (generated by Dr N. Fusenig, DKFZ, Heidelberg and obtained from the I. A. Z. Munich, Germany) were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10 % fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (all PAA, Coelbe, Germany).
Cytotoxicity measurement
Cell viability was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay(Reference Ernst, Wagner and Lipinski17).
RNA isolation and real-time PCR
HaCaT cells were pre-cultured for 24 h (1 × 106 cells/6 well). Subsequently, cells were treated with SFN (10 μmol/l), cyanidin (1, 10 and 100 μmol/l) or SFN plus cyanidin for 6 h. Each experiment was performed in duplicate in two separate passages (passage nos. 13 and 14).
RNA was isolated with TRIsure following the manufacturer's protocol (Bioline, Luckenwalde, Germany). The primers were designed by primer3 software (Whitehead Institute for Biomedical Research, Cambridge; Steve Rozen, Heler Skaletsky) with the following sequences: HO-1: F-5′-CCAGGCAGAGAATGCTGAGT-3′, R-5′-GTAGACAGGGGCGAAGACTG-3′; γGCS: F-5′-GGCGATGAGGTGGAATACAT-3′, R-5′-CCTGGTGTCCCTTCAATCAT-3′; β-actin: F-5′-GGATGCAGAAGGAGATCACTG-3′, R-5′-CGATCCACACGGAGTACTTG-3′; Nrf2: F-5′-AAACCAGTGGATCTGCCAAC-3′, R-5′-GCAATGAAGACTGGGCTCTC-3′ (MWG Biotech, Ebersberg, Germany). Real-time PCR was performed using Sensi-Mix one-step kit (Bioline).
siRNA transfection
For transfection with siRNA, 0·2 × 106 HaCaT cells were seeded in twenty-four-well plates and pre-cultured for 24 h. Subsequently, the cells were transfected with 7·7 nmol/l siGENOME SMARTpool for Nrf2, GAPDH and non-targeting siRNA using DharmaFECT 1 (Fisher Scientific, Schwerte, Germany), following the manufacturer's instructions, for 42 h. Transfected and untransfected cells were either treated with the vehicle or SFN (10 μmol/l) for 6 h. Subsequently, RNA was isolated as described above.
Western blotting
For γGCS, HO-1 and NQO1 detection, the cells (passage nos. 13 and 14) were treated with the test compounds for 24 h. For Nrf2 detection in nucleic fractions cells were treated for 6 h. Sample preparation, electrophoresis and blotting were performed as described by Wagner et al. (Reference Wagner, Ernst and Iori12). The membranes were probed with HO-1 (1:1000; Stressgen, MI, USA), γGCS (1:10 000; kindly provided by Dr Dianne Botta and Dr Terance J. Kavanagh, University of Washington, Seattle, WA, USA), NQO1 (1:500), Nrf2 (1:200), TATA binding protein (TBP) (1:200) or actin (1:800; all Santa Cruz, CA, USA) at 4°C overnight. Subsequently, the membranes were incubated with a secondary antibody (1:4000 anti-rabbit (Bio-Rad, Munich, Germany): HO-1, γGCS, Nrf2, actin; 1:3000 anti-goat (Santa Cruz): NQO1) for 1 h. The bands were visualised using ECL reagent (Fisher Scientific) in a ChemiDocXRS system (Bio-Rad). The molecular weight of the protein bands was estimated using the WesternC protein standard (Bio-Rad).
Statistical analysis
Statistical calculations were performed with PASW 18 (IBM, Chicago, USA). If data were not normally distributed (Kolmogorov–Smirnov), ln-transformation was used. ANOVA with a Dunnett post hoc test or in the case of not normally distributed data, the non-parametric Mann–Whitney U test was applied.
Results
SFN alone (10 μmol/l) and SFN plus cyanidin (100 μmol/l) significantly increased Nrf2 nuclear protein levels. However, cyanidin (100 μmol/l) alone did not increase Nrf2 levels.
Nrf2 controls its own target gene expression(Reference Kwak, Itoh and Yamamoto18). SFN treatment increased Nrf2 mRNA levels in HaCaT cells. Importantly, following transfection with siRNA targeting Nrf2, Nrf2 mRNA was significantly down-regulated in SFN-treated cells (Fig. 1).
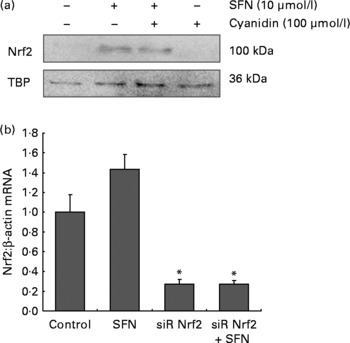
Fig. 1 (a) Nucleic Nrf2 protein levels in HaCaT cells following 6 h incubation with 100 μmol/l cyanidin in the absence ( − ) and presence (+) of sulforaphane (SFN; 10 μmol/l). (b) Nrf2 mRNA in HaCaT cells increased following 6 h incubation with 10 μmol/l SFN. Nrf2 mRNA was decreased following transfection with siRNA against Nrf2. Values are means, with their standard errors of two independent experiments in triplicate represented by vertical bars. * Mean values were significantly different compared with the control cells (P < 0·05).
MicroRNA 144 down-regulates Nrf2 expression(Reference Sangokoya, Telen and Chi19). Therefore, miRNA 144 levels in response to SFN (10 μmol/l), cyanidin (100 μmol/l) and SFN plus cyanidin treatment were determined. A moderate decrease of miRNA 144 levels (approximately 25 %) was found following SFN treatment. MiRNA 144 was not affected by cyanidin treatment (data not shown).
γGCS and HO-1 mRNA levels were significantly induced by 10 μmol/l SFN (Fig. 2(a) and (b)). Incubation with cyanidin only (100, 10 and 1 μmol/l) did not affect γGCS and HO-1 gene expression. Following co-incubation with SFN plus cyanidin, γGCS and HO-1 mRNA levels were not changed compared with HaCaT cells treated with SFN only.

Fig. 2 (a) γ-Glutamylcysteine synthetase (γGCS) and (b) haem oxygenase-1 (HO-1) mRNA levels in HaCaT cells following 6 h incubation with cyanidin (100, 10 and 1 μmol/l) in the absence ( − ) and presence (+) of sulforaphane (SFN; 10 μmol/l) compared with untreated control cells. Values are means, with their standard errors represented by vertical bars of two independent experiments. * Mean values were significantly different compared with the control cells (P < 0·05). Western blotting of (c) γGCS and NAD(P)H:quinone oxidoreductase 1 (NQO1), (d) HO-1 in HaCaT whole cell extracts following 24 h incubation with cyanidin (100, 10 and 1 μmol/l) in absence ( − ) and in presence (+) of SFN (10 μmol/l).
Similar to the gene expression data, we also found an increase in γGCS, HO-1 and NQO1 protein levels by SFN alone (10 μmol/l) and SFN plus cyanidin but not by cyanidin (100, 10 and 1 μmol/l) per se (Fig. 2(c) and (d)).
Discussion
It has been recently shown that secondary plant metabolites may antagonise Nrf2 gene expression. As demonstrated in hepatocytes, breakdown products of neoglucobrassicin, an indole glucosinolate, suppressed SFN-induced Nrf2 target gene expression(Reference Haack, Lowinger and Lippmann20), and ascorbic acid partly antagonised the Nrf2-dependent HO-1 induction by resveratrol(Reference Wagner, Boesch-Saadatmandi and Breckwoldt21). However, following co-incubation of the HaCaT cells with SFN plus cyanidin, the SFN-mediated induction of HO-1, γGCS and NQO1 was not affected.
This may be at least partly related to the low stability of cyanidin under cell culture conditions(Reference Ernst, Wagner and Lipinski17). In a previous study, we showed that cyanidin is taken up by the HaCaT cells and that its concentration decreases rapidly in the incubation medium. Only 1 % of the cyanidin concentration applied was recovered by HPLC-DAD measurements(Reference Ernst, Wagner and Lipinski17). Therefore, cellular concentrations of cyanidin in the present study may have been too low to activate Nrf2.
Interestingly, at a concentration of 25 μmol/l, the breakdown product of cyanidin, protocatechuic acid, was shown to exert Nrf2-activating properties(Reference Vari, D'Archivio and Filesi22). However, our previous studies indicate that in a cell culture medium, supplemented with 100 μmol/l cyanidin, protocatechuic acid concentration was only about 2 μmol/l (data not shown). Thus, under the conditions that were investigated, the concentrations of protocatechuic acid were possibly again not high enough to mediate Nrf2 activation in the HaCaT cells.
Cyanidin naturally occurs as glycoside and not in its less stable aglycone form as used in the present cell culture study. Considering the rat feeding study by Hwang et al. (Reference Qiu, Luo and Yao7), who found that Nrf2 is activated by an anthocyanin-rich extract, it is possible that either the conjugated form of cyanidin or other anthocyanins present in the extract may have activated Nrf2. Furthermore, glycosylated anthocyanins were shown to enhance reactive oxygen species production in cultured cells that possibly drive Nrf2 target gene expression(Reference Feng, Ni and Wang23).
In conclusion, the present data indicate that SFN is a potent inducer of Nrf2-dependent gene expression in the skin. Given that Nrf2 antagonises NFκB(Reference Wagner, Ernst and Iori12), the induction of Nrf2 target genes by SFN and other isothiocyanates may result in the protection of the skin against inflammatory stimuli such as UV, which needs to be confirmed in further studies in vivo. The present data also show that cyanidin does not induce Nrf2 targets such as HO-1, γGCS and NQO1. Furthermore, there was neither a synergistic nor an antagonistic interaction between cyanidin and SFN in terms of Nrf2 activity.
Acknowledgements
We are grateful to the DFG Cluster of Excellence ‘Inflammation at Interfaces’ for financial support. The authors state no conflict of interest. I. M. A. E., A. E. W. and P. H. performed the experiments. A. E. W., P. H. and G. R. designed the experiments. All authors wrote the manuscript and approved the final version.




