Introduction
Parrots (family Psittacidae) are one of the most threatened bird families (Marsden and Royle Reference Marsden and Royle2015, Olah et al. Reference Olah, Butchart, Symes, Medina Guzmán, Cunningham, Brightsmith and Heinsohn2016). Of the world’s 380 extant species, 28% are classified as ‘Threatened’ and 15% as ‘Near Threatened’ (BirdLife International 2021a), primarily as a result of habitat loss and degradation along with excessive capture for the pet trade (Olah et al. Reference Olah, Butchart, Symes, Medina Guzmán, Cunningham, Brightsmith and Heinsohn2016, Berkunsky et al. Reference Berkunsky, Quillfeldt, Brightsmith, Abbud and Aguilar2017, BirdLife International 2021a). The Neotropical region’s macaws (genera Ara, Anodorhynchus, Cyanopsitta, Diopsittaca, Orthopsittaca and Primolius) are perhaps the most seriously affected group within the family: of the 18 extant species, 50% are classified as ‘Threatened’ (BirdLife International 2021a). Bolivia is the country with the greatest number of macaw species, with 13 recorded in the country (Herzog et al. Reference Herzog, Terrill, Jahn, Remsen, Maillard, García-Solíz, MacLeod, Maccormick and Vidoz2019). Of these, two are ‘Critically Endangered’ Bolivian endemics, Red-fronted Macaw Ara rubrogenys and Blue-throated Macaw Ara glaucogularis, and each is restricted to a distinct ecological region, i.e. seasonally dry rain-shadowed valleys in the south-central Andes and the Llanos de Moxos grassland floodplain in the northern lowlands of Bolivia, respectively (Herzog et al. Reference Herzog, Maillard, Embert, Caballero and Quiroga2012, Reference Herzog, Terrill, Jahn, Remsen, Maillard, García-Solíz, MacLeod, Maccormick and Vidoz2019, BirdLife International 2021a).
Both regions are remote, inaccessible and characterized by poor road infrastructure. For mobile, wide-ranging birds such as macaws, this poses serious logistical and financial challenges for the obtention of rigorous, reliable data on global and breeding population sizes and temporal trends therein. This is particularly true for developing tropical countries like Bolivia, where in-country funding for such work is virtually inexistent – and where biodiversity data gaps consequently are greatest (Collen et al. Reference Collen, Ram, Zamin and McRae2008). A case in point is the Blue-throated Macaw, for which the first-ever reliable global population size estimate was determined only recently (Herzog et al. Reference Herzog, Maillard, Boorsma, Sánchez-Avila, García-Soliz, Paca-Condori, Vaílez de Abajo and Soria-Auza2021). Population size and trend data are vital, however, for evaluating the extinction risk of threatened species and devising successful, effective conservation actions or management plans.
Estimates of the Red-fronted Macaw’s global population size based on field work suggest that the species has undergone a severe and very rapid population decline from 3,000–5,000 birds in 1981–1982 (Lanning Reference Lanning1991) to 2,000–4,000 individuals in 1991–1992 (Pitter and Christiansen Reference Pitter and Christiansen1995) to only 807 macaws in 2011–2012 (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). Further, Ridgely (Reference Ridgely and Pasquier1981; also see Lanning Reference Lanning1991) reported that several hundred Red-fronted Macaws were trapped annually for the international pet trade in the late 1970s (most likely continuing at the same rate through to at least the early 1980s), which could add up to well over 1,000 birds removed from the wild population during this period. Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), however, found no clear evidence of strong genetic erosion in the population as a whole during recent decades, but rather during the last centuries or millennia, possibly coinciding with the expansion of the Incan Empire in the 15th century and concomitant intense habitat transformation in Bolivia’s inter-Andean dry valleys. Today, main threats to the species are continued habitat loss and degradation (due to forest conversion to agriculture, overgrazing, domestic and industrial firewood cutting), nest-poaching and trapping of adults for local and national pet supply and persecution of the species by local farmers as a crop pest, fuelling a presumed on-going rapid population decline (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, Pires et al. Reference Pires, Schneider, Herrera and Tella2016, BirdLife International 2021b).
Contrary to the uncertainties about the magnitude and velocity of the Red-fronted Macaw’s global population decline, the species’ natural history is well documented. Within its restricted extent of occurrence of about 21,200 km2 (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) to 27,350 km2 (Herzog et al. Reference Herzog, Maillard, Embert, Caballero and Quiroga2012, BirdLife International 2021b), it inhabits tropical dry forest, thorn and cactus scrub at altitudes of 900–3,100 m (Fjeldså and Krabbe Reference Fjeldså and Krabbe1990, Lanning Reference Lanning1991, Pitter and Christiansen Reference Pitter and Christiansen1995, Collar Reference Collar, del Hoyo, Elliot and Sargatal1997, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, Herzog et al. Reference Herzog, Terrill, Jahn, Remsen, Maillard, García-Solíz, MacLeod, Maccormick and Vidoz2019, Collar et al. Reference Collar, Boesman, Sharpe, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). Tropical dry forests are among the most threatened ecosystems worldwide (Miles et al. Reference Miles, Newton, DeFries, Ravilious, May, Blyth, Kapos and Gordon2006), and the macaw’s habitat is often highly degraded. It feeds on a variety of fruits, seeds and flower buds of at least 19 native tree species and contributes to seed dispersal in 11 of these species (Blanco et al. Reference Blanco, Hiraldo, Rojas, Dénes and Tella2015). The Red-fronted Macaw is socially monogamous and breeds semi-colonially in small cavities of tall vertical cliffs in river valleys in the Mizque, Caine, Grande and Pilcomayo watersheds during austral summer and autumn from about December to May (Boussekey et al. Reference Boussekey, Saint-Pie and Morvan1991, Lanning Reference Lanning1991, Christiansen and Pitter Reference Christiansen and Pitter1993, Rojas et al. Reference Rojas, Zeballos, Rocha, Balderrama, Aguirre, Aguayo, Balderrama, Cortez and Tarifa2009, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). Locally, in the El Palmar Natural Integrated Management Area, a few pairs also breed in cavities of the Pasopaya palm Parajubaea torallyi (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, Rojas et al. Reference Rojas, Yucra, Vera, Requeja and Tella2014).
During the non-breeding season, the species frequents cultivated areas, which are often located outside the macaw’s breeding areas (Pitter and Christiansen Reference Pitter and Christiansen1995, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). Here, they feed primarily on maize and peanut crops, resulting in local conflicts with farmers, and occur in flocks of usually up to several dozen, but occasionally up to 135 or even 200 individuals (Nores and Yzurieta Reference Nores and Yzurieta1984, Lanning Reference Lanning1991, Pitter and Christiansen Reference Pitter and Christiansen1995, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). At night, birds gather in communal roosts that can contain up to about 100 macaws (Pitter and Christiansen Reference Pitter and Christiansen1995, Herzog et al. Reference Herzog, Kessler, Maijer and Hohnwald1997). Roost sites are also used during the breeding season by non-breeding adults and immatures and by some breeding males during the incubation period (Christiansen and Pitter Reference Christiansen and Pitter1993, Herzog et al. unpubl. data).
Red-fronted Macaw life history traits are typical of long-lived and slowly reproducing species (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). In the wild, pairs raise 1–3 fledglings (Pitter and Christiansen Reference Pitter and Christiansen1995, Zeballos Reference Zeballos2006, Bonilla Reference Bonilla2007). In captivity, the species has a maximum lifespan of 36.2 years and a median age at first breeding of 4.5 years (Young et al. Reference Young, Hobson, Bingaman Lackey and Wright2012). Consequently, a large proportion of the global population, the so-called ghost fraction (Negro Reference Negro2011), is expected to be comprised of non-breeders. Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) reported a range-wide ghost fraction of about 80% (based on a minimum of only 67 and a maximum of 136 breeding pairs, and low proportions of juveniles observed after the breeding season), but this may be an overestimate as they were unable to observe the behaviour of 34 pairs at seven breeding cliffs. Pitter and Christiansen (Reference Pitter and Christiansen1995) possibly observed a smaller proportion of non-breeders in the Río Caine valley. Although they did not calculate the ghost fraction per se for this area, it can be roughly inferred from the data reported by Pitter and Christiansen (Reference Pitter and Christiansen1995). They estimated a total local population of 100 macaws, one third of which were juveniles, and 56% of all pairs they observed were accompanied by fledglings, which may suggest that roughly 35% of the local population was comprised of breeding pairs (i.e. a ghost fraction of 65%).
A number of conservation actions have been implemented over the past two decades (BirdLife International 2021b), but whether they have been successful at halting or even reversing the Red-fronted Macaw’s rapid population decline from as many as 5,000 birds in the early 1980s (Lanning Reference Lanning1991) to 807 birds in 2011–2012 (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) is unknown. With a ghost fraction as high as estimated by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), natural population recovery will almost inevitably be slow, even in the absence of threats such as the continued local trapping or persecution of macaws. On the other hand, Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) remarked that although they discovered new nesting sites, the existence of additional sites cannot be discounted. Discovery of such sites could increase population size and alter ghost fraction estimates. Here, based on a thorough range-wide breeding season survey, we reassess the Red-fronted Macaw’s breeding and total population sizes, globally and separately for watersheds, breeding areas (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) and genetic clusters (Blanco et al. Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), and its conservation status category 10 years after the 2011 breeding season survey (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). Further, we discuss the implication of our results for future conservation planning and actions. We largely followed field methods employed by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), but with greater survey effort and geographic coverage and adequate behavioural observations of almost all pairs detected at nesting cliffs. This resulted in the discovery of previously undocumented nesting sites and breeding areas, an improved accuracy and upward correction of population size estimates and a downward correction of the ghost fraction, thus providing justification for downlisting the species from ‘Critically Endangered’ to ‘Endangered’ in accordance with IUCN Red List criteria (BirdLife International 2021a) despite its fine-scale, philopatry-related genetic structure (Blanco et al. Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021).
Methods
Survey area
Our study covered the entire known breeding range of the Red-fronted Macaw on the east slope of the south-central Bolivian Andes in the departments of Chuquisaca, Cochabamba, Potosi and Santa Cruz: seasonally dry rain-shadowed valleys in the Caine, Grande, Mizque and Pilcomayo river watersheds at altitudes of 950–2,900 m (Figure 1). The natural vegetation in these valleys, which are characterized by high plant endemism, is tropical dry forest in more humid areas and thorn or cactus scrub with scattered taller trees in drier areas, but many forest areas have been heavily degraded or transformed to thorn scrub as a result of long-term human activities (Herzog and Kessler Reference Herzog and Kessler2002, Ibisch et al. Reference Ibisch, Beck, Gerkmann, Carretero, Ibisch and Mérida2003, López Reference López2003, López and Zambrana-Torrelio Reference López and Zambrana-Torrelio2006, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, BirdLife International 2021b).

Figure 1. Study area, main rivers (watersheds), Red-fronted Macaw breeding areas (white polygons), 2021 nesting sites (black dots) and potential nesting sites (grey diamonds) that were unoccupied in 2021. Numbering of breeding areas follows that of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013; see Table 2); areas 1-14 were reported by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), whereas numbers 15-18 indicate areas newly discovered by this study. Parentheses indicate breeding areas that correspond to each of the four genetic clusters (gc1-4) identified by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021; see Table 3).
Field surveys
Data collection took place mainly between 14 and 27 March 2021, with additional local follow-up surveys in two areas between 7 and 13 April (Caine River), 16 and 22 April and on 14 May 2021 (Grande River). Surveys were conducted by six teams of at least two field ornithologists each, simultaneously covering different sections of the macaw’s breeding range during the main survey in March. Field ornithologists were properly trained in data collection methods and thoroughly familiar with the field identification of all psittacid species in the survey area prior to the onset of field work. One team each covered the Caine, Grande and Pilcomayo watersheds, respectively, two teams surveyed the Mizque watershed and the sixth team surveyed areas in both the Mizque and Grande watersheds. Teams visited all past and present breeding sites and communal roosts located within the species’ breeding range that were known to the authors at the time of the survey (based on >15 years of Red-fronted Macaw field work and implementation of conservation actions by the three lead authors), and they also explored potentially suitable sites in adjacent areas. In addition, field teams interviewed inhabitants of local villages and farmsteads to obtain new information on potential breeding cliffs previously unknown to ornithologists, which then were visited. Most cliffs were accessed via unpaved roads using four-wheel-drive vehicles, but several sites could only be reached by hikes of up to several hours. Location and altitude of all sites was determined with hand-held GPS units.
Following Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), nesting sites were observed with spotting scopes and binoculars from a safe distance (to avoid disturbance) during one afternoon (mostly between 15h00 and 18h30) and one morning (mostly between 06h30 and 10h00 hrs) on consecutive days. Eighty-one per cent of all sites were observed for a total of at least four hours each. Due to logistic constraints, nine sites could only be observed for 1.0–3.5 hours each on a single day. Mean (± SD) observation time per site was 433 ± 219 minutes. The behaviour of all Red-fronted Macaw pairs associated with nesting cliffs and palms was studied closely. Each pair was then assigned to one of five mutually exclusive categories: A) non-breeding: perched near or on cliff/palm tree, but not entering or inspecting any nesting cavities; B) possibly breeding: repeatedly inspecting potential nesting cavities, but apparently not incubating or feeding chicks; C) potentially breeding: repeatedly entering and leaving the same cavity or spending substantial time inside the cavity or at the cavity entrance (typically, only one member of a pair entered the cavity, while the other member perched nearby displaying vigilant behaviour); D) confirmed breeding: feathered chicks seen at cavity entrance; E) confirmed breeding: recently fledged chicks with parents at or in the vicinity of the cliff/palm tree. Category B corresponds to non-breeding pairs, categories C to E to breeding pairs and categories B to E to the maximum number of breeding pairs as defined by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). However, given that our main survey in March took place about one month earlier than the breeding survey of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), at least some category B pairs likely would have been assigned to category C in April or May.
Like Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), we also counted numbers of non-breeding macaws that were not associated with nesting cliffs or palms and that gathered in foraging groups, at mid-day resting sites and in communal nocturnal roosts. Macaws that flew past or over nesting sites also were noted. Following Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), we recorded only the maximum number of birds observed simultaneously when several counts were available for a single site. In all cases, we took precautions to avoid double-counting of non-breeding individuals not associated with nesting cliffs. For example, for sites near nocturnal roosts, only the maximum count at the roost (obtained at dawn or dusk) was taken into account, discarding counts of macaws flying by cliffs during the day or observed foraging nearby. For nesting sites in close proximity to each other, only the maximum count of birds observed simultaneously for all sites combined was taken into account.
Our total survey effort amounted to 466.3 hours of observation time during 138 person-days of field work: 339.2 hours at nesting sites; 78.0 hours at potentially suitable cliffs where no breeding pairs were found during our survey; and 49.1 hours at roost, resting and foraging sites and cliffs considered unsuitable for Red-fronted Macaw breeding. For comparison, the total survey effort of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) was 284.5 hours, including breeding and non-breeding season surveys in seven different months over two consecutive years.
Data analysis
We used Pearson correlations to compare the number of macaw pairs associated with nesting sites (breeding pairs, non-breeding pairs, maximum number of pairs) in the eight breeding areas of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) between 2011 (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) and 2021 (this study). Data were square-root-transformed to normality where necessary.
Results
The total number of Red-fronted Macaws encountered from March to May 2021 at nesting sites, nocturnal roosts, daytime resting and foraging sites combined was 1,160 (Table 1). Nesting macaws were found in 47 distinct cliffs and eight palm trees (Figure 1, Table 1). Nineteen additional cliffs observed appeared suitable for Red-fronted Macaw nesting but were not occupied by the species (Figure 1, Table 1). A total of 206 Red-fronted Macaw pairs (412 individuals, 35.5% of the global population) were associated with nesting sites, 47 of which were assigned to category A (non-breeding, not inspecting cavities), 21 to category B (possibly breeding, only inspecting cavities), 134 to category C (showing characteristic breeding behaviour), one to category D (feathered chicks seen at cavity entrance) and three to category E (recently fledged chicks with parents at or near nesting site). Thus, categories B-E added up to 159 pairs (Table 1) that showed reproductive behaviour, whereas categories C-E added up to 138 pairs. At one cliff, a very narrow canyon, the topographic relief did not permit adequate observation of macaw behaviour, and three pairs were conservatively assigned to category A but may actually have been inspecting cavities or nesting.
Table 1. Results of the Red-fronted Macaw population survey during the 2021 breeding season in seasonally dry rain-shadowed valleys on the east slope of the south-central Bolivian Andes.

1 Distinct nesting cliffs or palm trees
2 Cliffs seemingly suitable for Red-fronted Macaw nesting, but not occupied when visited during this survey
3 Category B-E pairs as defined in Methods
4 Category A pairs as defined in Methods
5 Macaws unassociated with nesting sites
6 Includes eight nests in palm trees in the El Palmar Natural Integrated Management Area
Just over three quarters (76%) of all nesting cliffs held fewer than five breeding pairs (categories B-E), with almost half of all cliffs (43%) holding only a single pair (Figure 2A). Only two cliffs held >10 pairs; combined with three cliffs holding nine pairs each (Figure 2A), these five cliffs (11%; four in the Mizque, one in the Grande watershed) accounted for almost one third (31%) of all breeding pairs (categories B-E). The altitudinal range of nesting cliffs was 980–2,690 m and that of nesting palms 2,670–2,890 m. The altitudinal distribution of all nesting sites (Figure 2B) was fairly uniform, with small peaks at 1,300–1,599 m (almost exclusively in the Mizque watershed), 2,000–2,099 m (Grande and Pilcomayo watersheds) and 2,800–2,899 m (palm trees in the Grande watershed) and a mean (± SD) of 1,875 ± 551 m (1,711 ± 416 m for nesting cliffs only). The altitudinal distribution of the number of breeding pairs largely paralleled that of the number of nesting sites except for a major peak at 1,300-1,599 m (55% of all breeding pairs), which was much more pronounced than the corresponding nesting cliff peak (Figure 2B).

Figure 2. Frequency distribution of the number of Red-fronted Macaw breeding pairs (categories B-E, equivalent to “maximum pairs” of Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) per nesting cliff (A); and (B) altitudinal distribution in 100-m bands (900 = 900-999 m, 1000 = 1000-1099 m, etc.) of the number of nesting cliffs and palms (open squares, solid line) and breeding pairs (solid dots, dashed line) during the 2021 breeding season.
Timing of breeding activities varied considerably across the species’ breeding range both locally and regionally. In the Caine watershed, for example, pairs with characteristic breeding behaviour were observed at two cliffs at the Caine River (1,960 m) on 18–19 March, with at least one pair probably feeding young chicks inside the cavity. Only 13 km to the west-northwest of this site, in the main canyon of Torotoro National Park, no pairs were observed during the previous two days at the canyon’s principal nesting cliff (2,630 m), and it wasn’t until three weeks later, between 7 and 13 April, that the presence of pairs with characteristic breeding behaviour was confirmed in the canyon. In the Mizque watershed, on the other hand, breeding activities commenced much earlier locally: two chicks were seen at the entrance of a nesting cavity on 15 March, one fledgling foraging in trees alongside its parents on 18 March, and two fledglings were observed taking flight from the entrance of a nesting cavity on 22 March. In the Grande watershed, a fledgling was being fed by one parent just outside the nesting cavity on 16 April.
Nine nocturnal roost sites were encountered, three in the Mizque watershed and two in each of the other three watersheds. Maximum counts of roosting macaws ranged from 10 to 75, with a mean (± SD) of 40.8 ± 20.6 macaws per roost, and added up to a total of 367 individuals. Five sites were used exclusively for roosting (maximum counts: 43–75), whereas three nesting cliffs in the Mizque and one in the Pilcomayo watershed also served as roost sites (maximum counts: 10–49). The two roost sites in the Caine watershed held 65% of this watershed’s total population, and the main Pilcomayo roost held 58% of this watershed’s total population. Four daytime resting sites were detected, one in the Mizque and three in the Grande watershed, with maximum counts of 12–62 macaws.
The four watersheds differed substantially in their numbers of breeding pairs (categories B-E) and overall macaw numbers. The Mizque watershed had the greatest number of breeding pairs (84) and total macaw population (482 birds), followed by the Grande (46 and 398, respectively), Caine (20 and 181, respectively) and Pilcomayo (9 and 99, respectively) watersheds (Table 1). In relative terms, just over one half (53%) of the species’ breeding population and 41.5% of its global population occurred in the Mizque watershed, compared to only 6% and 8.5%, respectively, in the Pilcomayo watershed (Figure 3). The proportion of breeding birds ranged from 18.2% (14.1% when excluding category B pairs) in the Pilcomayo to 34.9% (30.7% when excluding category B pairs) in the Mizque watershed and amounted to 27.4% of the global population (Table 1), which corresponds to a ghost fraction of 72.6%. When taking into account only category C-E pairs (138 pairs, 23.8% of the global population), the ghost fraction increases to 76.2%.

Figure 3. Proportion of the Red-fronted Macaw breeding population (category B-E pairs; solid bars) and of the global macaw population (hatched bars) found in each of the four watersheds during the 2021 breeding season.
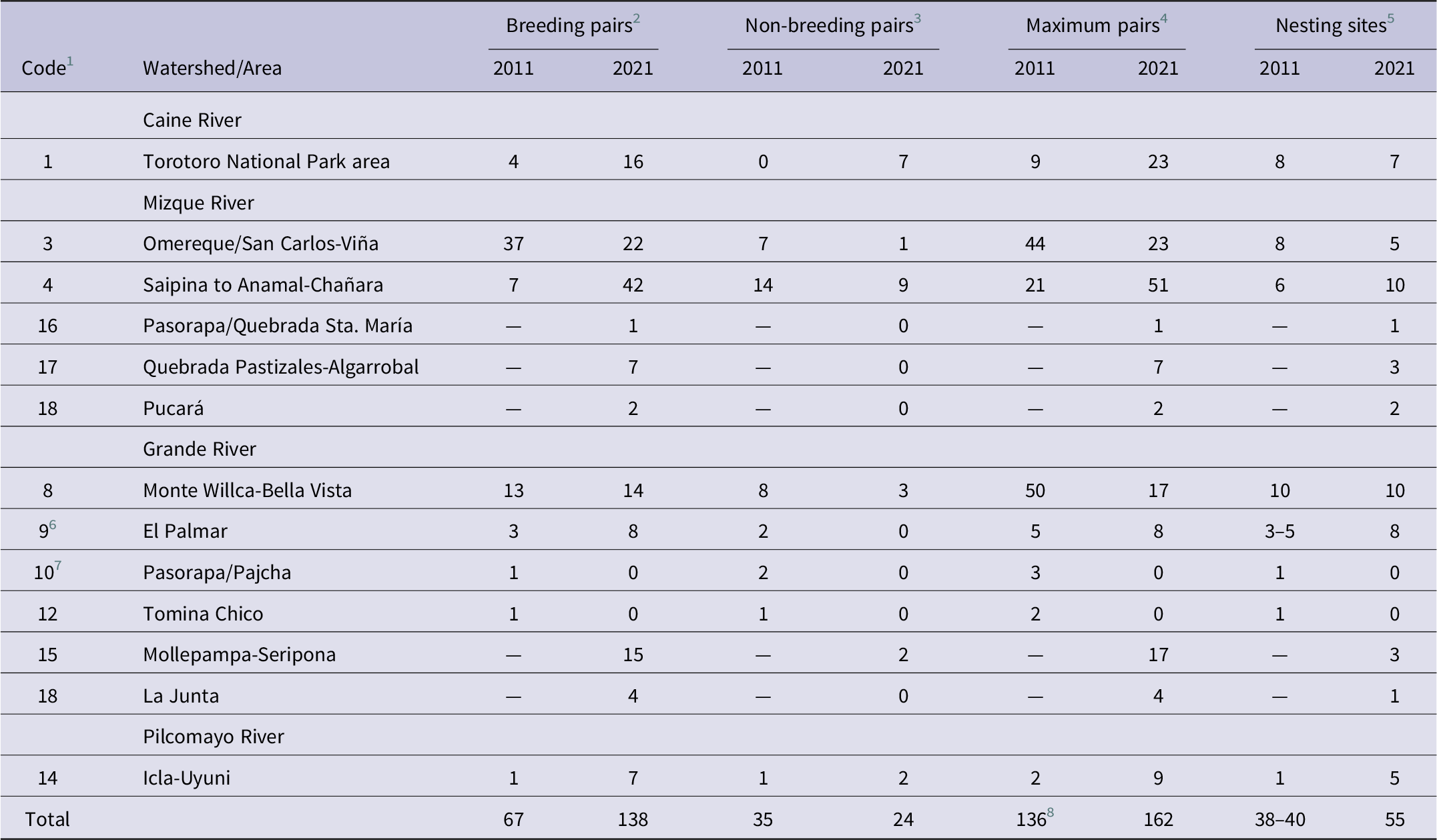
Nesting Red-fronted Macaws were found in six out of the eight breeding areas identified by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), and no macaws were detected at all in the Pasorapa/Pajcha and Tomina Chico areas (breeding areas 10 and 12, respectively, of Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013; Table 2). Additionally, 10 nesting sites in four areas not reported by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) were surveyed, where 29 breeding pairs (categories C-E; 31 pairs when including category B) were observed (Figure 1, Table 2). The Mollepampa-Seripona area (area 15; Figure 1, Table 2) consisted of three fairly dispersed nesting cliffs (8–9 km distance between adjacent sites) in the Grande River valley c.18–25 km north-east of breeding area 9 (El Palmar Natural Integrated Management Area) of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Pasorapa/Quebrada Sta. María (area 16; Figure 1, Table 2) is a somewhat isolated site c.11 km to the south-west of the nearest nesting cliff in breeding area 4 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Quebrada Pastizales-Algarrobal (area 17; Figure 1, Table 2) and Pucará (Table 2) are located in the lower Mizque River valley c.13–20 km south and 49 km south-east, respectively, of the nearest nesting cliff in breeding area 4 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). La Junta (Table 2) is situated in the Grande River valley close to the Mizque-Grande river confluence and only about 4 km north of non-breeding area 13 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). The distance between La Junta and Pucará was only 8.5 km, and the two can be treated as a single breeding area (area 18; Figure 1, Table 2). When considering all 10 breeding areas occupied in 2021, 60% of the maximum number of breeding pairs was concentrated in three breeding areas (Saipina to Anamal-Chañara, Omereque/San Carlos-Viña, Torotoro National Park), whereas five areas each held less than 10 pairs (Table 2).
Table 2. Direct comparison of the results of the 2011 Red-fronted Macaw breeding season survey reported by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013, table A2) and those obtained by the present study in the 2021 breeding season.
1 Breeding area codes corresponding to numbers in Figure 1: areas 1-14 follow Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013); areas 15-18 discovered by this study
2 Pairs showing characteristic breeding behaviour as defined by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), equivalent to category C-E pairs of this study
3 Pairs that inspected cavities and interacted with other pairs, but did not show characteristic breeding behaviour (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013), equivalent to category B pairs of this study
4 The sum of pairs showing characteristic breeding behaviour and apparently non-breeding pairs associated with nesting sites
5 Distinct nesting cliffs or palm trees
6 Erroneously identified as area 10 in Table A2 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013)
7 Erroneously identified as area 9 in Table A2 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013)
8 Includes 34 pairs (five in breeding area 1, 29 in breeding area 8) whose behaviour could not be observed by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) due constraints imposed by topographic relief
For the eight breeding areas of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) there was little coincidence between years (2011 versus 2021) in the number of breeding pairs (Pearson correlation, r = 0.61, P = 0.11), non-breeding pairs (r = 0.53, P = 0.17) and maximum number of pairs (r = 0.42, P = 0.29) (Table 2). Only the Monte Willca-Bella Vista area had similar numbers of breeding pairs in both years (13 in 2011, 14 in 2021; Table 2), but this result may be confounded by the large number of pairs in this area whose behaviour Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) were unable to observe (see Table 2). Particularly noteworthy are the shifts in the Omereque/San Carlos-Viña and Saipina to Anamal-Chañara breeding areas (Table 2), located adjacent to each other in the central Mizque watershed (Figure 1). In the former, the number of breeding pairs decreased from 37 in 2011 to 22 in 2021, whereas in the latter it increased by a factor of six from seven in 2011 to 42 in 2021 (Table 2).
With respect to the four genetic clusters identified by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), in 2021 the number of breeding pairs (categories B-E) per cluster varied from 64 (cluster 4) to nine (cluster 1), the number of nesting sites from 17 (cluster 3) to five (clusters 1 and 2) and the total Red-fronted Macaw population from 296 (cluster 3) to 99 (cluster 1) (Table 3). Clusters 3 and 4 both had substantially larger numbers of breeding pairs, nesting sites and greater total population sizes than clusters 1 or 2, and they both had over 50 breeding individuals (Table 3).
Table 3. Number of Red-fronted Macaw pairs associated with nesting sites, number of nesting sites and total population estimate in the distribution area of each of the four genetic clusters identified by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) during the breeding season 2011 (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, table A2) and 2021 (this study).

2 Pairs showing characteristic breeding behaviour as defined by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), equivalent to category C-E pairs of this study
3 Breeding pairs plus pairs that inspected cavities and interacted with other pairs at nesting sites, but did not show characteristic breeding behaviour (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013), equivalent to category B-E pairs of this study
4 Distinct nesting cliffs
5 Assumed to belong to genetic cluster 4 as these areas are geographically located between breeding area 4 and non-breeding area 11 (Tomina River valley, Grande watershed) of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), both of which were identified as genetic cluster 4 by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021)
Discussion
This study is the most thorough, time intensive and geographically complete breeding season survey of the ‘Critically Endangered’ Red-fronted Macaw to date. The results reported here substantially enhance our understanding of global, breeding and local population sizes, the degree of patchiness in the distribution of breeding areas and the conservation status of this Bolivian endemic. Our conservative global population size estimate of 1,160 macaws observed at nesting sites, nocturnal roosts, daytime resting and foraging sites combined is 43.7% higher and our observation of 138–159 breeding pairs about 40–60% higher than the corresponding estimates reported by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Similarly, the proportion of breeding birds in the global population (23.8–27.4%) is several per cent higher and more accurate than Tella et al.’s (Reference Tella, Rojas, Carrete and Hiraldo2013) inferred proportion of about 20% derived from their estimated number of breeding pairs (16.6–33.7% of the whole population) combined with the proportion of juveniles observed at the end of the breeding season (productivity). We also discovered four previously undocumented breeding areas holding 10 nesting cliffs (21% of all nesting cliffs) and 29–31 breeding pairs (about 20% of all breeding pairs). Finally, we found about twice as many breeding pairs nesting in Pasopaya palms than reported previously (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, Rojas et al. Reference Rojas, Yucra, Vera, Requeja and Tella2014).
Three mutually non-exclusive hypotheses are conceivable to explain these differences. First, the harder you look, the more you find. Our breeding season survey effort was at least twice that of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Especially in remote, inaccessible regions with poor road infrastructure, greater survey effort and geographic coverage are expected to result in greater numbers of macaws and nesting sites detected. Our discovery of new breeding areas supports this hypothesis, but even when disregarding all birds observed in these areas, the total numbers of macaws and of breeding pairs still are substantially greater than those reported by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Second, conservation efforts undertaken during the past two decades (BirdLife International 2021b) may have been sufficiently successful to result in some level of population recovery. However, growth as steep as ≥40% in both global and breeding populations over a 10-year period seems unrealistic, and population growth alone therefore is unlikely to explain the differences between our results and those of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013).
Third, with respect to the number and proportion of breeding pairs, interannual variations in that proportion, in the timing of breeding activities (Monterrubio et al. Reference Monterrubio, Enkerlin-Hoeflich and Hamilton2002) or in nesting success (Monterrubio et al. Reference Monterrubio, Enkerlin-Hoeflich and Hamilton2002, Renton and Salinas-Melgoza Reference Renton and Salinas-Melgoza2004, Rivera et al. Reference Rivera, Politi, Bucher and Pidgeon2014) could contribute to the differences between our results and those of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). Unfavourable climatic conditions and concomitant reduced food availability (Renton and Salinas-Melgoza Reference Renton and Salinas-Melgoza2004, Rivera et al. Reference Rivera, Politi, Bucher and Pidgeon2014) possibly could have limited the number of reproductively active pairs or overall nesting success in 2011, although we have no direct evidence to support this hypothesis. However, we observed fledglings and feathered chicks from three different nests in mid-March in the Mizque watershed and an additional fledgling in mid-April 2021 in the Grande watershed, whereas Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) observed only two feathered chicks and no fledglings in April-May 2011, suggesting that Red-fronted Macaw breeding may have started later or that nesting success may have been lower in 2011 than in 2021. Given the species’ incubation period of 25–26 days and a nestling period of 70–73 days (in captivity; Hollingshead and Hollingshead Reference Hollingshead and Hollingshead1989, Collar et al. Reference Collar, Boesman, Sharpe, del Hoyo, Elliott, Sargatal, Christie and de Juana2020), egg-laying and incubation in the Mizque watershed must have started in mid-December 2020 (as reported by Lanning et al. 1991). Opposed to this, Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) found that in January 2011 most pairs were only prospecting nest cavities. Overall, these considerations suggest that caution is in order when drawing conclusions about population sizes and ghost fractions (Negro Reference Negro2011) from single-breeding-season surveys as was done by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) and Pacífico et al. (Reference Pacífico, Barbosa, Filadelfo, Oliveira, Silveira and Tella2014).
Low coincidence between survey years in the number of pairs associated with nesting sites in each of the eight breeding areas of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) further suggests the existence of interannual and local variations in Red-fronted Macaw reproductive activity. In this regard, the pronounced decline in the number of nesting pairs in the Omereque/San Carlos-Viña breeding area and the even steeper increase in the number of nesting pairs in the adjacent (30 km distance) Saipina to Anamal-Chañara breeding area merit closer examination. In the former area, peanuts formed an important food source for nesting macaws each year from April to June/July (when macaws mainly fed on remaining peanut seeds in already harvested field; authors’ unpubl. data), but local farmers stopped producing peanuts between 2013 and 2015. Subsequently, from April 2017 to early 2018, road construction (widening and paving of a gravel road, including use of explosives for rock blasting) within minimum distances of only 150–200 m of the three San Carlos nesting cliffs in the Red-fronted Macaw Communal Nature Reserve exposed macaws to previously unexperienced levels of human disturbance. Both events may have caused some breeding pairs to abandon the site and emigrate to other breeding areas, and these circumstances therefore may have contributed to, or may even largely account for, the decrease in breeding pairs from 37 in 2011 to 22 in 2021. Emigration to the nearest breeding area seems most likely, and the six-fold increase in breeding pairs from seven in 2011 to 42 in 2021, and a parallel increase from six to 10 nesting cliffs, in the Saipina to Anamal-Chañara breeding area supports the hypothesis that emigrating San Carlos pairs settled in that breeding area.
The discovery of four new breeding areas during our field surveys shows that the Red-fronted Macaw’s breeding range is notably more continuous than previously thought (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). It further raises the question as to whether these areas form part of any of the genetic clusters identified by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), or if they are distinct population nuclei that should be treated as independent conservation units (Fraser and Bernatchez Reference Fraser and Bernatchez2001, Palsbøll et al. Reference Palsbøll, Bérubé and Allendorf2007). Two of these areas (13 breeding pairs, six nesting cliffs), situated in the lower Mizque River valley, extend the known breeding range in this watershed southward to the confluence of the Mizque and Grande rivers, where the altitudinally lowest (980 m) nesting cliff is found. It seems likely that these areas are part of Blanco et al.’s (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) genetic cluster 4 as they are geographically located between breeding area 4 (central Mizque River valley) and non-breeding area 11 (Tomina River valley in the Grande watershed) of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), both of which were identified as genetic cluster 4 by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021). The third area, which held only a single breeding pair, may also be assignable to genetic cluster 4 due to its close proximity to breeding area 4 of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013). The fourth area (15–17 breeding pairs, 3 nesting cliffs), in the central Grande River valley, is geographically located in-between genetic clusters 3 and 4 and between two small breeding areas that were not sampled genetically by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021). Its genetic affinity is therefore entirely uncertain.
Our new findings indicate that the genetic structure of the Red-fronted Macaw’s global population requires further study and much more complete sampling, which already was pointed out by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), who sampled only five of the eight breeding areas known at the time. Today, 12 breeding areas are known, nine of which held at least six breeding pairs each in 2021. The need for further study is particularly important if, as part of a comprehensive global conservation strategy, independent evolutionary units are to be defined and preserved as components of the species’ overall genetic integrity as was proposed by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021). Testing our hypothesis that cluster 2 breeding pairs immigrated into cluster 4 is especially relevant in this respect. If true, this event could lead to an intermixing of genetic clusters 2 and 4 and therefore alter the Red-fronted Macaw’s fine-scale, philopatry-related genetic structure. Clusters 2 and 4 already were the genetically most closely related population nuclei in 2011 (Blanco et al. Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), and current or future intermixing likely could further reduce or completely eliminate the already limited genetic differentiation between these two population nuclei.
In addition, it should be pointed out that genetic differences between all four clusters identified by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) were small. Their study detected genetic differentiation between population nuclei only when using sample location priors in their statistical analyses (i.e. inclusion of sample group information in genetic clustering algorithms), which, as pointed out by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) themselves, suggests a weak population structure. Models incorporating sample location priors were developed specifically to allow detection of weak population structure (Hubisz et al. Reference Hubisz, Falush, Stephens and Pritchard2009). Although this was acknowledged by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021), their discussion and conclusions emphasized only the differences detected, but not their magnitude. Further, Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) even suggested a possibly complete lack of genetic intermixing although their analyses supported a gene flow-drift equilibrium model over a drift-alone model to explain the genetic differentiation between clusters. To support this, they suggested that the apparently low gene flow detected between genetic clusters may simply be due to the sampling of transient individuals that moved between colonies in different population nuclei. This is largely speculative, however, and was not supported by any direct evidence by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021). Thus, while the existence of fine-scale genetic structure in the absence of geographic and ecological barriers is most certainly noteworthy and of scientific interest, given incomplete geographic sampling and the weak genetic differentiation encountered, Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) may have overstated the conservation relevance of their findings.
The lack of evidence of strong genetic erosion in the global Red-fronted Macaw population in recent decades reported by Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) is robust but somewhat unexpected in light of the reports of Ridgely (Reference Ridgely and Pasquier1981) and Lanning (Reference Lanning1991) that up to several hundred (300–400+) Red-fronted Macaws were trapped annually for the international pet trade in the late 1970s and early 1980s. These numbers did not include individuals accidentally killed or injured by trappers during capture attempts with cannon nets (Ridgely Reference Ridgely and Pasquier1981) or individuals that did not survive transport between Bolivia and destination countries, and mean annual totals of macaws removed from the wild population can therefore be expected to have exceeded 400. Trapping at these levels took place from 1978 (Ridgely Reference Ridgely and Pasquier1981) to at least 1983, when the Red-fronted Macaw was listed on Appendix 1 of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), or 1984, when the Bolivian government banned the export of all live wildlife (Jorgensen and Thomsen Reference Jorgensen and Thomsen1987, Lanning Reference Lanning1991). However, lack of or delayed government enforcement of this ban probably resulted in the continued trapping of macaws and illicit trafficking to neighbour countries at similar levels for at least another year or two (see Silva Reference Silva1989). Thus, removal of an average of 400 birds annually by professional trappers would have added up to a conservative estimate of about 3,200 Red-fronted Macaws over the eight-year period from 1978 to 1985. Lanning (Reference Lanning1991) studied the species between late December 1981 and early March 1982, estimating a global population of 3,000–5,000 birds, albeit based on a partial census extrapolated to the potential distribution of the species. If, prior to Lanning’s (Reference Lanning1991) survey, about 1,500 macaws were removed by trappers between 1978 and 1981, the Red-fronted Macaw’s global population size prior to the onset of large-scale professional trapping would have consisted of 4,500–6,500 birds. Consequently, removal of approximately 3,200 macaws between 1978 and 1985 would have corresponded to a global population size reduction of about 50–70%. Although the degree of uncertainty around this estimate is high, in the absence of more accurate data on the global population size prior to professional trapping and actual numbers of macaws exported or accidentally killed by trappers and dealers, obtaining a more accurate population reduction estimate is probably not possible.
Presently, poaching for the illegal domestic trade (locally and nationally) is presumed to be one of the major threats to Red-fronted Macaws (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013, Pires et al. Reference Pires, Schneider, Herrera and Tella2016, BirdLife International 2021b). However, up-to-date quantitative information is lacking. The number of Red-fronted Macaws traded annually in the Los Pozos market in Santa Cruz de la Sierra, which was Bolivia’s most important market for illegal wildlife trade, was 26–32 birds (Herrera and Hennessey Reference Herrera and Hennessey2007, Pires et al. Reference Pires, Schneider, Herrera and Tella2016), but this information dates back to 2004–2005, and the number of macaws traded in more recent years is unknown. About five years ago, open trade of parrots and other wildlife in the Los Pozos market was shut down thanks to law enforcement efforts of wildlife authorities of the regional government of the department of Santa Cruz. It is noteworthy that all or almost all Red-fronted Macaws traded in 2005, which included both adults (66%) and nest-poached juveniles, were captured in a single municipality in Santa Cruz department (Vallegrande; Pires et al. Reference Pires, Schneider, Herrera and Tella2016). Although the species is known to move long distances within its distributional range outside the breeding season (Meyer Reference Meyer2010), this indicates that not all breeding areas were equally affected by the Los Pozos market trade. The spatially closest breeding areas in the Mizque watershed likely were the most affected, whereas those in the more distant Caine and Pilcomayo watersheds were unlikely to be affected in any significant way. In recent years, however, trapping and nest poaching appear to have increased in the Caine watershed (Torotoro National Park area; T. Calahuma-Arispe unpubl. data, J. L. Tella in litt. 2022), perhaps as a result of the closure of the Los Pozos market and a resulting shift of illegal trade activity from Santa Cruz to Cochabamba. A detailed analysis of the current situation is urgently needed.
Considerable local demand for pet macaws in rural villages recently was reported as a previously overlooked threat by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013), who found 45 Red-fronted Macaws as pets in a fairly small sample of villages within the distributional range of the species. These birds originated from both nest poaching in five breeding areas and trapping of adults in two breeding and three non-breeding areas (Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013). This number has since increased to 103 as a result of continued pet surveys in additional villages in more recent years (J. L. Tella in litt. 2022). The total number of pet macaws in local villages therefore amounts to at least about 10% of the entire population in the wild, and Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) postulated that the impact of local pet demand seems to be greater than that of the illegal trade in large cities. Although they are a reason for concern, these raw numbers are of limited value to conservation assessments because the time span over which these birds were extracted from the wild population is unknown. Given that Red-fronted Macaws are relatively long-lived pets (maximum lifespan of 36.2 years in captivity; Young et al. Reference Young, Hobson, Bingaman Lackey and Wright2012), these birds could have been poached or trapped over a period of over 30 years, which would correspond to an average extraction rate of only about three macaws per year. On the other hand, the actual number of pet macaws in all villages throughout the species’ range can be expected to be at least 2–3 times higher than the number of detected cases. In addition, some macaws are likely killed or seriously injured during capture attempts each year. Based on these preliminary considerations, we venture to estimate that local demand for pet macaws in rural villages causes the removal of about 10–15 birds annually from the wild population, less than 50% of the number of birds traded in the Los Pozos market in 2005. Persecution and deliberate killing of Red-fronted Macaws by local farmers who consider them a crop pest possibly pose a greater threat, but no data exist on this issue.
Conservation implications and outlook
The Red-fronted Macaw is currently listed as ‘Critically Endangered’ based on IUCN Red List criterion C2a(i) (BirdLife International 2021b), i.e. “population size estimated to number fewer than 250 mature individuals; and a continuing decline, observed, projected or inferred, in numbers of mature individuals; and no subpopulation estimated to contain more than 50 mature individuals.” Our results demonstrate that this criterion no longer applies to the Red-fronted Macaw. First, our record of 138–159 breeding pairs demonstrates that the species’ global population contains at least 276–318 mature individuals, which is a conservative estimate as certainly not all reproductively active pairs were detected by our field survey. Additional undiscovered breeding areas or nesting cliffs are likely to exist in highly inaccessible areas within or just outside the species’ known breeding range. Particularly the Icla-Uyuni breeding area in the Pilcomayo watershed holds potential for the discovery of additional nesting cliffs to the south of the currently known sites. Further, as has been shown for the Blue-throated Macaw Ara glaucogularis (Berkunsky et al. Reference Berkunsky, Daniele, Kacoliris, Díaz-Luque, Silva Frias, Aramburu and Gilardi2014), not all pairs that breed in any given year may attempt to nest the following year, which inevitably would lead to an underestimation of the total number of mature individuals by single-breeding-season surveys. Finally, given the species’ extended 6–7-month reproductive season and the spatial variation observed in the timing of breeding activities, single 2-day ‘snapshot’ visits to specific nesting sites inevitably underestimate the total number of breeding pairs in any given season. This is an important variable that should be factored into future monitoring plans, which should consider repeated visits at regular intervals, spanning the entire breeding season, to at least the most important nesting sites in each watershed (see below).
Second, we found that two of the four genetic clusters (subpopulations, i.e. geographically or otherwise distinct groups in the population between which there is little demographic or genetic exchange; BirdLife International 2021a) of Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021) each held over 50 mature individuals. In cluster 3 (the Torotoro National Park and Monte Willca-Bella Vista breeding areas) we recorded at least 30–40 breeding pairs (60–80 mature individuals) and in cluster 4 (the Saipina to Anamal-Chañara breeding area) at least 42–51 breeding pairs (84–102 mature individuals). If two of our newly discovered breeding areas (Quebrada Pastizales-Algarrobal, Pucará-La Junta) also belong, as we suspect, to cluster 4, the total number of breeding pairs in this cluster amounts to 55–64 breeding pairs (110–128 mature individuals). Third, although we do not have direct evidence that the Red-fronted Macaw’s global population is no longer in decline, the fact that our population size estimates are ≥40% higher than those of Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) strongly suggest that the observed or inferred population decline between about 1975 and 2010 (Ridgely Reference Ridgely and Pasquier1981, Lanning Reference Lanning1991, Pitter and Christiansen Reference Pitter and Christiansen1995, Tella et al. Reference Tella, Rojas, Carrete and Hiraldo2013) has been largely halted.
As we cannot fully rule out that the species’ population continues to decline, particularly in light of ongoing nest poaching and trapping for illegal trade and local pet demand combined with persecution by local farmers, we propose downlisting the Red-fronted Macaw to ‘Endangered’ under IUCN Red List criterion C2a(i) (BirdLife International 2021a), i.e. “population size estimated to number fewer than 2,500 mature individuals; and a continuing decline, observed, projected or inferred, in numbers of mature individuals; and no subpopulation estimated to contain more than 250 mature individuals.” Such downlisting by no means implies that on-going conservation efforts can be relaxed. The number of mature macaws still is dangerously close to the threshold of <250 mature individuals required for a listing of ‘Critically Endangered,’ and the main threats to the species documented by Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) have not ceased. We agree with Tella et al. (Reference Tella, Rojas, Carrete and Hiraldo2013) that awareness and education programs are key conservation actions to reduce local demand for pet macaws and other parrot species. Perhaps even more important is working jointly with farmers who are killing macaws as crop pests to determine and implement win-win solutions beneficial to both farmers and Red-fronted Macaws. Alternative livelihood opportunities such as nature-based tourism are a promising approach that already has converted local farmers into conservation stewards such as in the Red-fronted Macaw Communal Nature Reserve (Omereque municipality), which was established with the support of Asociación Armonía and is the species’ most important local breeding site (19–20 breeding pairs in three adjacent cliffs in 2021).
Particularly encouraging in all respects are fairly recent subnational public conservation and sustainable management efforts in the form of municipal protected areas (MPAs) within the species’ breeding and foraging range, i.e. the MPAs Jardín Cactáceas (Comarapa municipality) and Pasorapa (Pasorapa municipality) and the integrated natural management areas Monte Willca (Sucre municipality) and Lagarpampa-Mollepampa (Aiquile municipality). The Red-fronted Macaw is a focal species of conservation efforts in all areas. The same is true for the two national-level protected areas that hold breeding populations of the species, i.e. Torotoro National Park and El Palmar Natural Integrated Management Area. When including the Red-fronted Macaw Communal Nature Reserve, 22 nesting sites (14 cliffs, 8 palm trees) and 56–63 breeding pairs were located inside these subnational and national protected areas in the 2021 breeding season. An additional 14 nesting cliffs and 55–65 breeding pairs were located within 1 km of the borders of these protected areas. Thus, about 80% of all breeding pairs could be safeguarded through effective protection, management and monitoring by these conservation areas in collaboration with local communities. First and foremost, such protection should include the immediate implementation of effective actions (e.g. surveillance or regular patrolling of nesting sites, local education campaigns) to eliminate or at least reduce nest poaching and trapping.
Spearheaded by Asociación Armonía and Fundación Natura Bolivia, the development of a national conservation action plan for the Red-fronted Macaw is currently underway, in which all aforementioned and additional important stakeholders are participating. This process will provide a comprehensive roadmap for coordinated, collaborative conservation and management actions across key public, communal and private stakeholders, hopefully leading to a documented population recovery and growth over the next few years. Regular population monitoring will be essential to evaluate whether such actions are successful in stabilizing and progressively increasing the species’ global and breeding population sizes. Range-wide breeding season surveys by multiple teams that simultaneously visit all known breeding sites, as carried out in the present study, are labour-intensive, logistically complex and costly, and they are therefore best implemented at longer (e.g. five-year) intervals to optimize limited conservation resources. In addition, as pointed out above, because this approach requires single snapshot visits to specific nesting sites it almost inevitably will underestimate the total number of breeding pairs in any given season.
To quickly detect population trends or changes therein, we recommend conducting annual monitoring of a smaller geographic sample focusing only on the most important Red-fronted Macaw nesting areas in each of the four watersheds. These include the Torotoro National Park area in the Caine watershed; the Omereque/San Carlos-Viña and Saipina to Anamal-Chañara areas in the Mizque watershed; the Monte Willca-Bella Vista, El Palmar and Mollepampa-Seripona areas in the Grande watershed; and the Icla-Uyuni area in the Pilcomayo watershed. We recommend monthly or ideally bi-weekly afternoon and consecutive morning visits (this optimizes the use of time as the unproductive hot mid-day hours can be used to move from one site to the next) to each breeding site, or at a bare minimum to those sites with five or more breeding pairs (12 cliffs plus the El Palmar area in 2021), throughout the entire breeding season from December to May. This would provide an almost complete picture of the total number of pairs breeding or attempting to breed at each site during a given season, and it would allow for estimations of reproductive success and, in many cases, even productivity (number of fledged or almost fledged chicks per nesting pair). If financial constraints do not permit bi-weekly monitoring throughout the entire breeding season, we recommend monthly visits during the first half (December to February) and bi-weekly visits during the second half (March to May) of the season. Involving local stakeholders (e.g. park guards of the El Palmar Natural Integrated Management Area and Torotoro National Park), previously trained and equipped with binoculars and spotting scopes, would help reduce monitoring costs and generate local conservation stewardship and awareness.
Acknowledgements
We are grateful to all communities for allowing us to work on their lands; to the directors of Torotoro National Park (Felix Mamani) and El Palmar Natural Integrated Management Area (Carolina Martínez Mostajo) for supporting our field work in these protected areas; to the Museo de Historia Natural Alcide d’Orbigny, Cochabamba, and the autonomous regional governments of the departments of Cochabamba and Santa Cruz for institutional support; and to the following individuals for their help during field work: Celso Aguilar Juchasara, Juvenal Choque Flores, Juanito Escalante, Lucía Fernández Jauregui, Erlan Montero Montaño, Darly Pantoja Flores, Basilio Péres Guzmán, Beatriz Quispe Choque, Aly Rocha Valverde, José Vallejos Rachi and Eleuterio Yucra Reyes. This study was made possible thanks to the generous financial support provided by American Bird Conservancy, the Mohamed bin Zayed Species Conservation Fund and Fundación Natura Bolivia (special thanks to María Teresa Vargas and Nigel Asquith). Finally, we thank Jennifer R. A. Cahill for sharing her views on the genetic analyses of Blanco et al. (Reference Blanco, Morinha, Roques, Hiraldo, Rojas and Tella2021); and Daniel J. Lebbin, John C. Mittermeier and José L. Tella for careful revision and comments on the manuscript.








