Introduction
Plants may produce compounds that directly or indirectly affect their biological environment. These compounds are called allelochemicals and have a role in the growth, health, and behaviour of other organisms (Aissani et al. Reference Aissani, Tedeschi, Maietti, Brandolini, Garau and Caboni2013). Root-knot nematodes (Meloidogyne spp.) are among a vast array of pests continually attacking plants and causing approximately US$70 billion of crop losses in fruit and vegetable production annually (Aissani et al. Reference Aissani, Urgeghe, Oplos, Saba, Tocco, Luigi Petretto, Eloh, Menkissoglu-Spiroudi, Ntalli and Caboni2015). Meloidogyne spp., which is the most common and widespread group of root-knot nematodes in the world, increases the severity of soil borne diseases (Aissani et al. Reference Aissani, Urgeghe, Oplos, Saba, Tocco, Luigi Petretto, Eloh, Menkissoglu-Spiroudi, Ntalli and Caboni2015; Caboni et al. Reference Caboni, Ntalli, Aissani, Cavoski and Angioni2012). Many scientific studies have reported data on the biological activity of plant secondary metabolites on root-knot nematodes. Aldehydes from Ailanthus altissima as (E,E)-2,4-Decadienal and (E)-2-Decenal, methylisothiocyanate from caper were active on Meloidogyne javanica (Caboni et al. Reference Caboni, Ntalli, Aissani, Cavoski and Angioni2012a; Caboni et al. Reference Caboni, Sarais, Aissani, Tocco, Sasanelli, Liori, Carta and Angioni2012b), and allyliosthiocyanate from Armoracia rusticana was active against Meloidogyne incognita (Aissani et al. Reference Aissani, Tedeschi, Maietti, Brandolini, Garau and Caboni2013).
Buckwheat (Fagopyrum esculantum Moench.) is a well-established special crop that has been grown on Eastern prairies for the last 40 years. Seeds of common buckwheat, or sweet buckwheat, are usually consumed in Asia, Europe, North America, South Africa, and Australia (Li et al. Reference Li and Zhang2001). At the present time, the only attributes used to evaluate buckwheat quality are color and flavor related to volatile aldehydes (Przybylski et al. Reference Przybylski, Woodward, Eskin, Malcolmson and Mazza1995). Buckwheat contains high levels of nutritionally beneficial components and can be processed into various functional foods. There have been a large variety of buckwheat-based food products available in the market, such as buckwheat noodles, pasta, bread, tea, spirits, and vinegar (Ikeda et al. Reference Ikeda2002).
Recent research has found that buckwheat seed extract has strong antioxidant activity (Lin et al. Reference Lin, Cai and Liao2002). In addition, immunostimulant and antivirulant activities of seeds have been noted (Bai et al. Reference Bai, Ji, Feng, Hao, Zhong, Cui and Wang2015; Yuan et al. Reference Yuan, Yan, Ye, Wu and Ng2015). Besides the work of Sipes and Arakaki dealing with the nematicidal activity of buckwheat against Meloidogyne javanica (Sipes et al. Reference Sipes and Arakaki1997), there is no available report on the nematicidal activity of F. esculantum on M. incognita, the most widespread Meloidogyne species.
In the present investigation, we report for the first time (1) the chemical characterization of F. esculantum aerial part methanol extract by GC-MS, (2) the nematicidal activity of this latter on M. incognita with and without taking into account moisture, (3) the preliminary structure–activity relationship of the most abundant aldehyde with other selected ones, (4) the correlation between nematicidal activity and antioxidant power, and (5) their synergistic activity.
Material and methods
Chemicals
Aldehydes standards, fosthiazate of purity greater than 98%, Tween-20, and dimethylsulfoxide were obtained from Sigma-Aldrich. Methanol and water were high-performance liquid chromatography (HPLC)-grade.
Plant materials and extraction
Buckwheat seeds were purchased from a local market in Cagliari, Italy, in March 2016. Voucher specimens were deposited at the Laboratory of Functional Physiology and Valorization of Bioresources, Higher Institute of Biotechnology of Beja-University of Jendouba-Tunisia, for species identification. Seeds were germinated in cotton for 2 weeks at a temperature of 24 °C. Fresh aerial plant parts were collected and ground (100 g) and extracted with methanol or water (1:1 w/v) in a sonicator apparatus for 15 min, filtered through a Whatman no. 40 filter, and centrifuged for 15 min at 13,000 rpm. The extracts were then used to assess the nematicidal activity. The same protocol was repeated after drying plant aerial parts at 105 °C for 24 h and moisture determination.
Nematode population
A population of M. incognita originally reared from tomato roots (Solanum lycopersicum L.) cv. Belladonna, a cultivar that is very susceptible to root-knot nematodes, was collected from a greenhouse in Cagliari, Italy. All plants were maintained in plastic pots (18 cm diameter) in a growth chamber at 25–28 °C with 60% relative humidity and a photoperiod of 16 hours. Plants used for inoculations were 7 weeks old and at the five-leaf stage. After 40 days, the plants were uprooted, and the roots were washed free of soil and cut into 2-cm pieces. Eggs were extracted according to the sodium hypochlorite procedure, and second-stage juveniles (J2) were allowed to hatch in modified Baermann funnels at 28 °C. All J2 hatchings in the first 3 days were discarded, and thereafter, J2 collected after 24 h were used in the experiments (Caboni et al. Reference Caboni, Tronci, Liori, Tocco, Sasanelli and Diana2014).
Nematicidal assay
The nematicidal activity of methanol and aqueous extracts and compounds on nematode juvenile infestive stage (J2) paralysis was tested, and the EC50 values were calculated. Stock solutions were prepared by dilution with dimethyl sulfoxide, whereas working solutions were obtained by dilution with distilled water containing the polysorbate surfactant 20 (Tween-20). Final concentration of dimethyl sulfoxide in each well never exceeded 2% v/v because preliminary trials showed that paralysis of nematodes exposed at those concentration levels was similar to paralysis of nematodes maintained in distilled water (Aissani et al. Reference Aissani, Tedeschi, Maietti, Brandolini, Garau and Caboni2013). Distilled water, as well as a mixture of water with dimethyl sulfoxide and Tween-20 at concentrations equivalent to those in the treatment wells, served as a negative control, whereas fosthiazate at the same concentrations was used as a positive control. Twenty-five juveniles were used per treatment well in Cellstar 96-well plates (Greiner bio-one, Milan-Italy). The plates were covered to prevent evaporation and were maintained in the dark at 28 °C. Border wells containing plain water with nematodes were placed around the wells of each treatment to check the vapor drift among wells that could possibly interfere with the efficacy results. Juveniles were observed with the aid of an inverted microscope (Zeiss, West Germany- Oberkochen) at 40x after 24 h and were ranked into two distinct categories: motile or paralyzed. After the assessment, the nematodes were transferred into plain water, after washing in tap water through a 20 μm pore screen to remove the excess of tested compounds, and they were assessed again after 24, 48, and 72 h for the re-obtainment of motility. Dead nematodes were characterized by the presence of internal vacuoles. In another experiment, nematodes were treated with single aldehydes found by GC-MS analysis or for synergistic activity.
GC-MS analysis
A gas chromatograph HP 5890 Series with a mass spectrometric detector (MSD) 5972 from Hewlett-Packard (Palo Alto, CA, USA) was used. Column: VOCOL, 60 m 0.25 mm (i.d.), film thickness 1.5 μm (Supelco, Bellefonte, PA, USA). Temperature program: starting temperature 50 °C (2 min), heating rate 5 °C /min, final temperature 210 °C (40 min). Tinj: 250 °C; Tdet: 280 °C; injection volume: 1 μl. Carrier gas: He, flow 1 ml/min. MS conditions: electron impact mode, total ion current (TIC) recorded. Mass spectra of the compounds were compared to the spectra from the NIST02 spectral library and with mass spectra of reference compounds. Identities of compounds were confirmed by comparison of their retention times with the retention times of reference compounds.
Antioxidant assay
The antioxidant capacity of the tested compounds was performed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity, as described previously by Grzegorczyk et al. (2007). Briefly, various concentrations of aldehydes (0.3–200 mg/ml) were added to 1 ml of 0.1-mM methanol solution of DPPH and incubated at 27 °C for 30 min. The optical density of the sample was quantified at 517 nm. DPPH radical-scavenging activity (RSA), expressed as percentage, was estimated utilizing the following formula:
Ascorbic acid (Sigma-Aldrich) was used as a reference molecule in the same concentrations as the tested extract. All the analyses were carried out in triplicates. The EC50 value was determined as the concentration of the compound required to scavenge 50% of the DPPH radicals.
Statistical analysis
Treatments of paralysis experiments were replicated six times, and each experiment was performed twice. The percentages of paralyzed J2 in the microwell assays were corrected by elimination of the natural death/paralysis in the water control according to the Schneider Orelli formula, corrected (Puntener et al. Reference Puntener1981):
They were analysed (ANOVA) after being combined over time. Because ANOVA indicated no significant treatment by time interaction, means were averaged over experiments. Corrected percentages of paralyzed J2 treated with test compounds were subjected to nonlinear regression analysis using the log-logistic equation proposed by Seefeldt et al. (Reference Seefeldt, Jensen and Fuerst1995):
where C = the lower limit, D = the upper limit, b = the slope at the EC50, and EC50 = the test compounds concentration required for 50% paralyzed nematodes after elimination of the control (natural death/paralysis). In the regression equation, the test compounds concentration (% w/v) was the independent variable (x), and the paralyzed J2 (percentage increase over water control) was the dependent variable (y). The mean value of the six replicates per compound concentration and immersion period was used to calculate the EC50 value.
Results
Moisture determination
The two weeks aged aerial part plants moisture was calculated at 60.5% dealing with the richness of this plant on water and allowing the assessment of the nematicidal activity of the extract before and after drying the plant.
J2 paralysis bioassays
Without taking into account plant compound bioavailability or the synergetic effect when the extract was tested against M. incognita, a linear dose–response relationship was established, and significant paralysis/death of nematodes was evident after 48 and 72 hours of exposure to methanol extract with an EC50 calculated value of about 62.6 ± 26.0 (R2 = 0.99) and 40.8 ± 26.1 μg/ml (R2= 0.96), respectively (Figure 1). Nematicidal activity of the methanol extract of fresh plant was EC50 = 127.7 ± 67.2 and 98.3 ± 54.0 μg/ml after the same period of immersion, respectively, whereas aqueous extracts of the fresh and dried plant were not active, with EC50 > 1000 μg/ml during the same period (Table 1).

Figure 1. Curves of nematode death after 48 and 72 hours of immersion in 10, 20, 60, and 100 μg/ml of buckwheat methanol extract. No death was noted in control (Tween 20).
Table 1. EC50 of tested extracts on M. incognita J2

GC-MS analysis of methanol extract
Using GC-MS of these experimental conditions, we were able to identify and quantify 17 aliphatic and aromatic aldehydes. The most abundant aldehyde was salicylaldehyde at 16 % (Table 2).
Table 2. Nematicidal activity of the tested compounds (n = 3)
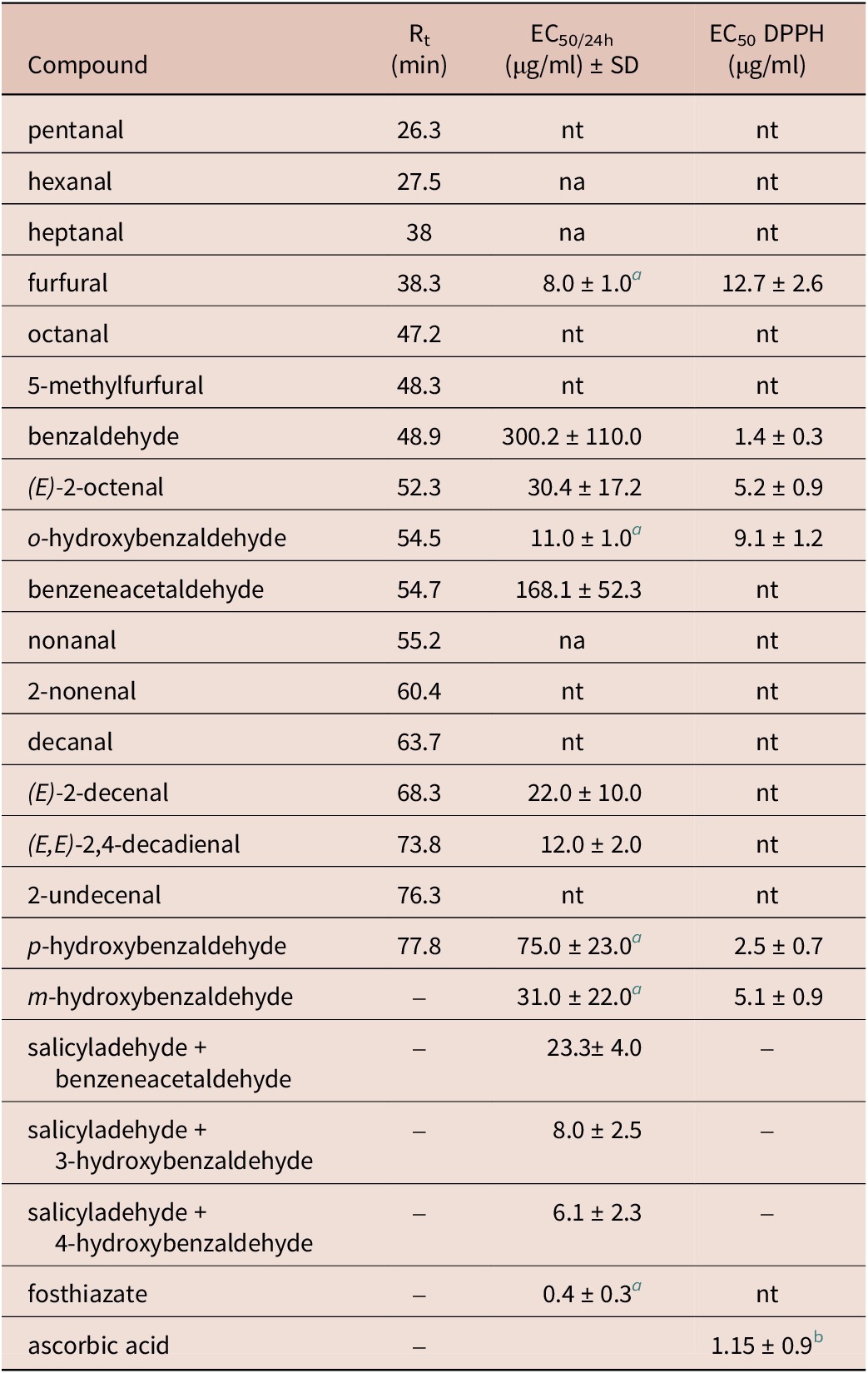
a Result reported by Caboni et al. (Reference Caboni, Aissani, Cabras, Falqui, Marotta, Liori, Ntalli, Sarais, Sasanelli and Tocco2013)
b Data reported by Aissani et al. (Reference Aissani, Balti and Sebai2017)
J2 paralysis of the tested compounds
Compared with the selected aldehydes previously tested against J2, o-hydroxybenzaldehyde was the most active one, followed by m-hydroxybenzaldehyde (3), p-hydroxybenzaldehyde (4), benzeneacetaldehyde (5), and then benzaldehyde (1) with an EC50 after 24 hours of immersion of about 11.0 ± 1.0, 31.0 ± 22.0, 75.0 ± 23.0, 168.1 ± 52.3, and 300.2 ± 110.0 μg/ml, respectively (Figure 2, Table 2).
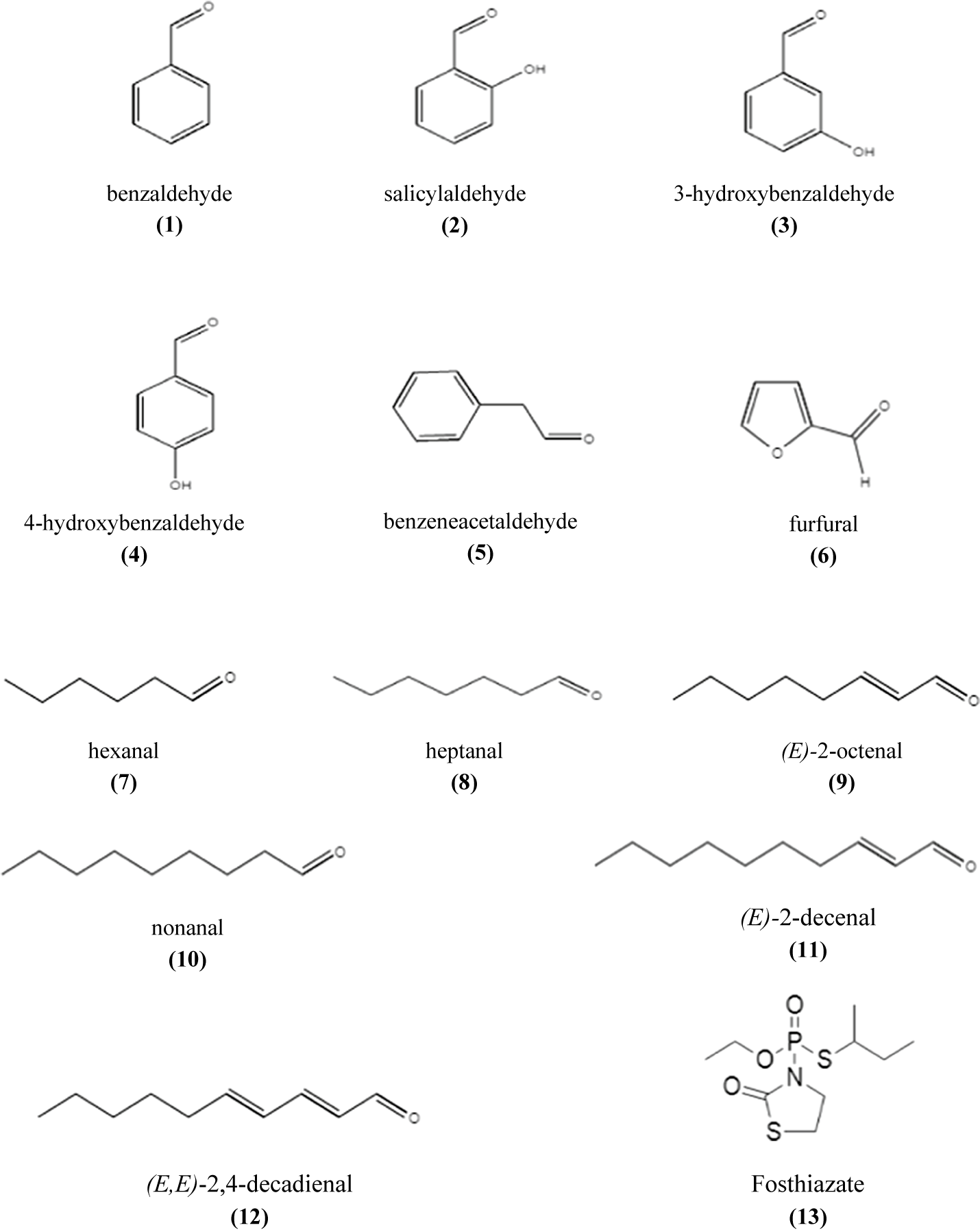
Figure 2. Chemical structure of tested compounds.
Synergistic activity assessment
A synergistic activity of o-hydroxybenzaldehyde and m-hydroxybenzaldehyde; o-hydroxybenzaldehyde and p-hydroxybenzaldehyde was observed with EC50 of 8.0 ± 2.5 and 6.1 ± 2.3 μg/ml, respectively. The addition of o-hydroxybenzaldehyde to benzeneacetaldehyde significantly enhances the activity of this latter to become 23.3 ± 4.0 μg/ml after the same immersion period.
Antioxidant assay
The DPPH radical-scavenging activity method showed that the most antioxidant compound was benzaldehyde followed by p-hydroxybenzaldehyde and (E)-2-octenal with EC50/24h values of 1.4 ± 0.3, 3.5 ± 0.7, and 5.2 ± 0.9 μg/ml, respectively. However, the least antioxidant compound was furfural followed by o-hydroxybenzaldehyde and m-hydroxybenzaldehyde with EC50/24h values of 12.7 ± 2.6, 9.1 ± 1.2, and 5.1 ± 0.9 μg/ml, respectively.
Discussion
In this study, aerial fresh and dried parts of common buckwheat F. esculentum were extracted using methanol and water, and the nematicidal activity of the extracts was assessed against juveniles of M. incognita. Results showed that the methanol extract had more activity when the plant was dried, with EC50 = 40.8 ± 26.1 μg/ml after 72 hours. This nematicidal activity is high, taking into account the effect of Capparis spinosa stems methanol extracts with an EC50 value of 215.0 ± 36.0 μg/ml (Caboni et al. Reference Caboni, Sarais, Aissani, Tocco, Sasanelli, Liori, Carta and Angioni2012b). The aqueous extract was not active, with EC50>1000 μg/ml.
Using GC-MS analysis, we were able to identify 17 aliphatic and aromatic aldehydes,with salicylaldehyde (o-hydroxybenzaldehyde) being the most abundant at 16%. In the same fashion, the analysis of buckwheat volatilome by headspace technique showed that this plant is rich with aldehydes and ketones (Prosen et al. Reference Prosen, Kokalj, Janeš and Kreft2010).
According to Janes and Kreft (Reference Janes and Kreft2008) and Janes et al. (Reference Janes, Prosen and Kreft2012), salicylaldehyde is a characteristic aroma component of common buckwheat and presents the highest concentration with 1.6 μg/ml. Aldehydes from Ailanthus altissima presented a strong nematicidal activity against root knot nematodes (Caboni et al. Reference Caboni, Ntalli, Aissani, Cavoski and Angioni2012a).
In our previous study, salicylaldehydes presented a nematicidal activity against the J2 stage of M. incognita of 11.0 ± 1.0 μg/ml after 24 h of immersion (Caboni et al. Reference Caboni, Aissani, Cabras, Falqui, Marotta, Liori, Ntalli, Sarais, Sasanelli and Tocco2013). Table 2 summarizes the nematicidal effect of tested compounds found by GC-MS analysis.
The structure-activity study showed that salicyaldehyde (o-hydroxybenzaldehyde) is three times more active than m-hydroxybenzaldehyde and seven times more active than p-hydroxybenzaldehyde. Thus, position 2 of the hydroxyl group in the benzene ring seems to be very important for nematicidal power, followed by positions 3 and 4. Using aldehydes with linear chains, our results clearly indicate that α,β,γ,δ-unsaturated aldehydes are generally more nematicides than aromatic counterparts against M. incognita confirming results found by Caboni et al. (Reference Caboni, Ntalli, Aissani, Cavoski and Angioni2012a) using M. javanica. In a recent study, (E)-2-decenal degenerated the nematode pseudocoel cells and caused malformations of somatic muscles (Ntalli et al. Reference Ntalli, Ratajczak, Oplos, Menkissoglu-Spiroudi and Adamski2016). Caboni et al. (Reference Caboni, Aissani, Cabras, Falqui, Marotta, Liori, Ntalli, Sarais, Sasanelli and Tocco2013) showed that nematodes treated with salicylaldehyde and aromatic aldehydes presented serious cuticle damages with marked macroscopic fractures and paralysis in a straight shape and evident internal vacuolization compared to the controls (Caboni et al. Reference Caboni, Aissani, Cabras, Falqui, Marotta, Liori, Ntalli, Sarais, Sasanelli and Tocco2013). This observation was noted also after immersion of nematodes in buckwheat methanolic extract (Figure 3). Previous studies reported similar results when nematodes were treated with isothiocyanates and some vacuolar-type proton-translocating adenosine triphosphatase (V-ATPase) inhibitors such as pyocyanin (Aissani et al. Reference Aissani, Tedeschi, Maietti, Brandolini, Garau and Caboni2013; Aissani et al. Reference Aissani, Urgeghe, Oplos, Saba, Tocco, Luigi Petretto, Eloh, Menkissoglu-Spiroudi, Ntalli and Caboni2015). Conversely, nematodes treated with the organophosphorous fosthiazate were paralyzed in a coiled shape (Aissani et al. Reference Aissani, Urgeghe, Oplos, Saba, Tocco, Luigi Petretto, Eloh, Menkissoglu-Spiroudi, Ntalli and Caboni2015). Salicylaldehyde produced an increased pH in lysosomal-like organelles on HeLa human cell line, and this alteration was most likely related to a V-ATPase impairment (Caboni et al. Reference Caboni, Tronci, Liori, Tocco, Sasanelli and Diana2014). In another experiment, synergistic activity of o-hydroxybenzaldehyde and m-hydroxybenzaldehyde; o-hydroxybenzaldehyde and p-hydroxybenzaldehyde was observed with EC50 of 8.0 ± 2.5 and 6.1 ± 2.3 μg/ml, respectively, after 24 h. Interestingly, the addition of o-hydroxybenzaldehyde to benzeneacetaldehyde significantly enhances the activity of this latter from 168.1 ± 52.3 to 23.3 ± 4.0 μg/ml after the same immersion period.

Figure 3. Nematodes before (A) and after (B) treatment with Buckwheat methanol extract (internal vacuole is evident).
Remarkably, salicylaldehyde showed the highest nematicidal activity with the corresponding lowest antioxidant activity with EC50= 9.1 ± 1.2 μg/ml. Conversely, benzaldehyde presented the lowest nematicidal activity with the corresponding highest antioxidant effect (Table 1, Figure 4). These results confirm those found in our previous studies when we correlated nematicidal and antioxidant activities of selected isothiocyanates and phenolic compounds (Aissani & Sebai Reference Aissani and Sebai2022; Aissani et al. Reference Aissani, Balti and Sebai2017).

Figure 4. Correlation between nematicidal activity and antioxidant effect of the tested aldehydes.
This study is the first to investigate the nematicidal activity of common buckwheat methanol extract against M. incognita. This plant is rich in aldehydes, such as salicylaldehyde, which has nematicidal activity against this root-knot nematode and allows us to use this plant for crop protection against pests. Comparison with other aldehydes showed that position 2 in the phenolic ring of the aldehyde is important for nematicidal activity. This gives insight into the development of new potent nematicides.
Acknowledgements
The author extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the General Research Project under Grant number (RGP.2/83/44).
Competing interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical standard
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national guidelines on the care and use of laboratory animals and have been approved by the institutional committee of the Institut Pasteur, Tunis.








