Introduction
Conventional in vitro fertilization has had poor success for horses (Squires et al., Reference Squires, Carnevale, McCue and Bruemmer2003; Leemans et al., Reference Leemans, Gadella, Stout, De Schauwer, Nelis, Hoogewijs and Van Soom2016). However, assisted fertilization using intracytoplasmic sperm injection (ICSI) resulted in experimental pregnancies and limited offspring by the mid-1990s (Squires et al., Reference Squires, Carnevale, McCue and Bruemmer2003; Galli et al., Reference Galli, Colleoni, Duchi, Lagutina and Lazzari2007). Subsequently, ICSI was developed for clinical use in horses, with the production of pregnancies and offspring from subfertile mares and/or stallions with limited or poor sperm quality (Carnevale et al., Reference Carnevale, Stokes, Squires, Campos-Chillon, Altermatt and Suh2007; Colleoni et al., Reference Colleoni, Barbacini, Necchi, Duchi, Lazzari and Galli2007; Hinrichs, Reference Hinrichs2013). As the demand for ICSI increased in the equine industry, methods for maturing oocytes and culturing embryos in vitro were explored (Carnevale & Sessions, Reference Carnevale and Sessions2012; Hinrichs, Reference Hinrichs2013; Galli et al., Reference Galli, Duchi, Colleoni, Lagutina and Lazzari2014). However, our understanding of equine fertilization and early embryo development is still limited.
Oocytes can be matured in vivo or in vitro for equine assisted reproduction. Oocyte maturation is induced in vivo by administration of compound(s) to the donor mare that initiate follicle and oocyte maturation within the dominant follicle during the follicular phase, and the oocyte can be collected from the follicle before ovulation (Carnevale, Reference Carnevale and Ginther2016). Theoretically, the resulting oocytes should be of optimal quality, and collection, oviductal transfer, and fertilization in vivo of similar oocytes result in high pregnancy rates (Carnevale & Ginther, Reference Carnevale1995). In vitro maturation of oocytes has been widely used in domestic animals and is of interest in human reproduction (Edwards, Reference Edwards1965; Hinrichs et al., Reference Hinrichs, Schmidt, Friedman, Selgrath and Martin1993; Arlotto et al., Reference Arlotto, Schwartz, First and Leibfried-Rutledge1996; Galli et al., Reference Galli, Colleoni, Duchi, Lagutina and Lazzari2007; Lonergan & Fair, Reference Lonergan and Fair2016). In the horse, immature oocytes are collected from live mares or excised ovaries by collecting oocytes from numerous follicles of various sizes for in vitro maturation, fertilization, and foal production (Galli et al., Reference Galli, Colleoni, Duchi, Lagutina and Lazzari2013; Hinrichs, Reference Hinrichs2013; Carnevale, Reference Carnevale and Ginther2016). The extent to which the artificial environment, associated with in vitro maturation, affects the oocyte has not been fully determined. In addition, immature oocytes from small follicles are removed from their natural environment prior the development of conditions associated with follicle growth and hormonal stimulation. Consequently, oocytes from immature follicles are more variable in quality and developmental competency (Hinrichs, Reference Hinrichs1991; Hyttel et al., Reference Hyttel, Fair, Callesen and Greve1997).
An understanding of differences in zygotes developing from oocytes matured in vivo (IVO) and in vitro (IVM) would further our knowledge of the normal progression of postfertilization events and of potential alterations in cytoskeletal and nuclear maturation prior to the first mitotic division. In our study, we used confocal microscopy to examine equine zygote development at timed intervals after ICSI of IVO or IVM.
Materials and Methods
Oocyte Collections
IVO were collected between April and August in Fort Collins, CO, USA (40° latitude) from light-horse mares between 4 and 16 years (mean±SEM of 10.8±0.7 year). Reproductive tracts were imaged using ultrasonography to evaluate follicular growth. Oocytes were collected from the dominant follicle(s) during the follicular phase and between 18 and 25 h (21±0.3 h) after administration of human chorionic gonadotropin (1,500 IU, iv; Intervet Inc., Millsboro, DE, USA) and deslorelin acetate (SucroMate™, 0.75 mg, im; Bioniche Life Sciences Inc., Belleville, Ontario, Canada). Oocytes were collected by ultrasound-guided, transvaginal follicle aspirations as previously described (Carnevale et al., Reference Carnevale, Maclellan, Coutinho da Silva, Scott and Squires2000), but using a commercial embryo flush solution (ViGRO™ Complete Flush, Bioniche Animal Health USA, Inc., Pullman, WA, USA) to lavage the follicle. Upon collection, the oocytes were cultured for 19.5–27.0 h (22.0±0.3 h) in Tissue Culture Medium 199 with Earle’s salts (Gibco, Life Technologies, Grand Island, NY, USA) with additions of 10% fetal calf serum (FCS, Cell Generation LLC, Fort Collins, CO, USA), 0.2 mM sodium pyruvate, and 25 μg/mL gentamicin sulfate (Sigma Aldrich, St. Louis, MO, USA) at 38 or 38.5°C in a humidified atmosphere of 6% CO2 and air.
IVM were collected from excised ovaries in Cremona, Italy (45° latitude) during the natural breeding season. Ovaries were obtained from mares of diverse breeds and unknown ages from a local abattoir and transported at 24°C for <4 h before collection of oocytes at the laboratory. Retrieved oocytes were placed in culture medium [Dulbecco’s modified Eagle’s medium (DMEM)/F12 (D8900; Sigma Aldrich, Milan, Italy) with 10% serum replacement (Life Technologies, Monza, Italy) and 0.1 IU/mL of human menopausal gonadotropin (Menopur 75, Ferring, Milan, Italy)] for 28 h at 38.5°C in humidified atmosphere of 5% CO2 and air.
ICSI and Zygote Culture
Prior to ICSI of IVO or IVM, cumulus cells were removed and extrusion of the first polar body was confirmed. For both labs, ICSI was performed using a piezo drill. Frozen-thawed semen from one stallion in each laboratory was used for all sperm injections, and a motile sperm with normal morphology was selected for each injection. After ICSI, potential zygotes were cultured under standard conditions for each laboratory. For IVO, potential zygotes were cultured individually in 30-μL drops of medium [DMEM/F12 (Sigma Aldrich, St. Louis) with 10% FCS] under mineral oil at 38.5°C and in an atmosphere of 5% CO2, 5% O2, and 90% N2. Potential zygotes from IVM were placed as a group into 300 μL of a modified synthetic oviductal fluid medium with bovine serum albumin (BSA; Sigma Aldrich, Milan) and modified Eagle Medium amino acids (Sigma Aldrich, Milan) in four-well dishes at 38.5°C and in 5% CO2, 5% O2, and 90% N2 (Lazzari et al., Reference Lazzari, Wrenzycki, Herrmann, Duchi, Kruip, Niemann and Galli2002; Colleoni et al., Reference Colleoni, Lagutina, Lazzari, Rodriguez-Martinez, Galli and Morrell2011).
Samples Fixation and Immunostaining
Presumptive zygotes were fixed at room temperature in a microtubule stabilizing buffer (MTSB-XF, 0.1 M Pipes, 5 mM MgCl2. 6H2O, 2.5 mM EGTA at pH 6.9) containing 2% formaldehyde, 1 µM taxol, 10 units/mL aprotinin, 50% deuterium oxide and modified with 0.1% Triton X-100, and 1 mM 1,4-Dithiothreitol (Messinger & Albertini, Reference Messinger and Albertini1991). Zygotes were fixed at 4 (n=5), 6 (n=6), 8 (n=5), 12 (n=5), and 16 h (n=7) (ICSI=0 h) for IVO and at the same time points 4 (n=8), 6 (n=8), 8 (n=8), 12 (n=10) and 16 h (n=10) for IVM. After fixation, oocytes were rinsed in wash solution [(phosphate buffered saline containing 0.2% powdered milk, 2% normal goat serum, 0.1 M glycine, and modified to contain 1% BSA and 0.1% Triton X-100 (Messinger & Albertini, Reference Messinger and Albertini1991)] for IVO or a modified wash solution (phosphate buffered saline containing 1% BSA and 0.1% Triton X-100) for IVM, and stored at 4°C until immunostaining. The same procedure for immunostaining was used for IVO and IVM.
Oocytes were incubated with diluted primary antibodies in wash solution and positioned in four-well plates on rotating platform shaker for 4 h at 37°C at the following concentrations: α/β tubulin cocktail (1:100, mouse; Sigma Aldrich, St. Louis) and human-anti centromere antibody-CREST/ACA (1:100; Life Technologies, Grand Island). After primary incubation, oocytes were rinsed in wash solution for a minimum of 12 h at 4°C, then incubated with secondary antibodies conjugated to either Alexa 488 or Alexa 647 (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted in wash solution for 4 h. When secondary incubation was complete, the oocytes were held in wash solution for 5 h and then incubated with phalloidin (Alexa 561; Life Technologies) and Hoechst 33258 (1 μg/mL; Life Technologies, Grand Island, NY, USA) for another 5 h at 37°C. For confocal imaging, samples were mounted onto coverslips in 50% glycerol in phosphate buffered saline with 25 mg/mL sodium azide and 1 μg/mL of Hoechst 33258 (Barrett & Albertini, Reference Barrett and Albertini2007).
Confocal images were acquired on an Olympus IX81 microscope (Waltham, MA, USA) fitted with a Yokogawa spinning disk (CSU22 head) using either a 60X/1.42 NA, DIC Planapochromatic or a 40X/1.35NA planapochromatic oil lens. Entire oocytes were imaged in 1-µm intervals at 40× and 0.2-µm intervals at 60×. Images were captured with a Photometrics Cascade II EM CCD camera (Tucson, AZ, USA) and analyzed using SlideBook software (Intelligent Imaging Innovations, Denver, CO, USA).
Determination of Zygote Development Stages and Abnormalities After ICSI
Five sequential developmental events were categorized for presumptive equine zygotes: (1) condensed sperm chromatin with maternal chromosomes and extruded first polar body (PB1); (2) anaphase to telophase transition including anaphase/telophase shift of maternal chromosomes, condensed sperm chromatin, PB1, and second polar body (PB2) in the process of extrusion or fully extruded; (3) formation of the male pronuclei with maternal chromosomes, PB1 and PB2; (4) male and female pronuclei at distant positions; (5) male and female pronuclei in close apposition. Pronuclei (presumed male and female) were identified by localization of DNA and a human anticentromere antibody (CREST polyclonal antibody).
Development of presumptive zygotes were considered abnormal if the following images were observed: (1) premature chromosome condensation of the sperm with male chromosomes flanked by a bipolar spindle; (2) multiple pronuclei; (3) sperm chromatin induced ectopic polar body; (4) multipolar spindles with incorrect separation of chromosomes and presence of multiple centrosomes; (5) scattered maternal chromosomes and intact sperm head.
Statistical Analysis
χ 2 Analysis was used to determine overall effects across all time points, and Fisher’s exact test was used within each category for comparisons among time points if the overall difference was significant at p<0.05.
Results
Presumptive Zygote Developmental Events and Pronuclei Assessment
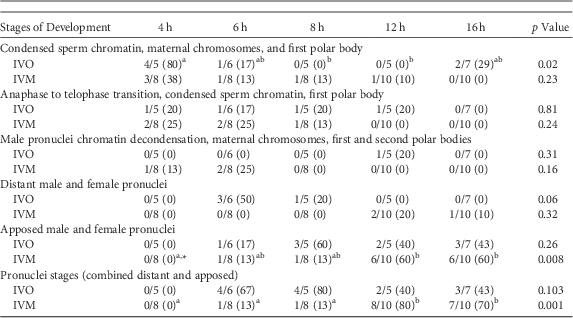
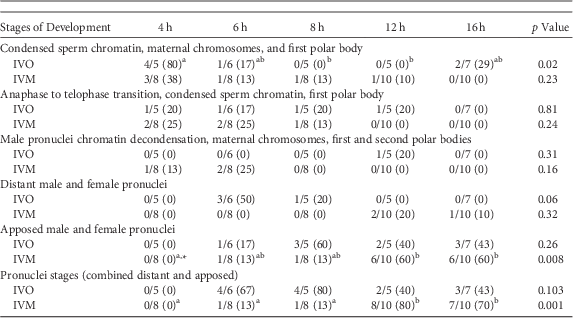
A similar series of developmental events were observed during zygote development after ICSI of IVO or IVM (Fig. 1, Table 1). Because of the relatively low number of samples, differences in the number of potential zygotes at specific developmental stages were observed over time only for IVO with condensed sperm chromatin, maternal chromosomes and PB1 (p=0.02, Figs. 1a–1c), IVO for presence of distant pronuclei (p=0.06, Fig. 2b), and IVM for apposed pronuclei (p=0.008, Fig. 2a, Table 1). The number of zygotes with pronuclei (distant or apposed) was significantly elevated at 12 and 16 h when compared with earlier time points for IVM, with the numerically highest percentage of pronuclei observed at 12 h (80%). Although not different over time, the highest numerical percentages of pronuclei were observed at 6 (67%) and 8 h (80%) for IVO (Table 1).
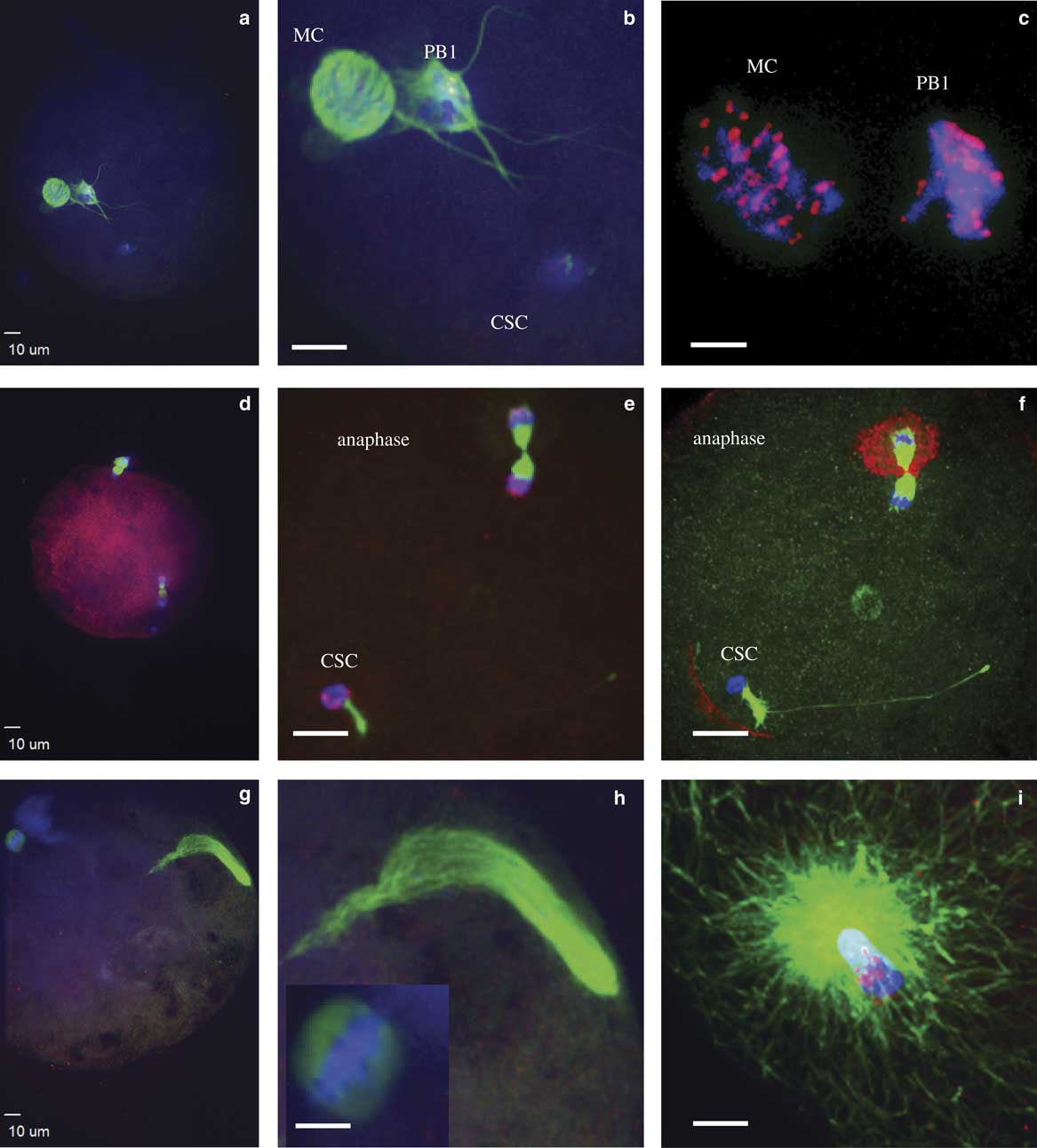
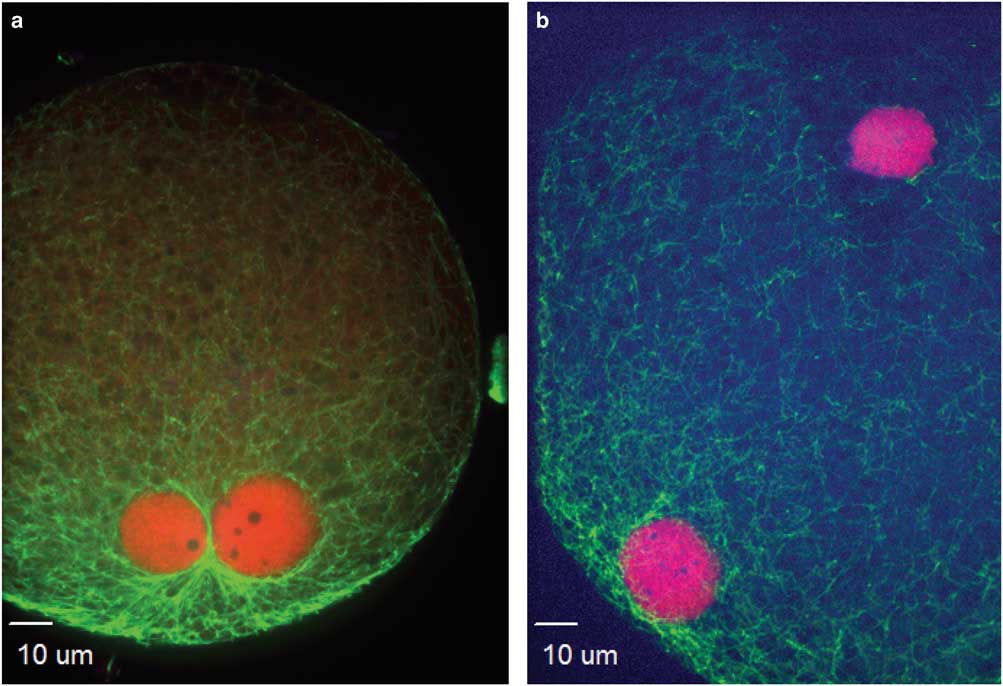
Figure 1 Events during zygote development observed between 4 and 16 h after intracytoplasmic sperm injection. a,b: Condensed sperm chromatin (CSC, lower right), maternal chromosomes (MC, left), and first polar body (PB1, just to right of MC); (c) representation of the kinetochores localized on MC and PB1; (d,e) anaphase to telophase transition with CSC with acrosome portion of the sperm (lower left), anaphase/telophase of MC, extruding the second polar body; (f) actin cap surrounding the set of chromosomes being extruded in the second polar body, sperm tail visible next to CSC; (g,h) formation of the male pronucleus (indicated by elongated tubulin structure), MC (h, left insert); and (i) tubulin aster formation around the sperm head at the time of sperm DNA decondensation (blue, DNA; red, centromeres and kinetochores, except in panel f red indicates actin; green, tubulin).

Figure 2 Nucleolus precursor bodies were observed within pronuclei at apposed (a) or distant (b) locations (red, centromere; green, tubulin).
Table 1 Numbers of Potential Equine Zygotes at Different Points of Development at 4, 6, 8, 12, and 16 h From All Oocytes Matured In Vivo (IVO) or In Vitro (IVM) And Fertilized by Intracytoplasmic Sperm Injection (0 h).
At the initial observation after ICSI, many zygotes had a female spindle arrested at metaphase II with aligned and compact chromosomes, an extruded first polar body, and paternal DNA as identified by the presence of the sperm head and, in some samples, the tail (Figs. 1a, 1b). Kinetochores were associated with maternal chromosomes at the metaphase plate and in the extruded first polar body (Fig. 1c). This early phase of development was observed predominantly at 4 h after ICSI, including 80 and 38% of presumptive zygotes derived from IVO and IVM, respectively. One zygote from each of the two groups had not progressed past this developmental stage at 6 h after ICSI, and two zygotes from each group appeared delayed or arrested in development and had not progressed from this stage by ≥8 h after ICSI.
The anaphase to telophase transition was imaged as maternal chromosomes approaching anaphase or extruding the second polar body during telophase, with the presence of a sperm head (Figs. 1d–1f). This stage was observed as early as 4 h after ICSI for IVO and IVM, with some presumptive zygotes arrested at this point in later hours.
Decondensation of male chromatin with a microtubule array around the sperm head nuclei, female chromosomes aligned at the metaphase plate, and PB1 with or without PB2 (Figs. 1g–1i) was only imaged in three oocytes, including IVO oocytes that appeared delayed in development at 12 h after ICSI.
The presence of two pronuclei, presumed male and female, were imaged as early as 6 h after ICSI (Fig. 2b). At 8 h for IVO and 12 h for IVM, 80% of zygotes had pronuclei. When the pronuclei were in different areas of the ooplasm (distant), they were surrounded by a complex tubulin net, which diffused throughout the developing zygote. When the two pronuclei were close, the tubulin net was concentrated and contracting at the site of apposition (Fig. 2a). The number of nucleolus precursor bodies (NPB) in both parental pronuclei varied from three to seven; NPB were imaged in all pronuclei when PB2 was not extruded and three pronuclei were present (Fig. 3b).
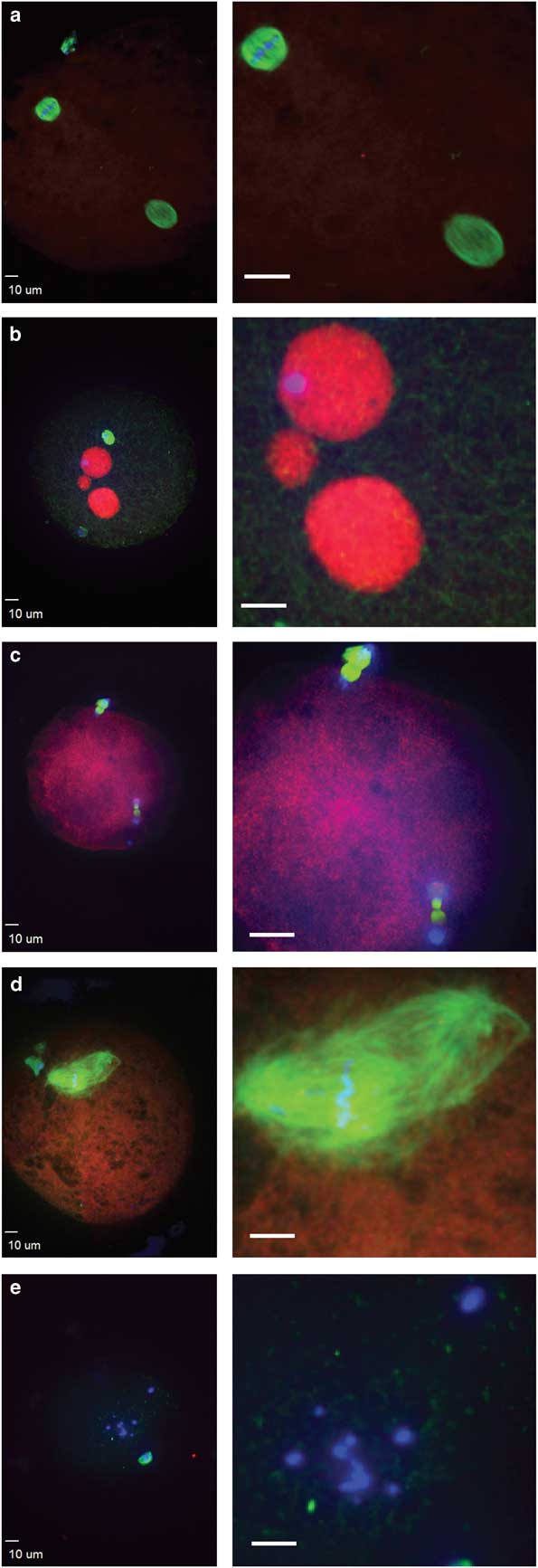
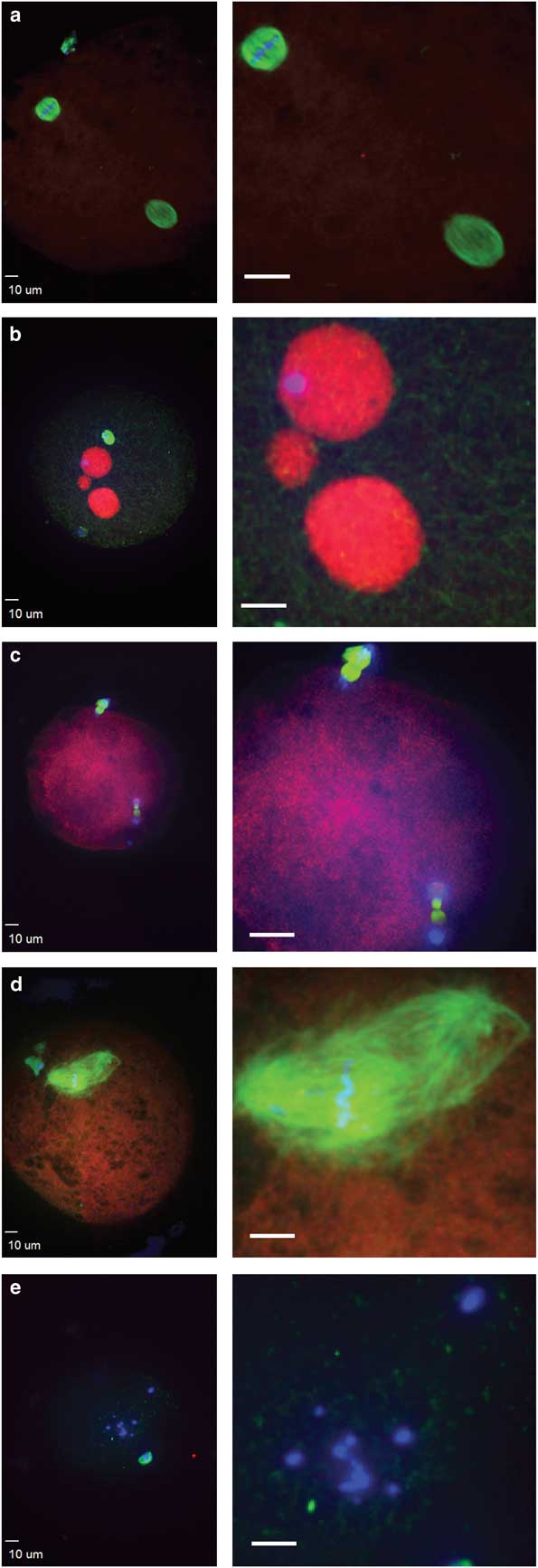
Figure 3 Abnormal morphologies of potential equine zygotes after intracytoplasmic sperm injection include (a) premature chromosome condensation, with female spindle (top left) and male chromosomes flanked by a spindle structure (lower right); (b) multiple pronuclei; (c) sperm chromatin induced ectopic polar body, represented by the two sets of chromosomes undergoing anaphase; (d) multipolar spindle; (e) scattered maternal chromosomes (blue, DNA; red, centromeres and kinetochores; green, tubulin).
Abnormalities Observed in Presumptive Equine Zygotes After ICSI
Failure of zygote development was observed for IVO and IVM and was associated with abnormal cytoskeletal and chromatin configurations (Figs. 3, 4). IVO had an incidence of abnormalities during zygote development of 3/28 (11%), with no significant differences for the various phenotypes over time (Table 2). Of the 44 potential zygotes from IVM, 13 (30%) had abnormal phenotypes, with the most common abnormalities being premature chromosome condensation and sperm chromatin induced ectopic polar body. Overall, potential zygotes from IVM had a higher number of abnormal phenotypes per total injected oocytes than IVO (p=0.04, Table 2).
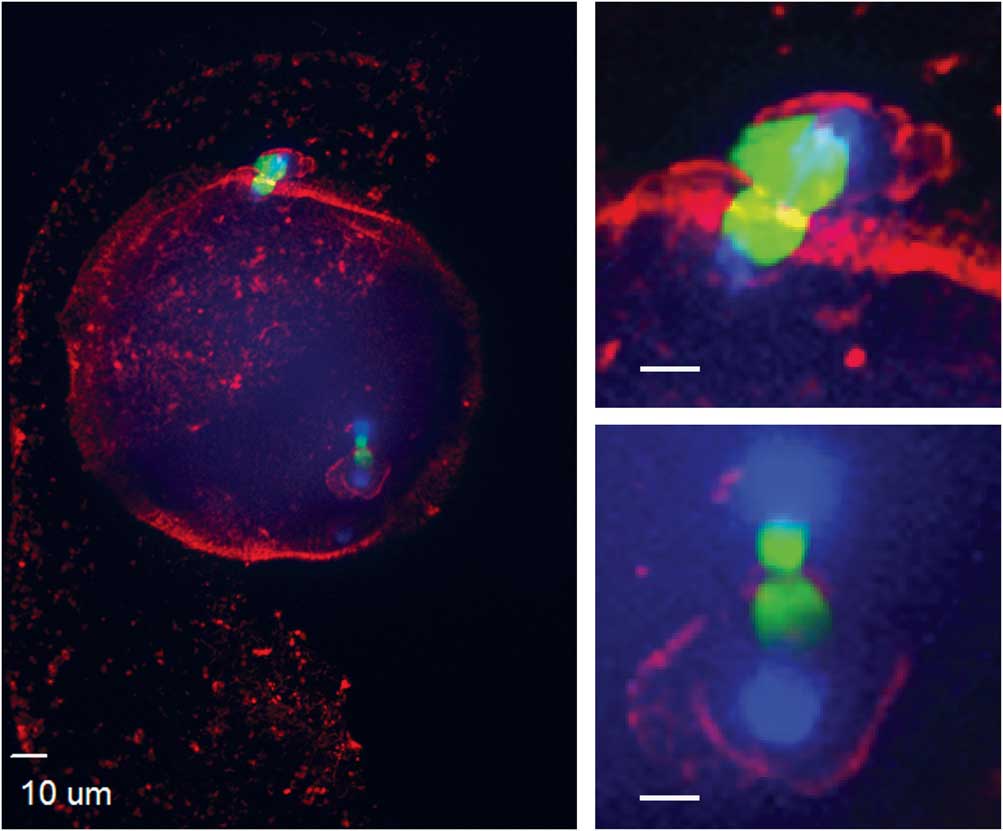
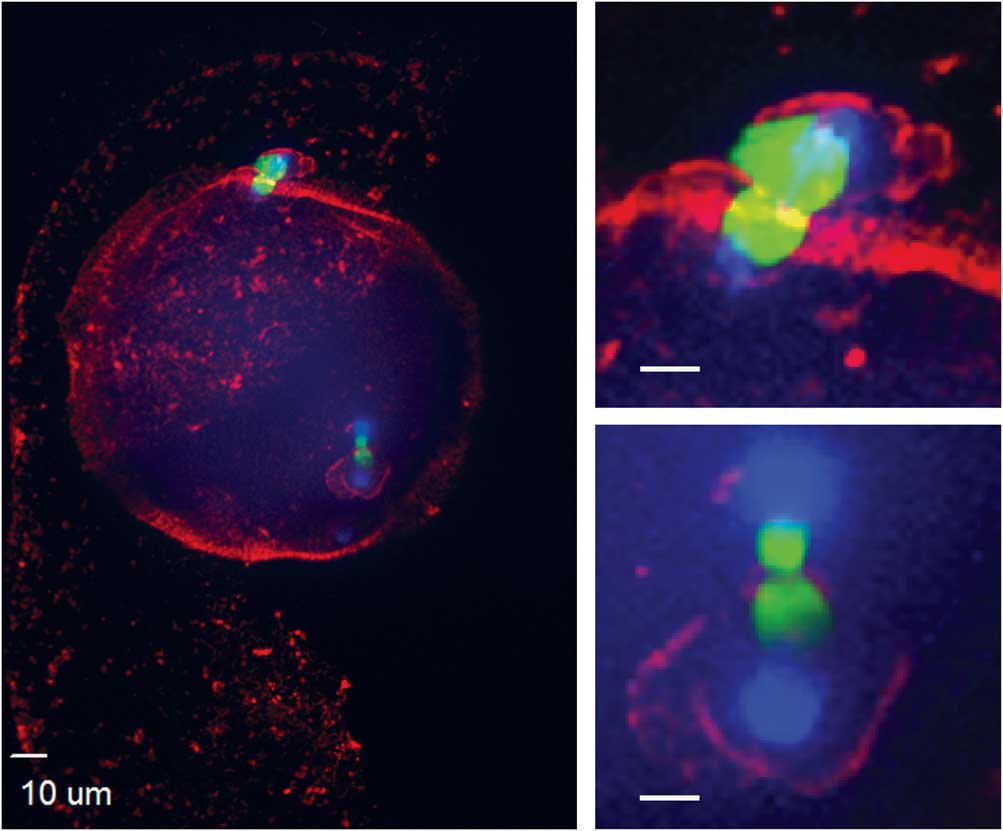
Figure 4 Actin cytoskeleton localization during ectopic polar body extrusion. In the left panel, cortical actin is concentrated around the two points of DNA extrusion. Top right and bottom right panels are magnified images of the two points of extrusion (blue, DNA; red, actin; green, tubulin).
Table 2 Number of Potential Equine Zygotes with Abnormal Morphologies at 4, 6, 8, 12, and 16 h After Intracytoplasmic Sperm Injection (0 h) Per Total Injected Oocytes Matured In Vivo (IVO) or In Vitro (IVM).

The total number of zygotes with morphologic abnormalities was higher (p=0.04) for IVM when compared with IVO (as denoted by bold numbers and different superscripts).
Discussion
Equine zygotes are difficult to obtain, and confocal microscopy provides a potentially efficient method to analyze the limited numbers of samples. The equine oocyte is a large cell with a high lipid content (Ambruosi et al., Reference Ambruosi, Lacalandra, Iorga, De Santis, Mugnier, Matarrese, Goudet and Dell’aquila2009). The ooplasm measures ~85 µm in diameter and is surrounded by the zona pellucida, a glycoprotein layer about 12-µm in thickness; the outer zona pellucida is ~117 µm in diameter (Altermatt et al., Reference Altermatt, Suh, Stokes and Carnevale2009). The size and properties of the equine oocyte affect immunostaining and confocal imaging, adding difficulty to the procedures. In addition, relatively few antibodies have been tested with the equine oocyte and zygote. However, we were able to successfully incorporate antibodies that had previously been used in other species.
Historically, development of the equine zygote and early embryo has been challenging to study. The equine conceptus will remain in the oviduct for 5–6 days before entering the uterus as a morula or early blastocyst (Freeman et al., Reference Freeman, Weber, Geary and Woods1991). Initial observations of equine zygotes were done using transmission electron microscopy, with limited numbers of samples collected from oviducts based on timing of breeding or administration of ovulation inducing compounds (Enders et al., Reference Enders, Liu, Bowers, Lantz, Schlafke and Suarez1987; Grondahl & Hyttel, Reference Grondahl and Hyttel1996). Later, ICSI were used as a method to fertilize equine oocytes matured in vitro, prior to analysis by electron microscopy (Grondahl et al., Reference Grondahl, Hansen, Hossaini, Heinze, Greve and Hyttel1997) or confocal microscopy (Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). However, at the time of these observations, procedures for equine oocyte maturation, ICSI and embryo culture were not yet easily repeatable or proven to produce viable embryos and foals, and timing of embryo development after ICSI was not always optimal. In the present study, proven methods were used in laboratories with records of producing viable foals after ICSI of IVO and IVM (Carnevale et al., Reference Carnevale, Stokes, Squires, Campos-Chillon, Altermatt and Suh2007; Galli et al., Reference Galli, Colleoni, Duchi, Lagutina and Lazzari2007).
In our study, we observed zygote development at timed intervals after ICSI and noted similar events after IVO or IVM. In previous studies using ICSI and microscopic evaluations of zygotes, oocytes comparable to our IVM group were used, with oocytes collected from ovaries that were obtained from abattoirs and matured in vitro (Grondahl et al., Reference Grondahl, Hansen, Hossaini, Heinze, Greve and Hyttel1997; Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). Our first observations were done 4 h after ICSI, and in most presumptive zygotes, the maternal metaphase II spindle with aligned chromosomes, first polar body, and sperm head were imaged. In the study by Tremoleda et al. (Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003), oocytes remained at metaphase II directly after sperm injection, with signs of oocyte activation noted in most oocytes at 6 h and leading to the second meiotic division with extrusion of the second polar body. We observed the anaphase to telophase transition, prior to extrusion of a second polar body, by 4 h in approximately one quarter of injected oocytes.
In our study, IVO seemed to develop to pronuclei sooner after ICSI than IVM, with the highest percentages of pronuclei noted after ICSI at 6 and 8 h for IVO, in comparison with 12 and 16 h for IVM. Within pronuclei were distinct areas with no CREST anticentromere staining, signifying NPB (Fig. 2a). The timing of pronuclei formation agreed with previous reports using IVM oocytes, with half of sperm-injected oocytes reaching the pronuclear stage by 12 h, while the remaining presumptive zygotes were still in earlier stages of development (Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). In another study, half of the sperm-injected IVM oocytes had two pronuclei at 20 h after ICSI, but none had two pronuclei at 10 h after ICSI (Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). A significant percentage (36%) of these sperm-injected, IVM oocytes did not cleave into two cells until 48 h after ICSI (Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). In our laboratory, we would expect cleavage or indicators or impending cleavage for IVO and IVM oocytes by 24 h after ICSI (Carnevale, personal communication, 2017).
The timing of pronuclei formation for IVO and IVM could be impacted by many factors other than maturation method, including differences in sperm, procedures, inherent oocyte quality, and total time of maturation. However, oocyte quality and cytoplasmic maturation could also differ between IVO and IVM oocytes (Sutton et al., Reference Sutton, Gilchrist and Thompson2003; Gilchrist & Thompson, Reference Gilchrist and Thompson2007). Oocytes collected from all follicles from excised ovaries represent a heterogenous group of oocytes in different stages of competence, maturation, and atresia (Hinrichs, Reference Hinrichs1991). In contrast, oocytes collected from dominant, maturing follicles are in a relatively consistent stage of maturation, with ovulation of a metaphase II oocyte anticipated ~38 h after administration of induction drugs (Figueiredo et al., Reference Figueiredo, Paiva, Kozicki, Kaercher, Weiss, Santos and Muradas2011). In this study, IVO oocytes were collected during the process of maturation, probably at metaphase I, and cultured in medium for the completion of maturation, in a system that has been proven to result in developmentally competent oocytes (Carnevale et al., Reference Carnevale, da Silva, Maclellan, Seidel and Squires2004). In contrast IVM oocytes were collected from follicles of various sizes and from ovaries of mares of unknown ages or stages of their reproductive cycle or pregnancy. Oocytes collected from small- or medium-sized, immature follicles can be less developmentally competent, as they complete maturation in artificial conditions over a limited time, in contrast to natural conditions within the dominant follicle (Hyttel et al., Reference Hyttel, Fair, Callesen and Greve1997). In addition, synchronization of nuclear and cytoplasmic maturation can be impacted when oocytes are matured in vitro. Epigenetic modifications occur in gametes and in early embryos (Reik et al., Reference Reik, Dean and Walter2001; Bromfield et al., Reference Bromfield, Messamore and Albertini2007; Cantone & Fisher, Reference Cantone and Fisher2013), and ART procedures and in vitro culture could cause epigenetic disturbances that affect oocyte competence, pregnancy, and offspring health (Bromfield et al., Reference Bromfield, Messamore and Albertini2007; El Hajj & Haaf, Reference El Hajj and Haaf2013). In our study, no definitive differences in zygote development were noted with the limited numbers of IVO and IVM oocytes, although zygotes from IVM subjectively appeared to develop slower. However, oocyte maturation groups differed in that significantly more zygotes from IVM, than IVO, had abnormalities suggestive of developmental failure. One potential risk of maturation in vitro is that nuclear and cytoplasmic maturation are not synchronized and can result in collateral effects on embryonic development (Smitz et al., Reference Smitz, Thompson and Gilchrist2011; Sanfins et al., Reference Sanfins, Plancha and Albertini2015). Transcriptional profiling after in vitro maturation of oocytes from cattle, women and mice has confirmed changes in genes and pathways, which could affect postfertilization events (Rinaudo & Schultz, Reference Rinaudo and Schultz2004; Mamo et al., Reference Mamo, Carter, Lonergan, Leal, Al Naib, McGettigan, Mehta, Evans and Fair2011; El Hajj & Haaf, Reference El Hajj and Haaf2013). The extent that maturation in vitro affected oocyte quality in this study could not be directly determined, primarily because of the inherent variability within the population of immature oocytes.
After sperm injection, factors associated with the sperm and oocyte are critical for oocyte activation, decondensation of the sperm chromatin, and initiation of embryo development (Choi et al., Reference Choi, Love, Varner and Hinrichs2004; Galli et al., Reference Galli, Colleoni, Duchi, Lagutina and Lazzari2007). Alterations in these processes can result in abnormal development, and abnormal morphologies were observed in some potential zygotes. Sperm-transmitted DNA damage leads to diverse abnormal reproductive outcomes and paternal genome loss, and it can be caused by different sperm chromatin defects, including premature sperm condensation and ectopic polar body extrusion after ICSI (Schmiady et al., Reference Schmiady, Tandler-Schneider and Kentenich1996; Marchetti & Wyrobek, Reference Marchetti and Wyrobek2005; Deng & Li, Reference Deng and Li2009; Marchetti et al., Reference Marchetti, Bishop, Gingerich and Wyrobek2015). After aneuploidy, the most common cause of fertilization failure in human in vitro fertilization and ICSI is premature sperm condensation (Edirisinghe et al., Reference Edirisinghe, Murch, Junk and Yovich1997; Moghbelinejad et al., Reference Moghbelinejad, Mozdarani and Rezaeian2013). Proper meiotic resumption of the oocyte is required to avoid premature condensation of the sperm head, which leads to DNA damage and aneuploidy in human oocytes (Manandhar & Toshimori, Reference Manandhar and Toshimori2003). In our study, premature sperm condensation was observed only in IVM, as previously reported (Tremoleda et al., Reference Tremoleda, Van Haeften, Stout, Colenbrander and Bevers2003). Another sperm-related abnormality, specific to IVM in our study, was sperm chromatin induced ectopic polar body extrusion, with a spindle forming around sperm chromatin and leading to failure of normal zygote development because of the loss of paternal chromatin (Deng & Li, Reference Deng and Li2009). Premature sperm chromatin condensation and sperm chromatin induced ectopic polar bodies were only observed in IVM, suggesting that progression to the pronuclei stage was disrupted during oocyte activation in IVM oocytes or alterations of sperm DNA specific packaging and protamine deficiency (Schmiady et al., Reference Schmiady, Tandler-Schneider and Kentenich1996; Deng & Li, Reference Deng and Li2009). However, we cannot exclude the potential that intrinsic oocyte quality or the stallion affected these results, as these variables were different for the two ICSI systems. Additionally, the two post insemination culture conditions differed between laboratories where the experiments were performed. Both DMEM/F12 and SOF media are used clinically with high success rates, but we acknowledge that diverse culture media, and therefore the environment during embryo development, could have affected the results observed in this study.
Additional zygote abnormalities included multiple pronuclei and multipolar spindle or presence of scattered maternal chromosomes and intact sperm head (chromosome fragmentation). Multipolar spindles suggest failure in spindle assembly checkpoints, and they were observed in a single oocyte from IVO and IVM (Sluder et al., Reference Sluder, Thompson, Miller, Hayes and Rieder1997; Courtois et al., Reference Courtois, Schuh, Ellenberg and Hiiragi2012). Scattered chromosomes and a sperm head were also observed in two potential zygotes from IVO and two from IVM. Multiple pronuclei suggest the failure of extrusion of the second polar body, possibly due to poor oocyte quality or sperm chromatin defects (Rosenbusch, Reference Rosenbusch2001). Overall, we observed more morphological abnormalities in zygotes from IVM than IVO. This could have been caused by alterations in normal oocyte maturation in vitro; however, the increased diversity of oocytes collected from equine ovaries is also likely to affect developmental potential.
Although the clinical use and success of ICSI has dramatically increased in the equine industry over the last decade, this is one of few studies that has focused on factors that impact zygote development. The lack of ongoing research is primarily due to our lack of knowledge regarding normal equine zygote formation and the expense and difficulty in obtaining equine oocytes, making projects that require large numbers of oocytes impractical and overly expensive. Often, the end points of projects studying equine ICSI are cleavage and blastocyst rates. Although these end points are important, they do not assess if fertilization was normal or why fertilization failure might have occurred. Therefore, more research is needed in this area to increase our understanding of equine fertilization and embryo development and to optimize success of ICSI. In this study, we demonstrated that confocal microscopy could provide meaningful data when using relatively low numbers of sperm-injected oocytes. Although multiple factors were different in regard to IVO or IVM, the results demonstrated some of the benefits and drawbacks for oocyte collection and maturation methods that are currently being used for clinical and research ICSI programs. The timelines provided by this research will allow future investigators to pick optimal time point(s) for observations of equine zygotes after ICSI of IVO or IVM. This information can be used for future investigations, especially as related to sperm and oocyte quality. The research demonstrates that confocal microscopy of individual zygotes can be a powerful tool in assessing factors impacting fertilization and zygote development in the horse, and it provides methodology that can be used by researchers to practically investigate treatment effects.
Conclusions
In conclusion, we used confocal microscopy to observe equine zygotes at timed intervals after ICSI of IVO or IVM. A similar progression of events during zygote development occurred in both groups. IVO when compared with IVM appeared to have a more rapid progression to pronuclei, although some of the potential zygotes were delayed or arrested in development. Abnormal zygote morphologies occurred more frequently in IVM than IVO. Confocal microscopy provided a feasible method to assess zygote development after in vivo or in vitro oocyte maturation using a limited number of samples.
Acknowledgments
Mare management, oocyte collection, and sample processing was done with the help of staff and students in the Assisted Reproduction Program at the Equine Reproduction Laboratory, Colorado State University (IVO), and ICSI was performed by JoAnne Stokes. The Cecil and Irene Hylton Foundation funded the study. Franc Stone and Abney Foundation scholarships and the Assisted Reproduction Program provided graduate support. Supplemental support came from a core infrastructure grant for microscope imaging and R01GM088371 (JGD) at Colorado State University and Ex Ovo Omnia project from Regione Sardegna and Lombardia at Avantea, Laboratory of Reproductive Technologies, Cremona, Italy (C.G.). The supply of ovaries was possible thanks to the collaboration of the Zerbini and Ragazzi slaughterhouse. The help of the technical staff at Avantea is also acknowledged.