Introduction
Infertility issues in men, including conditions such as oligozoospermia (low sperm count), asthenozoospermia (reduced sperm motility), and teratozoospermia (abnormal sperm shape), can have profound implications not only for reproductive outcomes but also for the overall well-being of affected individuals (Lu et al., Reference Lu, Xu, Wang, Sun, Xue, Hu, Xie and Ma2020). Oligozoospermia can significantly reduce the likelihood of spontaneous conception, necessitating assisted reproductive technologies for successful fertilization. Asthenozoospermia can impair the sperm’s ability to reach and fertilize the egg, often leading to infertility or subfertility. Teratozoospermia, characterized by abnormal sperm morphology, may affect the sperm’s capacity to penetrate the outer layers of the egg, further complicating the fertilization process (Lv et al., Reference Lv, Tang, Yu, Geng, Zhou, Shao, Li, Gao, Guo, Xu, Tan, Liu, Guo, Wu, Duan, Zhang, Wang, Hua and Fu2023). Collectively, these conditions can contribute to the psychological and emotional stress experienced by couples facing difficulties in conceiving, impacting their quality of life and causing distress. Moreover, these sperm abnormalities may sometimes reflect underlying health issues, necessitating comprehensive medical evaluations to identify and address potential systemic causes.
Calcium ions (Ca2+) are pivotal intracellular messengers involved in a myriad of physiological processes, including muscle contraction, neurotransmission, and cellular proliferation (Yamada et al., Reference Yamada, Tatsumi, Anraku, Suzuki, Kamejima, Uchiyama, Ohkido, Yokoo and Okabe2019). The calcium-sensing receptor (CaSR), a G-protein-coupled receptor, is instrumental in maintaining extracellular calcium homeostasis (Elajnaf et al., Reference Elajnaf, Iamartino, Mesteri, Müller, Bassetto, Manhardt, Baumgartner-Parzer, Kallay and Schepelmann2019). Expressed in various tissues, CaSR’s responsiveness to minute changes in ambient calcium concentrations underscores its critical role in systemic functions (da Silva Lopes and Abe, Reference da Silva Lopes and Abe2021). In the realm of human reproductive health, calcium signalling is paramount for sperm capacitation, acrosome reaction, and motility, processes essential for fertilization (Li et al., Reference Li, Luo, Li and Yan2020). Despite its established significance, the intricate workings of CaSR in sperm physiology and male fertility remain an area ripe for exploration.
CaSR expression and functional significance within human spermatozoa and spermatogenic cells are not as well characterized, presenting a critical gap in the field of reproductive biology. This lacuna in knowledge obstructs a complete understanding of the nuanced mechanisms by which CaSR influences male fertility, potentially obscuring its utility both as a diagnostic biomarker and a therapeutic target. The current study is designed to dissect the expression patterns of CaSR in both normal and pathological sperm samples, and to delineate its functional implications, particularly in the context of sperm motility and morphology. By bridging these knowledge gaps, this research endeavours not only to clarify the role of CaSR in male reproductive health but also to pioneer new avenues for the diagnosis and treatment of male infertility disorders. It is the aspiration of this work that by deepening the understanding of CaSR’s activity, more light can be shed on its potential to improve reproductive outcomes and alleviate male fertility complications.
Materials and methods
Sample collection and preparation
The study utilized semen samples from a cohort of 20 males aged between 25 and 40 years, presenting at the fertility clinic. The subjects were divided into two groups based on their semen analysis results according to the World Health Organization (WHO) criteria: a normal group (n = 10), exhibiting standard parameters of concentration, motility, and morphology; and an abnormal group (n = 10) characterized as either asthenozoospermia, oligozoospermia, or teratozoospermia.
Ethical approval for this study was granted by the Institutional Review Board (IRB) of Affiliated Hospital of Zunyi Medical University, and all participants provided written informed consent prior to sample collection. The consent procedure ensured confidentiality and the right to withdraw from the study at any point without any consequences for medical care.
Semen samples were collected by masturbation after a recommended 3–5 days of sexual abstinence and were allowed to liquefy at room temperature for 30 min. Following liquefaction, samples were processed immediately or stored at −80°C for subsequent analyses. For RNA and protein analyses, samples underwent centrifugation to separate spermatozoa from seminal plasma. The pellet was washed in phosphate-buffered saline (PBS) and then divided into aliquots for RNA extraction, protein lysates, and IHC. All procedures were conducted under strictly controlled conditions to prevent contamination and degradation of the samples.
Expression analysis of CaSR
For the quantification of CaSR mRNA levels, total RNA was extracted from the sperm samples using the TRIzol reagent, following the manufacturer’s instructions. The quality and concentration of RNA were determined using a spectrophotometer. Complementary DNA (cDNA) was synthesized using a reverse transcription kit. Quantitative PCR (qPCR) was then performed using SYBR Green PCR Master Mix on a real-time PCR system. Specific primers for CaSR and GAPDH (as a housekeeping gene for normalization) were utilized, and the relative expression levels of CaSR mRNA were calculated using the 2−ΔΔCt method.
Immunohistochemistry (IHC) was performed to localize CaSR in sperm and spermatogenic cells. Samples were fixed, permeabilized, and blocked to prevent non-specific binding. They were then incubated with a primary antibody against CaSR, followed by a biotinylated secondary antibody. The signal was amplified with an avidin-biotin complex and visualized with diaminobenzidine (DAB). Slides were counterstained with haematoxylin, dehydrated, and mounted. CaSR localization was observed under a light microscope, and images were captured for analysis. Positive staining was identified by a brown precipitate in the membrane region of the cells. Negative controls, in which the primary antibody was omitted, were included in each batch to confirm the specificity of staining.
Sperm functional assays
Assessing sperm motility with computer-assisted sperm analysis
Sperm motility was analyzed using a computer-assisted sperm analysis (CASA) system. Freshly liquefied semen samples were diluted in warm (37°C) buffer solution to achieve optimal sperm concentration for analysis. A small aliquot of the diluted sample was placed on a pre-warmed slide and assessed under a phase-contrast microscope connected to the CASA system. The system was calibrated to track sperm movement, providing detailed information on various parameters such as straight-line velocity (VSL), curvilinear velocity (VCL), and linearity (LIN). At least 200 spermatozoa per sample were analyzed to ensure statistical reliability.
Evaluating sperm morphology through differential staining
Sperm morphology was assessed using differential staining techniques, which allow the distinction of various sperm components. After washing the sperm samples, smears were prepared on glass slides and air dried. The smears were then stained using a rapid staining kit (e.g. Shorr’s staining), which differentially colours the head, midpiece, and tail of spermatozoa. Stained smears were examined under a light microscope at ×1000 magnification with oil immersion. At least 200 spermatozoa per sample were evaluated, and the percentage of normal morphology was calculated according to strict criteria, including head size, shape, acrosomal region, and tail integrity.
Statistical analysis
For normally distributed data, parametric tests were used. The independent t-test was used for comparing two groups (normal vs. abnormal sperm samples), while one-way analysis of variance (ANOVA) followed by post-hoc tests [e.g. Tukey’s honestly significant difference (HSD)] was used for comparisons involving more than two groups or conditions. In cases in which data did not follow a normal distribution, non-parametric tests such as the Mann–Whitney U-test (for two groups) and the Kruskal–Wallis test (for more than two groups) were utilized. For all tests, a P-value of less than 0.05 was considered statistically significant.
Results
Significant decrease in CaSR expression in abnormal sperm
Quantitative PCR analysis revealed significant differences in CaSR mRNA expression levels between normal and abnormal sperm samples. In normal sperm, the relative expression of CaSR mRNA was significantly higher compared with that in the abnormal group (P < 0.01). This difference indicated a substantial reduction in CaSR transcription in samples diagnosed with asthenozoospermia, oligozoospermia, or teratozoospermia (Figure 1). These findings suggest a possible correlation between diminished CaSR expression and impaired sperm function, underlying various forms of male infertility.
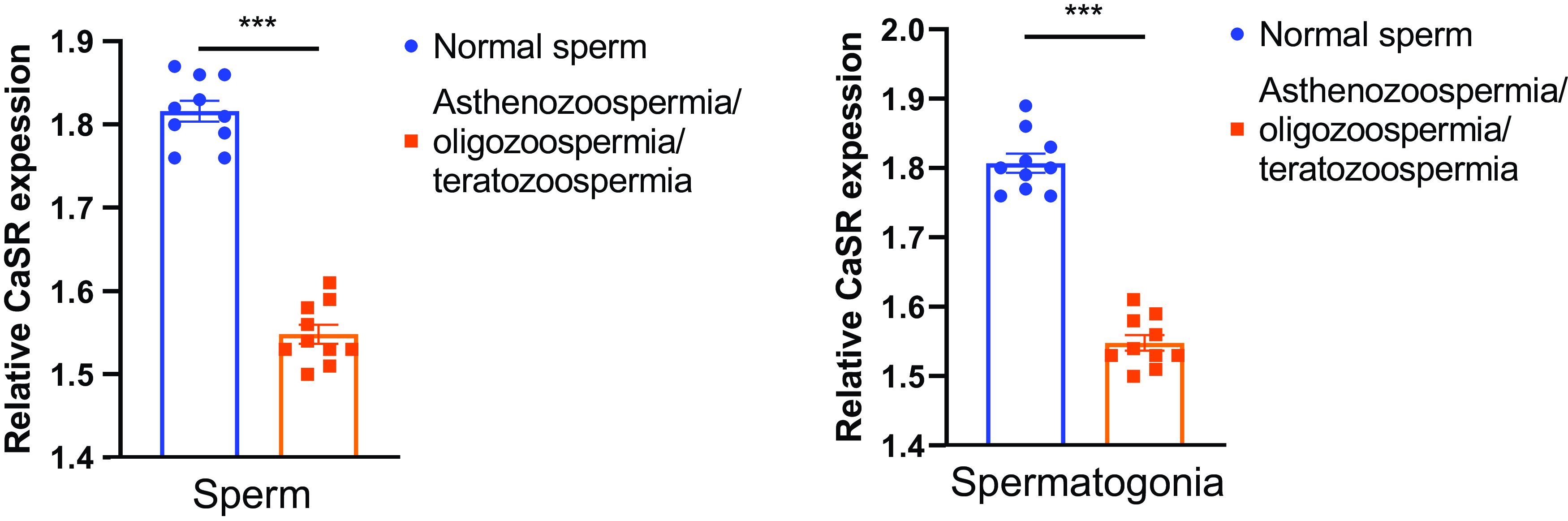
Figure 1. Reduced CaSR expression in sperm and spermatogonia of infertile patients compared with normal counterparts. (A) Relative CaSR expression levels in sperm samples. The graph displays the quantification of CaSR expression in normal sperm samples (n = 10) and sperm from patients diagnosed with asthenozoospermia, oligozoospermia, or teratozoospermia (n = 10). Expression levels are normalized to a control. Data are presented as mean ± standard error of the mean (SEM). Statistical significance is indicated by asterisks (***P < 0.001). (B) Relative CaSR expression levels in spermatogonia samples. This graph shows the quantification of CaSR expression in normal spermatogonia (n = 10) compared with spermatogonia from patients with asthenozoospermia, oligozoospermia, or teratozoospermia (n = 10). Expression levels are normalized to a control. Data are represented as mean ± SEM, with statistical significance denoted by asterisks (***P < 0.001).
Regulatory trends of CaSR expression in testicular and epididymal tissues
Immunohistochemical staining of testicular and epididymal tissues was conducted to discern the expression levels of the CaSR across different groups: control, CaSR agonist-treated, and CaSR antagonist-treated. The results depicted in Figure 2 illustrate a pronounced expression of CaSR in the control group, with intense brown DAB staining localized primarily in the spermatogenic cells of the testis and the epithelium of the epididymis, indicative of active CaSR expression. In comparison, Figure 3 reveals the effects of pharmacological modulation of CaSR on its expression in these tissues. Treatment with a CaSR agonist appeared to sustain the expression levels, as evidenced by the staining intensity comparable to that of the control. This suggests that activation of CaSR does not diminish its expression in the spermatogenic cells and the epididymal epithelium. Conversely, the application of a CaSR antagonist resulted in a noticeable decline in staining intensity, implying a reduction in CaSR expression. The decrease was more pronounced in the epididymal epithelium than in the testicular spermatogenic cells. These differential staining patterns provide a visual representation of the potential regulatory effects of CaSR activity on its own expression within the male reproductive tract. The results suggest that CaSR antagonism may downregulate receptor expression, which could be consequential in the context of sperm maturation and fertility. Contrastingly, CaSR agonism maintains receptor expression, affirming the receptor’s role in the normal functioning of testicular and epididymal cells. These findings contribute to our understanding of CaSR’s involvement in male reproductive health and may have implications for therapeutic strategies targeting male infertility.
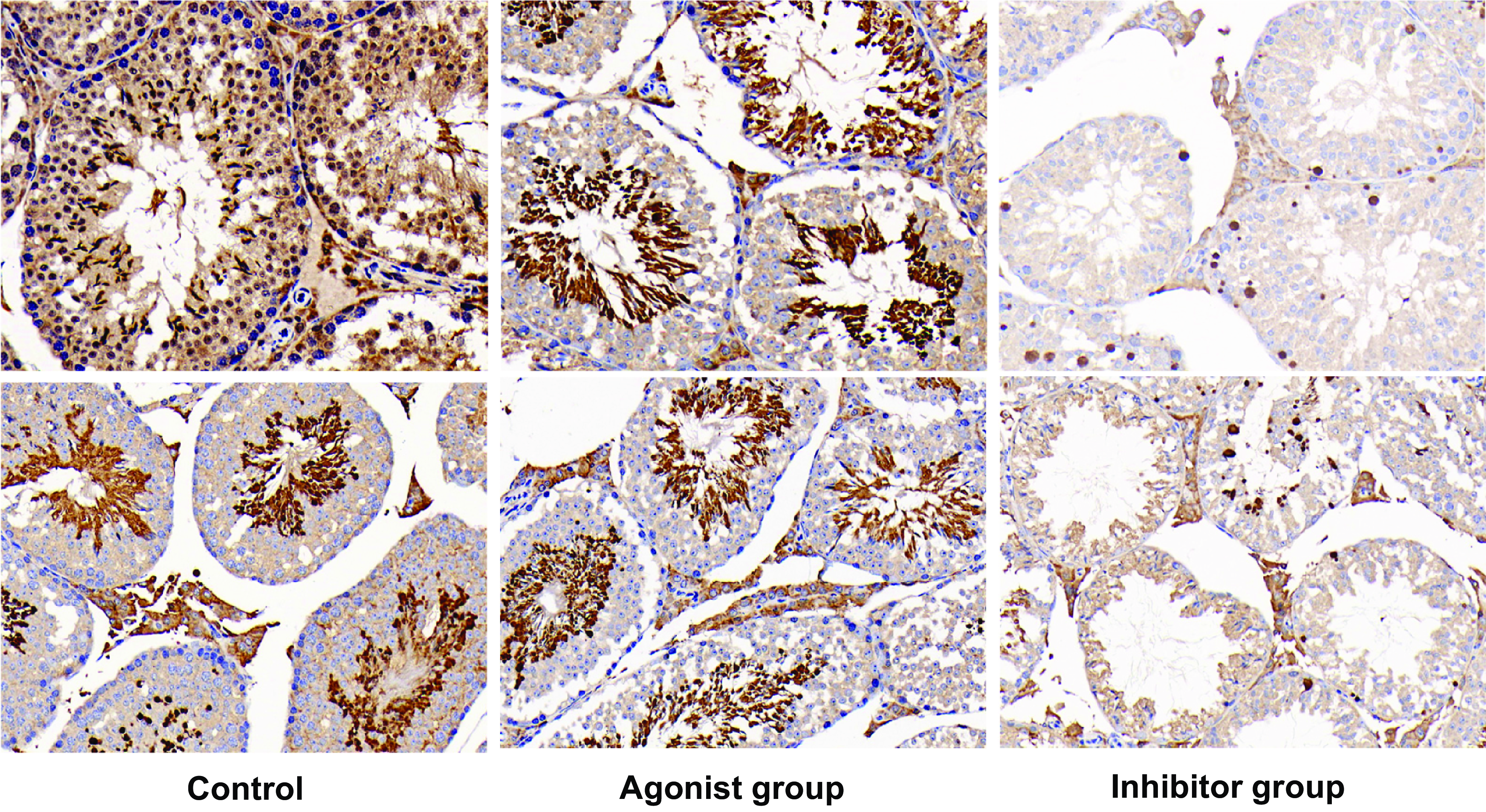
Figure 2. Differential CaSR expression in testicular tissue: effects of agonist and antagonist treatment. Immunohistochemical analysis of CaSR expression in testicular tissues. Left panel: Control group showing strong CaSR expression in spermatogenic cells, highlighted by intense dark brown DAB staining. Middle panel: CaSR agonist-treated group with maintained CaSR expression, evident from the comparable staining intensity to the control. Right panel: CaSR antagonist-treated group showing reduced CaSR expression, as indicated by the lighter DAB staining. Each image represents 1 of 10 independent samples per group.
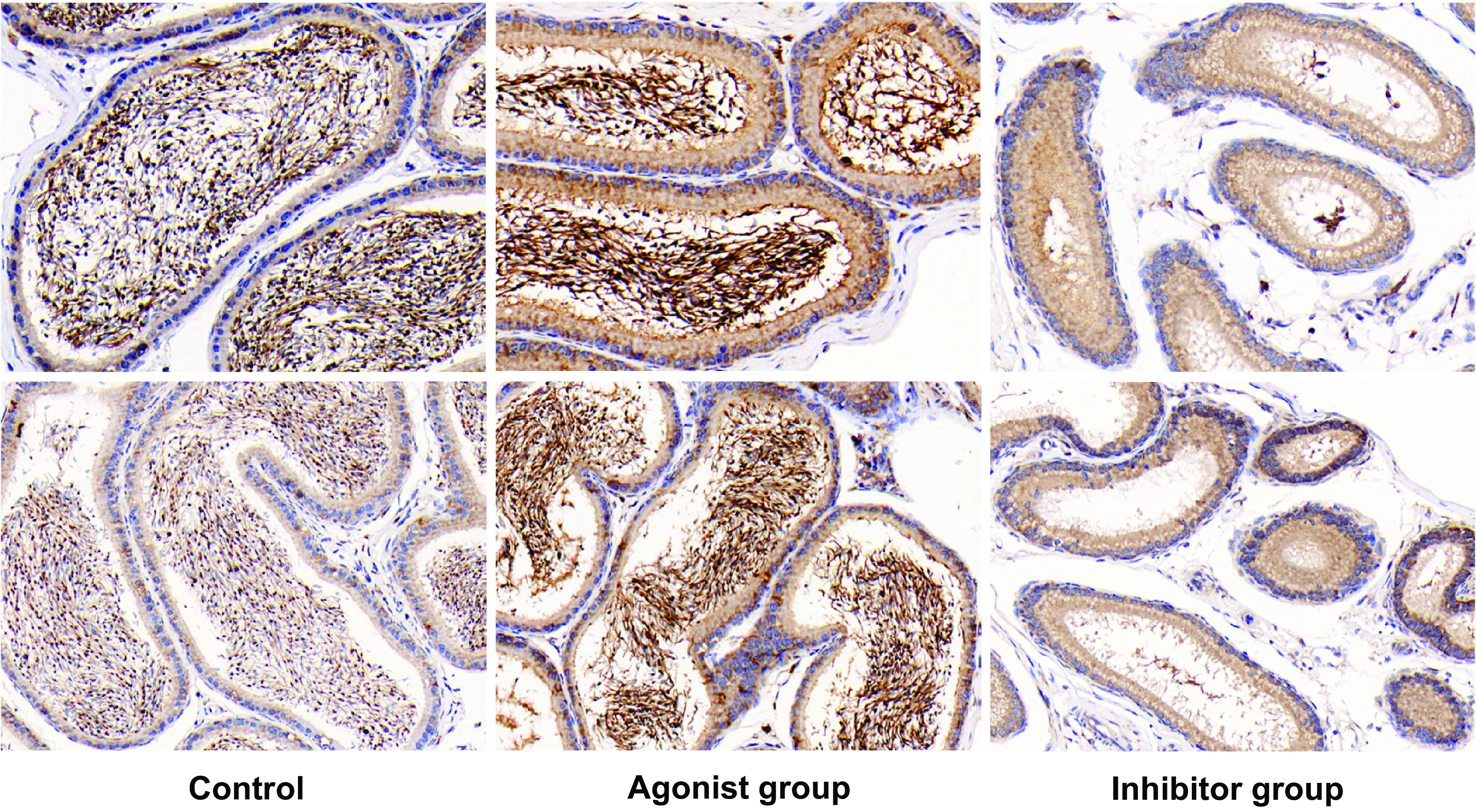
Figure 3. Modulation of CaSR expression in epididymal tissue by agonist and antagonist treatment. Immunohistochemical staining for CaSR expression in epididymal tissue. Left panel: Control group with intense CaSR staining in the epithelial cells of the epididymis. Middle panel: Tissue treated with a CaSR agonist showing maintained expression levels of CaSR, comparable to the control. Right panel: Tissue treated with a CaSR antagonist exhibiting diminished CaSR staining, indicating decreased expression. The images display representative staining from 10 individual samples per group, with magnified insets focusing on the epithelial cell layer where CaSR expression is assessed.
Varied influences of calcium channel modulators on sperm motility
This study aimed to elucidate the role of calcium channels in sperm function by analyzing the effects of various treatments on sperm motility and morphology across five distinct experimental groups: the normal control, the CaSR agonist-treated, the caffeine-treated, the high calcium with NiCl2, and the high calcium with CdCl3 groups, as illustrated in Figure 4. The normal control group served as the benchmark, with sperm exhibiting standard morphology and motility. Contrastingly, the CaSR agonist-treated group demonstrated enhanced sperm vitality, suggesting a stimulatory effect of CaSR activation on sperm motility. In the caffeine-treated group, a notable suppression of sperm activity was observed, contrary to the expected outcome, indicating a potential inhibitory mechanism of caffeine on sperm motility at the applied concentration. The two high calcium groups with channel blockers showed distinct effects. The high calcium with NiCl2 group presented a moderate decline in sperm motility. NiCl2, at 100 mM, is known to inhibit specific calcium channels selectively, which are implicated in the fine tuning of sperm motility. A stark contrast was evident in the high calcium with CdCl3 group. At 200 μM, CdCl3, a blocker of a wider array of calcium channels than NiCl2, caused a substantial reduction in sperm motility. This marked effect accentuates the critical function of these channels in sperm physiology and suggests their broader involvement in calcium homeostasis. The divergent effects of NiCl2 and CdCl3 can be attributed to their differential channel blocking profiles. NiCl2 tends to affect T-type calcium channels, which are involved in the acrosome reaction and sperm capacitation, while CdCl3 has been shown to inhibit L-type channels, which are essential for the hyperactivation of sperm motility necessary for fertilization (Lee et al., Reference Lee, Gomora, Cribbs and Perez-Reyes1999; Tredway et al., Reference Tredway, Guo and Chiappinelli1999). In summary, our results indicate that calcium channel activity is vital for maintaining normal sperm function, and that specific channel blockers can differentially modulate this activity.
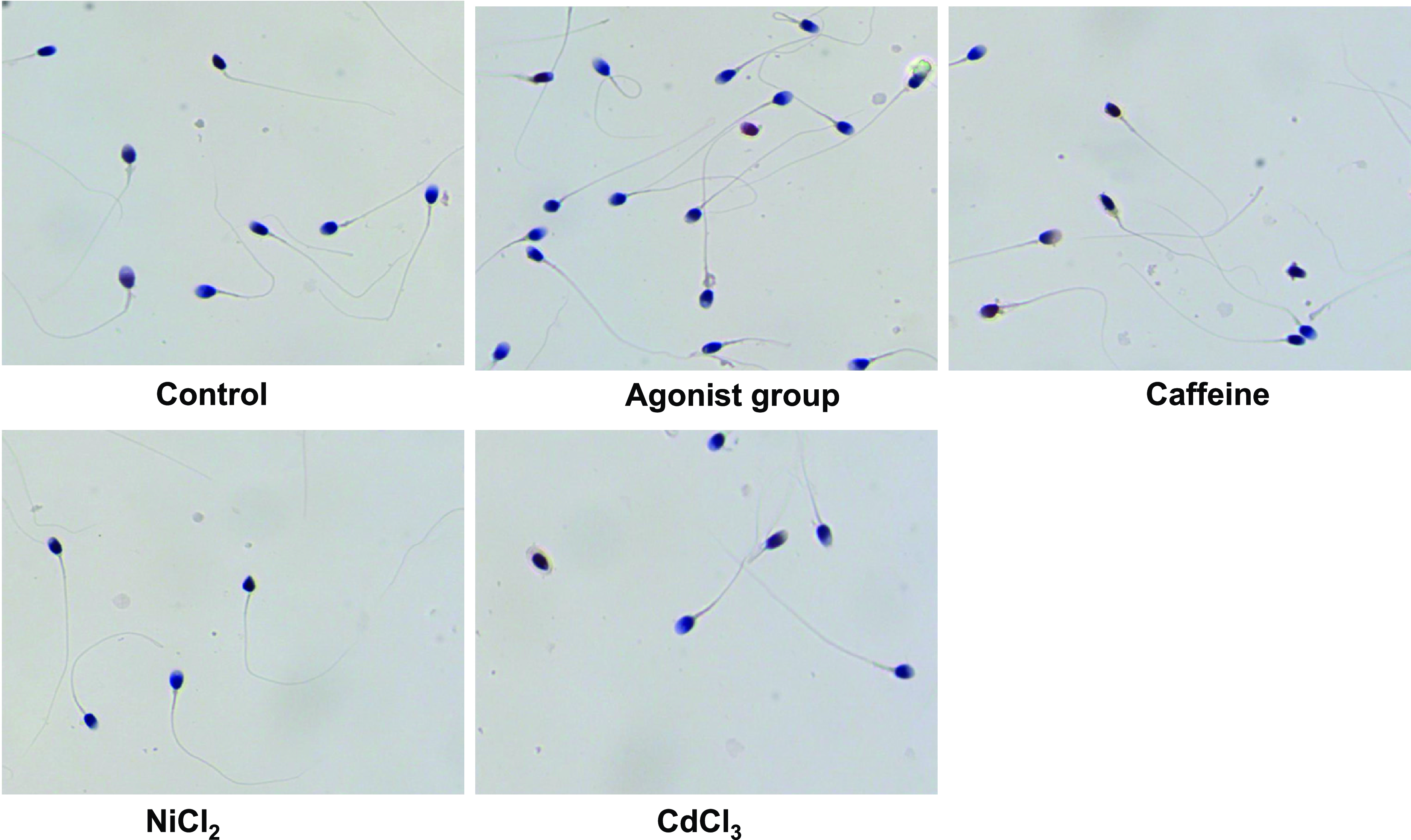
Figure 4. Comparative effects of CaSR modulation and calcium channel blockers on sperm motility. Microscopic analysis of sperm motility under various treatment conditions. Top left: Control group displaying normal sperm motility. Top centre: Sperm treated with a CaSR agonist, showing maintained motility. Top right: Sperm treated with caffeine, exhibiting reduced motility. Bottom left: Sperm in high calcium condition with a Ca2+ channel blocker (Ca-L), showing decreased motility. Bottom right: Sperm in high calcium condition with a Na+/Ca2+ exchanger inhibitor (Na-Ca), demonstrating significantly impaired motility. Each micrograph is representative of 10 independent experiments for each condition.
Discussion
This study embarked on a comprehensive exploration of the role of the CaSR in male reproductive health, particularly focusing on its expression and functionality in sperm cells and spermatogenic cells. Our findings revealed that CaSR is differentially expressed in normal versus abnormal sperm samples, with notably decreased expression observed in conditions associated with male infertility such as asthenozoospermia, oligozoospermia, and teratozoospermia. These observations were corroborated by functional assays, which demonstrated that modulation of CaSR, through inhibition, directly impacts sperm motility.
The significance of these findings cannot be understated, especially considering the critical role of calcium homeostasis in cellular functions across various tissues. In the realm of male reproductive health, the implications are substantial. The differential expression of CaSR in normal and abnormal sperm samples provides new insights into the molecular underpinnings of sperm physiology. This understanding could be pivotal in addressing male infertility, a condition that affects a significant portion of the population. According to the WHO, male factors account for ∼50% of all cases of infertility, highlighting the importance of research in this area (Du et al., Reference Du, Zhang, Chen, He and Lin2022). Furthermore, the ability of CaSR modulation to influence sperm motility opens up new avenues for therapeutic intervention. The potential of CaSR as a therapeutic target is especially relevant in light of the ongoing search for effective and safe fertility treatments.
CaSR’s role in sperm function and male fertility
The findings of our study shed light on the intricate role of the CaSR in the context of sperm physiology and male fertility. In normal sperm and spermatogenic cells, CaSR was found to be robustly expressed, indicative of its active role in these cells. The significant expression of CaSR in normal sperm suggests its involvement in the regulation of key processes such as motility and capacitation, which are essential for successful fertilization. Calcium signalling plays a crucial role in sperm function, including motility, chemotaxis, acrosome reaction, and capacitation (Correia et al., Reference Correia, Michelangeli and Publicover2015). Calcium influx is required for fertilization, and sperm-initiated calcium oscillations cease prematurely in the absence of external calcium (Wakai et al., Reference Wakai, Vanderheyden and Fissore2011). Two molecules, IP3R1 receptor in eggs and PLCζ in sperm, are central to the initiation and maintenance of calcium signalling during mammalian fertilization (Wakai et al., Reference Wakai, Vanderheyden and Fissore2011). Calcium signalling and the MAPK cascade are required for sperm activation in Caenorhabditis elegans (Liu et al., Reference Liu, Wang, He, Zhao and Miao2014). This study highlights the essential role of CaSR in the regulation of sperm function, emphasizing its importance in processes critical for fertilization and pointing to the broader relevance of calcium signalling pathways in reproductive biology.
Impact of CaSR modulation on sperm motility
Our functional assays provided valuable insights into how modulation of CaSR affects sperm motility. Activation of CaSR through agonists was seen to enhance sperm motility, suggesting that CaSR may play a role in promoting the physiological conditions necessary for effective sperm movement. Conversely, the inhibition of CaSR resulted in decreased motility, indicating a potential mechanistic pathway through which CaSR influences sperm function. These findings contribute to a deeper understanding of the molecular dynamics governing sperm motility, a critical factor in male fertility. According to the search results, the potential mechanisms through which CaSR agonists and antagonists influence sperm movement are likely to involve the regulation of intracellular calcium levels. The CaSR is important for the sensing of Ca2+, Mg2+, and HCO3 − in spermatozoa, and loss of function may impair male sperm function (Boisen et al., Reference Boisen, Rehfeld, Mos, Poulsen, Nielsen, Schwarz, Rejnmark, Dissing, Bach-Mortensen, Juul, Bräuner-Osborne, Lanske and Blomberg Jensen2021). Ca2+ ions have been shown to play roles in the initiation and maintenance of sperm hyperactivation movement, and Ca2+ ionophores can enhance sperm fertilizing capacity (Navarrete et al., Reference Navarrete, Alvau, Lee, Levin, Buck, Leon, Santi, Krapf, Mager, Fissore, Salicioni, Darszon and Visconti2016). However, the intracellular signalling pathways associated with the observed effect of CaSR on sperm motility and capacitation remain to be investigated.
Comparative analysis with other studies
The role of CaSR in reproductive health has been an area of growing interest, but its specific implications in male fertility have been less explored. Previous research has primarily focused on CaSR’s role in systemic calcium homeostasis and its implications for bone health and parathyroid function (Thakker, Reference Thakker2012; Conigrave, Reference Conigrave2016). Our study extends this understanding to the realm of male reproductive health, highlighting a novel aspect of CaSR’s function. In comparison with existing literature, our findings provide unique insights into CaSR’s specific role in sperm physiology. Our research bridges this gap by demonstrating the marked decrease in CaSR expression in cases of male infertility and the consequent effects on sperm function.
Potential therapeutic applications
The implications of our findings are profound in the context of therapeutic applications for male infertility. The possibility of targeting CaSR for therapeutic interventions opens up new avenues in the treatment of male infertility. Given the crucial role of CaSR in regulating sperm motility, modulation of this receptor could be a promising strategy for improving sperm function in infertile patients. The development of new treatments based on CaSR modulation could involve the use of specific agonists or antagonists to regulate sperm motility and functionality. These approaches could be particularly beneficial in cases in which traditional infertility treatments have been unsuccessful. For instance, in cases of asthenozoospermia in which sperm motility is compromised, CaSR agonists might be used to enhance motility and improve fertility outcomes. Understanding the role of CaSR in sperm function is also crucial for future drug development. This knowledge could guide the creation of targeted therapies that modulate CaSR activity to address specific fertility issues. Additionally, the insights gained from this study could be applied to broader reproductive health issues, potentially influencing the development of contraceptives or treatments for other conditions involving calcium signalling dysregulation.
Limitations of the current study
While our study provides important insights into the role of CaSR in male fertility, there are several limitations that must be acknowledged. First, the study was primarily conducted in vitro, which may not fully replicate the complexities of in vivo conditions. Therefore, the effects observed in our study might differ from those occurring in the natural physiological environment. Additionally, the study’s scope was limited to assessing the effect of CaSR modulation on sperm motility and morphology. Other aspects of sperm function, such as capacitation, acrosome reaction, and fertilization potential, were not explored. The effects of CaSR modulation on these critical aspects of sperm functionality remain to be investigated. Future research should aim to address these limitations, perhaps using animal models or conducting clinical trials, to better understand the role of CaSR in the broader context of male reproductive physiology.
Future research directions
Future studies should focus on elucidating the molecular mechanisms underlying CaSR’s role in sperm function. This could involve investigating the specific signalling pathways activated by CaSR in sperm cells and how these pathways influence various aspects of sperm physiology, including motility, capacitation, and the acrosome reaction. Additionally, research should be conducted to understand the long-term effects of CaSR modulation on male fertility. This is particularly important for assessing the potential use of CaSR modulators as therapeutic agents. Longitudinal studies could provide valuable information on the efficacy and safety of such treatments over time. Another potential area of research is exploring the role of CaSR in different subpopulations of sperm within an ejaculate. This could provide insights into whether CaSR expression varies among sperms with different motility patterns or morphological characteristics and how this might affect fertility outcomes. Finally, given the implications of CaSR in calcium homeostasis, it would be beneficial to study how systemic calcium levels and other related metabolic factors influence CaSR function in sperm and overall male reproductive health. This could lead to a more integrated understanding of male fertility that encompasses both systemic and local factors.
In conclusion, this study underscores the crucial role of the CaSR in male fertility, revealing its significant effect on sperm motility and morphology. The findings suggest CaSR as a potential target for treating male infertility, highlighting the need for further research to explore this promising avenue.
Data availability
All data in this study are true and reliable. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
This work was supported by grants from the Affiliated Hospital of Zunyi Medical University (2014) No. 48.
Author contributions
Conceptualization and Supervision, YGP; Investigation, LKY; Visualization, ZMZ; Writing – Original draft, QZL; Reviewing, Editing, and Funding Acquisition, QZL.
Competing interests
The authors declare no competing interests.







