Toxoplasma gondii (TG) affects one-third of the global human population and commonly involves the central nervous system (CNS)/brain more frequently in immunocompromised patients. Reference Graham, Fong, Naqvi and Lu1 Subclinical or asymptomatic cerebral toxoplasmosis is relatively common in immunocompetent individuals and occasionally seen in immunocompromised patients. The immunocompromised status can result from immunosuppressant and/or immunomodulatory treatment in patients after organ transplantation, and other immunosuppressive conditions such as human immunodeficiency virus infection. Reference Graham, Fong, Naqvi and Lu1–Reference Ramanan, Scherger and Benamu5 Focal CNS infectious lesions or brain abscesses are known complications of transplantation, and have been documented in 0.36–1% of organ transplant recipients. Reference Singh and Husain2 CNS toxoplasmosis occurs variably in transplant recipients with the highest incidence (13–53%) in cardiac transplantation and the lowest incidence in liver transplantation (rarely reported). After organ transplantation, common bacterial pathogens rarely cause intracranial abscesses, but CNS Nocardia infection has been noted in 1–6% of solid organ transplant recipients. Reference Singh and Husain2,Reference Chapman and Wilson6,Reference Selby, Ramirez and Singh7 Here, we report a unique case of a patient who developed brain abscesses with coexisting TG and bacterial infections 18 years after liver transplantation with continuous immunosuppressive treatment.
A 61-year-old female presented with a 3-month history of feeling generally unwell and a 2-week history of confusion and right-sided weakness. Her medical history was remarkable for Hepatitis C with liver cirrhosis and failure for which she underwent a liver transplant 18 years ago followed by immunosuppressive therapy with Tacrolimus (1 mg daily) and Mycophenolate (750 mg twice daily) thereafter. After liver transplantation, she was stable with regular follow-ups and no evidence of rejection. She also had a history of well-controlled type 2 diabetes mellitus, hypertension, and dyslipidemia. At the current presentation, neurological examination showed speech deficit and mild right-sided weakness. Head computerized tomography (CT) identified a large contrast enhancing lesion in the left frontal lobe. Subsequent brain magnetic resonance imaging (MRI) disclosed multiple T2/FLAIR hyperintense, and T1 post-contrast nodular or ring enhancing lesions in the brain parenchyma including the left frontal region (largest, 22.5 × 10.1 mm, with brain edema and mass effect causing a left-to-right midline shift), as well as left temporal, right frontal, and parietal regions (Figure 1). The imaging differential diagnosis included tumor metastases, multifocal primary brain tumor and, given her history, brain abscess. CT of the chest, abdomen, and pelvis showed no intra-thoracic or intra-abdominal mass lesions except a 4-mm nonspecific nodule in the right upper lobe of her lung. The nodule disappeared on the 12-week follow-up CT following below-mentioned treatment. Routine blood work revealed leukopenia (2.9, versus normal 4.0–11.0 × 109/L, on the operation day) with consistently low neutrophil (1.8, versus normal 2.0–7.5 × 109/L) and lymphocyte (0.7, versus normal 1.5–4.0 × 109/L) counts with normal liver function. The patient was referred to the neurosurgical service for further management. After assessment and discussion with the patient and family, the decision to proceed with a craniotomy for resection of the left frontal ring-enhancing lesion was made with the goal of pathological diagnosis and cytoreduction.
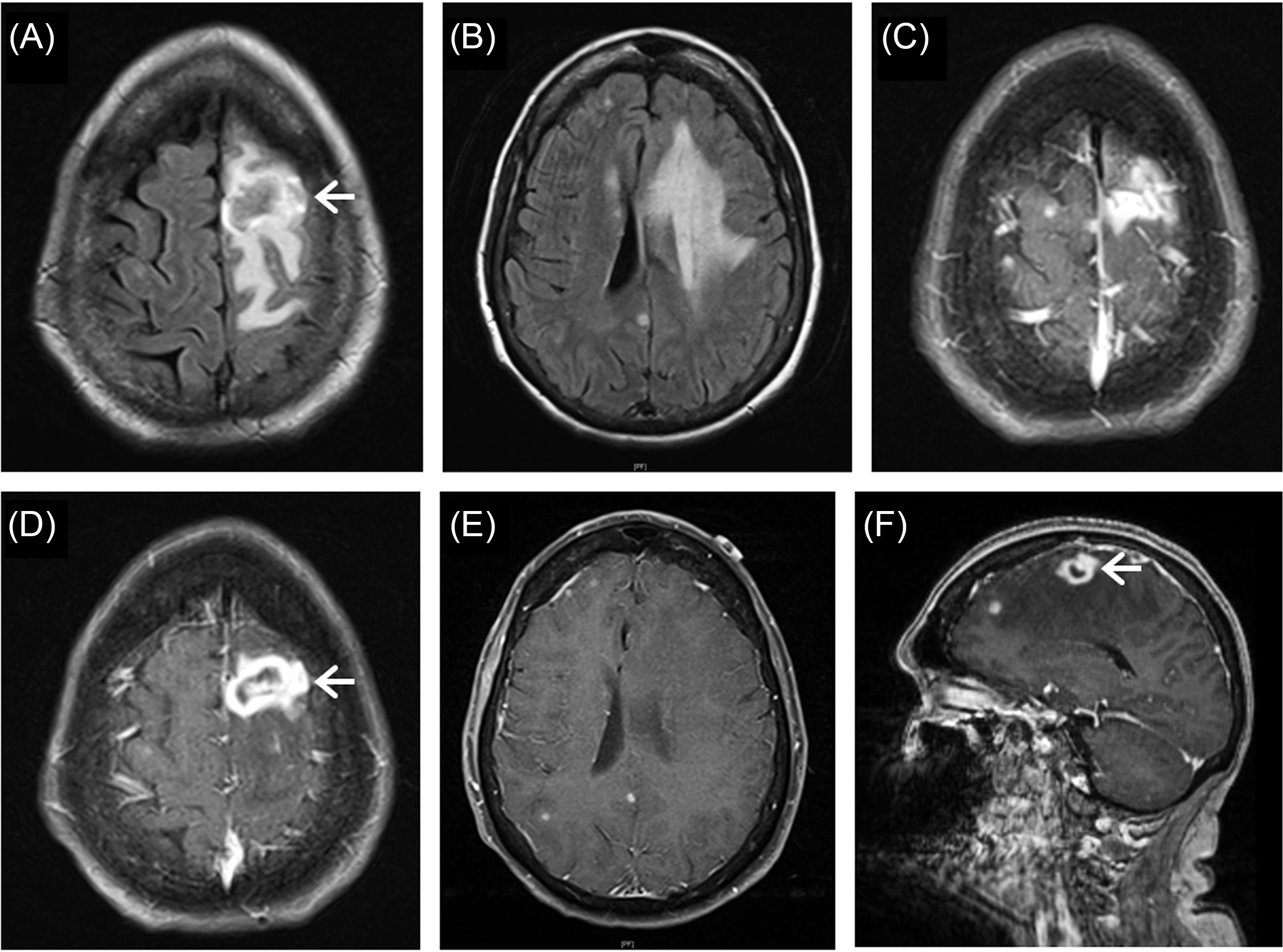
Figure 1: Magnetic resonance imaging of brain lesions. Axial FLAIR images show multiple hyperintense lesions in the brain parenchyma including the left frontal and temporal, as well as right frontal and parietal regions (A, B). T1 post-contrast images demonstrate multiple nodular and ring enhancing lesions in these brain regions (C–E, axial; F, sagittal). The largest lesion in the left frontal lobe is heterogeneous on FLAIR imaging (A, arrow) with brain edema and mass effect causing a right-sided midline shift (B), and ring-enhancing on T1 post-contrast imaging (D, F, arrows).
Pathological examination of the resected tissue revealed an encapsulated brain abscess with central necrosis surrounded by reactive changes such as astrocytic gliosis and abundant inflammatory cell infiltrates including neutrophils, CD68+ macrophages/microglia, CD3+, CD4+, and CD8+ T-cells, with fewer CD20+ B-cells (Figure 2). TG immunostaining demonstrated focally frequent positive tachyzoites and bradyzoites (Figure 2E); gram staining showed focally frequent variably positive beaded bacilli with vague branching, some of which were filamentous and also positive on Wade Fite staining (Figure 2F). The bacilli were morphologically consistent with Nocardia and focally coexisted with TG in this lesion. There was no evidence of other infection or neoplasm.

Figure 2: Photomicrographs of the left frontal ring-enhancing lesion show a brain abscess with central necrosis (A, hematoxylin & eosin, note area of necrosis in upper right) surrounded by reactive changes such as gliosis/fibrosis forming a capsule (B, reticulin stain, note area of increased reticulin deposition in lower left) and abundant inflammatory cell infiltrates including CD68+ macrophages/microglia (C) and CD3+ T-cells (D). The necrotic and adjacent inflamed tissue contains focally frequent Toxoplasma+ tachyzoites and bradyzoites (E, Toxoplasma immunostaining; an inset corresponding to the dotted-line rectangle) and beaded bacilli that are vaguely branching and variably positive on Gram staining (F, a right inset corresponding to the dotted-line rectangle; a left inset demonstrating the bacilli on Wade Fite staining). Scale bars: 50 µm (A–D) and 10 µm (E, F).
MRI completed after the craniotomy exhibited postoperative changes in the left frontal region. The patient was then treated with multiple medications including ceftriaxone (intravenous, 2000 mg daily for 20 days), trimethoprim/sulfamethoxazole (oral, 240 mg/1200 mg, four times per day for 1 month), and pyrimethamine (oral, 75 mg daily for 7 days) with leucovorin (oral, 25 mg daily). Five weeks post-operatively she was re-hospitalized for 4 weeks due to acute renal failure, seizures, and supraventricular tachycardia; after stabilization, she was discharged home and remained stable over the next 12 weeks. Follow-up head CT showed some encephalomalacia in the left frontal lobe, but no other acute abnormality.
Our present case is the first to demonstrate a brain abscess caused by TG and comorbid bacterial infections after liver transplantation and chronic immunosuppression. This patient’s CNS toxoplasmosis is seemingly long-standing but recently reactivated, given the presence of encapsulation and abundant T-cells that are thought to protect against TG infection and reactivation of chronic TG infection. Reference Graham, Fong, Naqvi and Lu1 The infection was not recognized until pathological examination and was presumably asymptomatic until its recent reactivation, which was likely associated with the brain bacterial infection (consistent with nocardiosis).
Nocardia infection is occasionally found in solid organ transplant recipients and usually occurs after prolonged immunosuppression, with most cases noted between 1 and 6 months post-transplantation and coexisting infections in 20% of the cases. Reference Singh and Husain2,Reference Chapman and Wilson6,Reference Selby, Ramirez and Singh7 CNS is the most frequent site of secondary dissemination and usually presents as a single or multiple brain abscesses that may be lobulated with nodular or ring enhancement on MRI. Multiple brain abscesses may be seen in up to 40% of Nocardia infection cases, and in chronically immunosuppressed patients, their development long after transplantation are predominantly due to Nocardia or TG infection. Reference Singh and Husain2,Reference Peleg, Husain and Qureshi8 A previous case report described an immunocompromised patient who developed a Nocardia abscess during treatment for CNS toxoplasmosis, Reference Soto-Hernández, Moreno-Andrade, Góngora-Rivera and Ramírez-Crescencio9 while the comorbidity of post-transplant brain toxoplasmosis and bacterial infection has not yet been reported. In our case, the brain nocardiosis may similarly result from further immunosuppression potentially caused by brain toxoplasmosis. After liver transplantation, CNS infections occurred in 1.2–18% of the recipients; fungal infection, in particular with aspergillosis, was reported in most of these cases Reference Bronster, Emre, Boccagni, Sheiner, Schwartz and Miller3 ; CNS toxoplasmosis has been reported in at least seven patients after liver transplantation. Reference Assi, Rosenblatt and Marshall4,Reference Ramanan, Scherger and Benamu5 Systemic TG infection is common after non-cardiac solid organ transplantation, although it may be subclinical or asymptomatic and CNS involvement is less common (18 ∼ 33%). Reference Ramanan, Scherger and Benamu5,Reference Galván-Ramírez, Sánchez-Orozco, Gutiérrez-Maldonado and Rodriguez Pérez10 Many cases of post-transplant TG infection are donor-derived, as transmission of TG can occur by transplantation of TG cysts residing in the donor organ. A systematic review of TG infection after liver transplantation revealed that IgM anti-Toxoplasma antibodies were found in 30% of the recipients, and Toxoplasma DNA or tachyzoites were reported in 67% of the seronegative recipients, Reference Galván-Ramírez, Sánchez-Orozco, Gutiérrez-Maldonado and Rodriguez Pérez10 while the pretransplant TG serology was usually negative. Reference Ramanan, Scherger and Benamu5 TG infection may be reactivated in the recipients due to their immunosuppressive treatment or other events that further suppress the immune system. These observations suggest that TG infection in post-transplant patients usually manifests days to months after organ transplantation; otherwise, it may be subclinical or asymptomatic for years until reactivation by worsening immune status as shown in our present case with comorbid bacterial infection. Although toxoplasmosis may be associated with other immunosuppressive conditions, Reference Graham, Fong, Naqvi and Lu1,Reference Soto-Hernández, Moreno-Andrade, Góngora-Rivera and Ramírez-Crescencio9 diabetes mellitus in our patient was well-controlled and she has no history of other immunosuppressive conditions beyond the liver transplantation.
CNS toxoplasmosis is a life-threatening infection particularly in immunocompromised individuals and remains under-recognized in the workup of patients with neurological manifestations. In post-transplant patients, CNS TG infection is relatively common and CNS nocardiosis is also occasionally seen, so radiological/clinical differential diagnoses for CNS lesions such as tumor-like lesions should include toxoplasmosis and nocardiosis. CNS toxoplasmosis with or without coexisting nocardiosis deserves more awareness in our practice to facilitate prompt diagnosis and treatment with perioperative prophylactic therapy.
Disclosures
The authors report no conflicts of interest and no financial disclosures.
Statement of Authorship
AKG and JQL designed the study. All authors provided the patient data and analysis. AKG, MKS, and JQL wrote the manuscript. SS, DY, and CM critically reviewed the manuscript. All authors approved the final manuscript.




