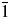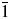I. INTRODUCTION
Metal iodates are an attractive inorganic compound system with a diversity of unusual structures and various physical properties, which mainly arise from the stereochemical active lone pair electrons on the I5+ ion and the asymmetric pyramidal coordination geometry of IO3 building units. Over the past two decades, the incorporation of d 0 transition-metal ions (Ti4+, Nb5+, V5+, and Mo6+) into ternary metal iodates afforded abundant new quaternary iodates, many of which exhibit non-centrosymmetric structures and promising nonlinear optical properties (Hu and Mao, Reference Hu and Mao2015; Chen et al., Reference Chen, Hu, Kong and Mao2021). The quaternary metal iodates containing magnetic transition-metal ions (Mn2+, Fe3+, Co2+, Ni2+, and Cu2+) have also been explored, and their magnetic properties have been studied, such as (LiFe1/3)(IO3)2 (Lan et al., Reference Lan, Chen, Tao, Xie, Jiang, Xu and Xu2002a, Reference Lan, Chen, Xie, Jiang and Lin2002b). The Cu2+ ion possesses a partially filled d-orbital and usually occupies a distorted octahedral CuO6 coordination geometry; hence, the combination of copper cations with iodate groups is expected to form novel structures with various connection fashion of CuO6 units, and may further afford interesting magnetic properties. As far, only seven Cu-containing quaternary iodates, NaCu(IO3)3 (Sen Gupta et al., Reference Sen Gupta, Ghose and Schlemper1987), AgCu(IO3)3 (Wang et al., Reference Wang, Ruan, Xu and Mao2017), KCu(IO)3 (Mitoudi-Vagourdi et al., Reference Mitoudi-Vagourdi, Rienmüller, Lemmens, Gnezdilov, Kremer and Johnsson2019), and LnCu(IO3)5 (Ln = La, Ce, Pr, and Nd) (Geng et al., Reference Geng, Meng and Yan2022), have been reported. In this study, a new Cu-containing quaternary iodate, namely, Ba2Cu(IO3)6, has been synthesized. The crystal structure and powder X-ray diffraction data of Ba2Cu(IO3)6 are reported.
II. EXPERIMENTAL
A. Sample preparation
Crystal samples of Ba2Cu(IO3)6 were synthesized by hydrothermal reactions of a mixture of BaCO3, CuO, and I2O5 sealed in a 25 ml Teflon-lined stainless-steel autoclave. The loaded compositions were BaCO3 (19.7 mg, 0.1 mmol), CuO (4.0 mg, 0.05 mmol), I2O5 (2670.4 mg, 8.0 mmol), and H2O (2.0 ml). The mixture was heated at 220 °C for 70 h, followed by slow cooling to ambient temperature at a rate of 3.0 °C/h. Colorless Ba2Cu(IO3)6 crystals were obtained as a single phase. The as-grown Ba2Cu(IO3)6 crystals exhibit long granular shapes with sizes ranging from 0.1 to 0.5 mm (Supplementary Figure S1). Elemental analyses were carried out on a field emission scanning electron microscope (FEI, NovaNanoSEM450) equipped with an energy-dispersive X-ray spectroscope (EDS; Oxford, X-MaxN). The Ba:Cu:I:O molar ratio based on the EDS analyses was given to be 2.08:1.00:6.11:18.58 (Supplementary Figure S2). The Ba2Cu(IO3)6 crystals were ground into powder and screened through 50 μm mesh for the X-ray powder diffraction measurement.
B. Powder diffraction data collection
X-ray powder diffraction data were collected at room temperature on a Bruker D8 ADVANCE X-ray diffractometer with a LynxEye detector and graphite-filtered Cu Kα radiation (Kα 1: 1.54059 Å, Kα 2: 1.54439 Å, Kα 2/Kα 1 ratio: 0.5). The X-ray generator operated with voltage and electric current set at 40 kV and 40 mA. The measurement was performed over the 2θ range from 5° to 95° with a scanning step width of 0.02° and a counting time of 1.5 s per step. The profile fitting and refinement of the experimental X-ray powder diffraction pattern were performed using the software package GSAS-II (Toby and Von Dreele, Reference Toby and Von Dreele2013). The Rietveld method (Rietveld, Reference Rietveld2014) was adopted for the refinement. In addition, the software package MDI Jade 7.5 (MDI, 2002) was used to fit the background, strip off the Cu Kα 2 component, and perform the assignment of Miller indices (h, k, l) to the observed peaks in the experimental X-ray powder diffraction pattern. The values of 2θ obs, d obs, (I/I o)obs, h, k, l, 2θ cal, d cal, (I/I o)cal, and Δ2θ were obtained (Supplementary Table SI). The Cu Kα 1 wavelength (λ = 1.5405981 Å) was used in converting observed line positions to d-spacing.
C. Single-crystal diffraction data collection
X-ray single-crystal diffraction data were collected on a Rigaku SCXMini CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 293(2) K. Data reduction was performed with the software CrystalClear, and absorption correction based on multi-scan method (Blessing, Reference Blessing1995) was applied. The structure was solved by the direct method and refined by the full-matrix least-squares fitting on F 2 using the structure solution program package SHELX (Sheldrick, Reference Sheldrick2015).
III. RESULTS AND DISCUSSION
Through the Rietveld refinements based on the experimental X-ray powder diffraction patterns using the software package GSAS-II, the profile fitting has been performed, and the unit cell and atomic coordinates have been refined. Ba2Cu(IO3)6 is identified to crystallize in the triclinic space group P ![]() $\bar{1}$ with unit-cell parameters of a = 7.48540(15) Å, b = 7.51753(19) Å, c = 7.64259(17) Å, α = 98.8823(7)°, β = 95.0749(7)°, γ = 97.6297(7)°, V = 418.528(9) Å3, Z = 1, ρ = 5.5055 g/cm3. The calculated XRD pattern of the Rietveld refinement fits well with the observed pattern (Figure 1), and the final reliability factors wR and GOF are 0.0529 and 2.52, respectively. All observed diffraction peaks of the experimental pattern are well indexed, and no detectable impurities were observed. Using the software package MDI Jade 7.5, the peak positions and intensities have been obtained. The Cu Kα 1 radiation (λ = 1.5405981 Å) has been used for the d-values calculation. The values of 2θ obs, d obs, (I/I o)obs, h, k, l, 2θ cal, d cal, (I/I o)cal, and Δ2θ are listed in Supplementary Table SI. The figure of merit is F 30 = 16.1 (0.0026, 647) (Smith and Snyder, Reference Smith and Snyder1979).
$\bar{1}$ with unit-cell parameters of a = 7.48540(15) Å, b = 7.51753(19) Å, c = 7.64259(17) Å, α = 98.8823(7)°, β = 95.0749(7)°, γ = 97.6297(7)°, V = 418.528(9) Å3, Z = 1, ρ = 5.5055 g/cm3. The calculated XRD pattern of the Rietveld refinement fits well with the observed pattern (Figure 1), and the final reliability factors wR and GOF are 0.0529 and 2.52, respectively. All observed diffraction peaks of the experimental pattern are well indexed, and no detectable impurities were observed. Using the software package MDI Jade 7.5, the peak positions and intensities have been obtained. The Cu Kα 1 radiation (λ = 1.5405981 Å) has been used for the d-values calculation. The values of 2θ obs, d obs, (I/I o)obs, h, k, l, 2θ cal, d cal, (I/I o)cal, and Δ2θ are listed in Supplementary Table SI. The figure of merit is F 30 = 16.1 (0.0026, 647) (Smith and Snyder, Reference Smith and Snyder1979).

Figure 1. The Rietveld refinement plot of the powder XRD patterns for Ba2Cu(IO3)6: experimental data (blue crosses), calculated data (green line), calculated Bragg positions (red tick marks), and difference curves (cyan line). The experimental XRD pattern was measured using the graphite-filtered Cu Kα radiation (Kα 1: 1.54059 Å, Kα 2: 1.54439 Å, Kα 2/Kα 1 ratio: 0.5).
The crystal structure of Ba2Cu(IO3)6 has also been determined using the X-ray single-crystal diffraction data, which gives the unit cell as a = 7.493(3) Å, b = 7.521(6) Å, c = 7.644(5) Å, α = 98.855(18)°, β = 95.060(16)°, γ = 97.62(2)°, V = 419.3(5) Å3, Z = 1, ρ = 5.495 g/cm3, and space group P ![]() $\bar{1}$. The detailed crystallographic information is summarized in Table I. As shown in Figure 2, the crystal structure of Ba2Cu(IO3)6, features isolated [Cu(IO3)6]4− anionic clusters separated by Ba2+ cations. The asymmetric unit contains one Ba, one Cu, three I, and nine O atoms [Figure 2(a)]. I(1), I(2), and I(3) atoms are all three-coordinated in IO3 trigonal-pyramidal geometry. The Cu(1) atom is located in a CuO6 distorted octahedral geometry. Each CuO6 unit is corner-sharing with six IO3 groups [two I(1)O3, two I(2)O3, and two I(3)O3] all in a monodentate fashion, leading to the formation of [Cu(IO3)6]4− clusters [Figure 2(b)]. Such [Cu(IO3)6]4− anions are discrete from each other and further connected by the Ba2+ counter cations [Figure 2(c)].
$\bar{1}$. The detailed crystallographic information is summarized in Table I. As shown in Figure 2, the crystal structure of Ba2Cu(IO3)6, features isolated [Cu(IO3)6]4− anionic clusters separated by Ba2+ cations. The asymmetric unit contains one Ba, one Cu, three I, and nine O atoms [Figure 2(a)]. I(1), I(2), and I(3) atoms are all three-coordinated in IO3 trigonal-pyramidal geometry. The Cu(1) atom is located in a CuO6 distorted octahedral geometry. Each CuO6 unit is corner-sharing with six IO3 groups [two I(1)O3, two I(2)O3, and two I(3)O3] all in a monodentate fashion, leading to the formation of [Cu(IO3)6]4− clusters [Figure 2(b)]. Such [Cu(IO3)6]4− anions are discrete from each other and further connected by the Ba2+ counter cations [Figure 2(c)].
TABLE I. Single-crystal crystallographic data for Ba2Cu(IO3)6.


Figure 2. (a) ORTEP representations of the asymmetric unit shown in thermal ellipsoid with 30% probability (Symmetry codes for the generated equivalent atoms: (a) = 1–x, 1–y, 1–z); (b) view of the [Cu(IO3)6]4−; (c) view of the 3D structure along [001] direction.
The lattice parameters obtained by the Rietveld refinement of experimental X-ray powder diffraction data are very close to these determined by the X-ray single-crystal diffraction data, and the deviations of the lengths of unit-cell axis a, b, c, and unit-cell volume are as minor as 0.10, 0.05, 0.02, and 0.18%, respectively. In addition, the experimental powder diffraction pattern and the simulated pattern derived from single-crystal data show excellent matching (Supplementary Figure S3). These results confirm the accuracy of the reported crystal structure and the X-ray powder diffraction of Ba2Cu(IO3)6.
IV. DEPOSITED DATA
The Crystallographic Information Framework (CIF) file and powder X-ray diffraction data were deposited with the ICDD. You may request this data from ICDD at [email protected].
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715623000258.
FUNDING STATEMENT
This work was financially supported by the Natural Science Foundation of Fujian Province (grant no. 2020J01893) and Fujian University of Technology (grant no. GY-Z19131).
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.