An inverse association between n-3 long-chain PUFA in tissues and risk of CVD and some cancers is a consistent finding of observational studiesReference Simon, Hodgkins, Browner, Neuhaus, Bernert and Hulley1–Reference MacLean, Newberry and Mojica4. An important element in judging the causal nature of this association is to understand the factors that influence the n-3 long-chain PUFA composition of tissue lipids.
Increased dietary intake of n-3 long-chain PUFA – EPA and DHA – increases the proportion of these fatty acids in plasma, blood cells and adipose tissueReference Holub, Bakker and Skeaff5–Reference Katan, Deslypere, van Birgelen, Penders and Zegwaard8. Furthermore, in cross-sectional studies, the proportion of tissue fatty acids as n-3 long-chain PUFA is correlated with n-3 long-chain PUFA intakeReference Andersen, Solvoll and Drevon9, Reference Hjartaker, Lund and Bjerve10. A number of non-dietary determinants such as sex and age may also affect n-3 long-chain PUFA composition. However, the extent to which non-dietary determinants influence tissue n-3 long-chain PUFA is not well described. A consequence of not considering the effect of non-dietary determinants of n-3 long-chain PUFA concentrations is that erroneous conclusions about n-3 biomarker–disease risk associations may be reached. For example, if sex, in addition to being an independent predictor of CVD risk, is also a biological determinant of tissue n-3 long-chain PUFA composition it may be more appropriate to examine the n-3 biomarker–disease risk association separately in men and women to minimise bias.
Evidence of sex differences in n-3 fatty acid metabolism has been reported. Results from two studies with small sample sizes showed that women, when fed 13C-labelled α-linolenic acid, had a greater capacity than men to synthesise 13C-labelled DHAReference Burdge, Jones and Wootton11–Reference Pawlosky, Hibbeln, Lin, Goodson, Riggs, Sebring, Brown and Salem14. The extent to which this sex difference in n-3 fatty acid metabolism, assessed under controlled conditions, is reflected in non-controlled conditions at a population level where dietary intake of preformed DHA may be the predominant determinant of tissue DHA composition is unclear. In three population-based surveys women had a higher percentage of total fatty acids as DHA in plasmaReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15, Reference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16 and adiposeReference Tavendale, Lee, Smith and Tunstall-Pedoe17 lipids than men; however, in another survey no sex difference was reportedReference Dewailly, Blanchet, Gingras, Lemieux and Holub18.
Age-related increases in n-3 long-chain PUFA composition of tissues have also been reportedReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15–Reference Cambien, Warnet, Vernier, Ducimetiere, Jacqueson, Flament, Orssaud, Richard and Claude19; however, in three of these studies in which fish intake was reported, the age-related changes in plasma DHA paralleled increases in fish consumptionReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15, Reference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16, Reference Dewailly, Blanchet, Gingras, Lemieux and Holub18.
The objective of the present study was to determine in a population-based sample of people living in New Zealand if n-3 long-chain PUFA differed between men and women and with age.
Subjects and methods
The sample from the 1997 National Nutrition Survey (NNS97) was taken from the 1996/97 New Zealand Health Survey, a population-based survey that assessed the health status of non-institutionalised New Zealanders aged 15 years and older. More detailed descriptions of the methods for the NNS97 have been publishedReference Quigley and Watts20–Reference Parnell, Wilson and Russell22. In brief, the New Zealand Health Survey used an area-based sampling frame with a three-staged stratified design that consisted of a selection of a set of 18 800 geographical areas (the primary sampling units), households within these primary sampling units and then a randomly chosen respondent within a household. The response rate for the New Zealand Health Survey was 74 % (n 7862); of these participants, 4636 provided dietary information in the NNS97 and 3223 gave a blood sample. The fatty acid composition of at least one serum lipid fraction was available for 2793 participants; thus, the fatty acid analysis represents 36 % of the original New Zealand Health Survey. Fourteen ethics committees throughout New Zealand approved the survey and all participants or the legal guardian of those under 18 years provided written informed consent. The procedures followed were in accordance with the ethical standards of the New Zealand Health Research Council.
The data collection for the NNS97 was undertaken over a 12-month period from December 1996 to November 1997. During a home visit, dietary information was collected from each participant using a computer-assisted 24 h recall methodReference Russell, Parnell and Wilson21. Intake of meat and fish fats were calculated from the food group analysis used in the NNS97. Freshwater fish is not sold in New Zealand, therefore all fish consumed by New Zealanders was assumed to be of marine origin. Age and ethnicity were self-reported and ethnicity was categorised into three groups: New Zealand Maori, Pacific People, and New Zealand European and other. The term ‘Pacific’ includes those who identify as Samoan, Tongan, Cook Island Maori, Niuean, Tokelauan, Fijian and other Pacific ethnic groups. All remaining participants were assigned to the New Zealand European and other group. Height and weight were measured according to standard techniques and BMI (kg/mReference Albert, Campos, Stampfer, Ridker, Manson, Willett and Ma2) was calculated. Participants were asked three questions pertaining to their smoking and categorised as smokers or non-smokers, the latter including former smokers. At the end of the home visit, blood was drawn from the participants from an antecubital vein into vacuum evacuated tubes with no anticoagulant. Participants were not required to fast. The tubes were centrifuged and the serum aliquoted into cryovials for storage at − 80°C.
Laboratory analysis
Details of the fatty acid analysis have been published elsewhereReference Holub, Bakker and Skeaff5, Reference Crowe, Skeaff, Green and Gray23. In brief, lipids were extracted from 400 μl serum according to the method of Bligh & DyerReference Bligh and Dyer24. Serum phospholipid, cholesterol ester and TAG were isolated using TLC and fatty acids were analysed using a DB-225 narrowbore column (30 m × 0·25 mm internal diameter; film thickness 0·25 μm; J&W Scientific, Deerfield, IL, USA) on a HP-6890 gas chromatograph with flame ionisation detection (Agilent, Palo Alto, CA, USA). The fatty acid results are reported as percentage of total fatty acids, on a molar basis (i.e. mol%). Precision of the fatty acid measurement was determined by repeat analysis of a pooled serum sample, one pooled sample was analysed for every twenty NNS97 samples. The CV for EPA (20 : 5n-3), docosapentaenoic acid (22 : 5n-3) and DHA (22 : 6n-3) in serum phospholipid (n 102) were 14, 12 and 13 %, respectively. The docosapentaenoic acid composition of serum cholesterol ester and TAG was not reported because the amount of this fatty acid in many serum samples was below the detection limits of the analytical methods.
Statistical analysis
All statistical analyses were carried out on Stata Statistical Software, Release 9 (Statacorp LP, College Station, TX, USA) using the survey commands where appropriate to control for the survey design or using weighted regression and adjusting the standard errors to account for the clustering. For the unadjusted analysis, age was coded as a categorical variable (15–24 years, 25–44 years, 45–64 years, 65+ years) and the survey commands were used to calculate the unadjusted means for the n-3 long-chain PUFA composition of serum phospholipid, cholesterol ester and TAG by sex and by sex and age category. Survey regression was used to test for sex differences and linear trends across the age categories for the n-3 long-chain PUFA. The adjusted means for each of the n-3 long-chain PUFA by sex and by sex and age were estimated using multiple fractional polynomial regressionReference Royston, Ambler and Sauerbrei25 controlling for potential confounders. All variables were entered into the model simultaneously using fractional polynomials for the continuous variables (age, BMI and fish fat (g/d)) or as indicator variables for the categorical variables (sex, ethnicity and smoking). The adjusted means were calculated for 20, 35, 54 and 73 years; the mean age of each age category. Sex-by-age and sex-by-BMI interactions were also tested in the models where appropriate and included if P < 0·05. The effects of sex and age on n-3 long-chain PUFA composition were considered statistically significant if P < 0·05. Similar analyses were conducted for the mean fish and meat fat intake by sex and by sex and age. All tests of significance were two-sided.
Results
The fatty acid composition of serum phospholipid, cholesterol ester and TAG were available for 2416, 2393 and 2402 participants, respectively, of the 3223 stored serum samples from the NNS97. The fatty acid composition of at least one serum lipid fraction was analysed in 2793 participants. The distribution of sex, ethnicity and smoking as well as the mean age, BMI, and intake of fish and meat fats were similar between participants for whom a fatty acid measurement was made (n 2793), and participants in the 1996/97 New Zealand Health Survey (n 7862) and NNS97 (n 4636) (Table 1).
Table 1 Characteristics of participants
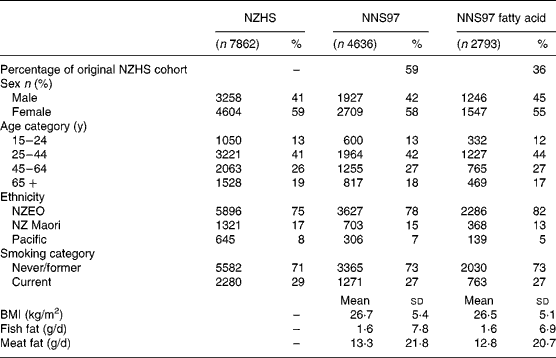
NNS97, 1997 National Nutrition Survey; NZEO, New Zealand European and others; NZHS, 1996/97 New Zealand National Health Survey.
The mean percentage of total fatty acids in serum phospholipid as EPA was 0·07 mol% (95 % CI 0·02, 0·12) lower in women compared with men; in cholesterol ester, EPA was 0·09 mol% (95 % CI 0·04, 0·14) lower in women (Table 2). Women also had a lower proportion of docosapentaenoic acid in serum phospholipid than men, by 0·10 mol% (95 % CI 0·07, 0·13). In contrast, the proportion of DHA in serum phospholipid and cholesterol ester was higher in women than in men, by 0·16 mol% (95 % CI 0·07, 0·25) and 0·02 mol% (95 % CI 0·01, 0·04), respectively. The sex differences in fatty acid composition were increased slightly after adjustment of the regression model for age, BMI, ethnicity and smoking. There were no significant differences in the fatty acid composition of serum TAG between men and women.
Table 2 n-3 Long-chain PUFA composition of serum lipids by sex and age
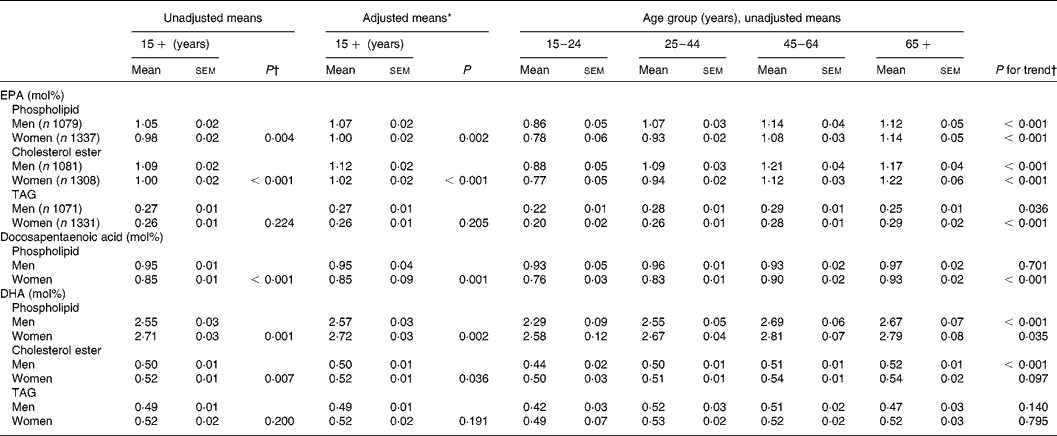
* Adjusted for age, BMI, ethnicity and smoking using multiple fractional polynomial regression.
† Calculated using survey regression.
The unadjusted means for the n-3 long-chain PUFA composition of serum phospholipid, cholesterol ester and TAG by sex and age are shown in Table 2. The proportion of EPA in serum phospholipid, cholesterol ester and TAG increased significantly across the age categories for both men and women (P < 0·05). The proportion of DHA in phospholipid for men and women and in cholesterol ester for men only increased across the age categories (P < 0·05), as did the proportion of docosapentaenoic acid in phospholipid for women only (P < 0·001).
The multivariate-adjusted means, after controlling for BMI, ethnicity and smoking, for n-3 long-chain PUFA in serum phospholipid by sex and age are shown in Fig. 1. Including fish fat intake (g/d) in the regression model had little influence on the adjusted means; therefore, it was omitted from the model. The age-related increase in the proportion of total fatty acids as EPA (sex by age interaction, P = 0·186) and DHA (sex by age interaction, P = 0·188) in serum phospholipid was similar in men and women. The proportion of EPA in serum phospholipid increased by 0·31 mol% between the ages of 20 and 73 years. The increase in DHA between 20 and 73 years was 0·28 mol%. At all ages, the proportion of EPA was lower and DHA higher in women compared with men. The docosapentaenoic acid composition of serum phospholipid increased with age in women (P < 0·001) but did not change with age in men (P = 0·591), the interaction of sex by age was significant (P = 0·002). The difference in docosapentaenoic acid composition of serum phospholipid between 20 and 73-year-old women was 0·18 mol%. Similar age-related increases for EPA and DHA in serum cholesterol ester and TAG were also found (results not shown).
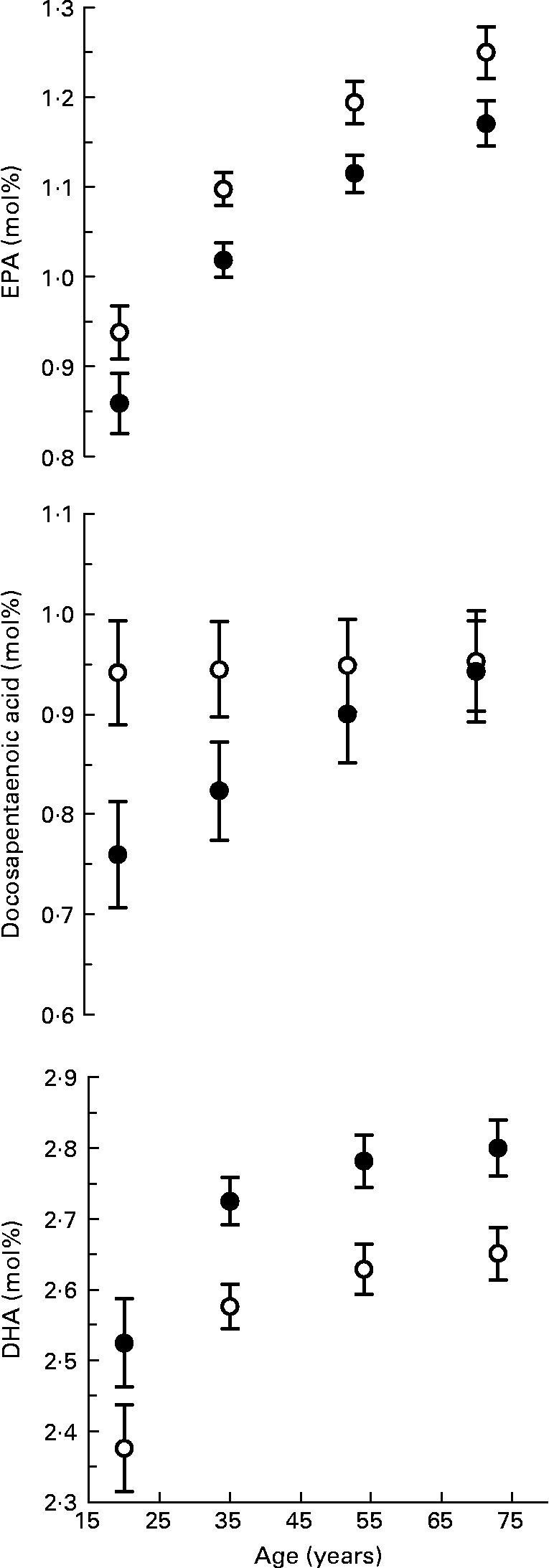
Fig. 1 n-3 Long-chain PUFA composition of serum phospholipid by age for men (○) and women (●). Values are means with their standard errors depicted by vertical bars. There was a positive association between age and EPA (P < 0·001) and DHA (P < 0·001) but not docosapentaenoic acid (P = 0·795) after adjusting for BMI, ethnicity and smoking using multiple fractional polynomial regression. Interactions between sex and age were significant for docosapentaenoic acid (P = 0·002) but not EPA (P = 0·186) or DHA (P = 0·188).
The mean intake of fish fat and meat fat for men and women who had a fatty acid value for at least one serum lipid fraction are presented in Table 3. There was no significant difference in the intake of fish fat between men and women, before (P = 0·106) or after (P = 0·106) adjusting for age, BMI, ethnicity and smoking. The age-related increase in fish fat intake was similar in men and women. The difference in fish fat intake between ages 20 and 73 years was 1·1 g/d for men and women. Intake of meat fat (unadjusted) was 8·2 (95 % CI 6·2, 10·1) g/d higher in men than women (P < 0·001). Age was a significant predictor of meat fat intake (P = 0·029). Mean intake of meat fat was 1·0 g/d higher in participants aged 20 years compared with 73 years.
Table 3 Dietary fat intake by sex and age
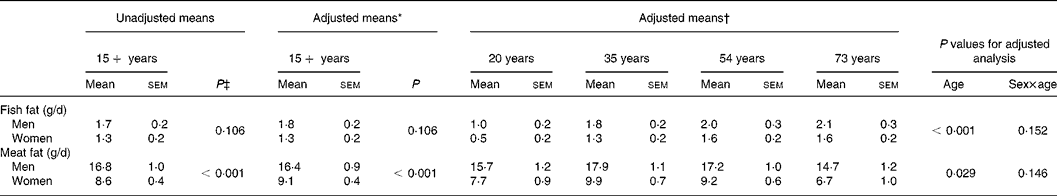
* Adjusted for age, BMI, ethnicity and smoking using multiple fractional polynomial regression.
† Adjusted for BMI, ethnicity and smoking using multiple fractional polynomial regression.
‡ Calculated using survey regression.
Discussion
We have shown that men and women differ in the relative proportions of n-3 long-chain PUFA in serum lipids in a population-based survey of New Zealanders. The most striking result was that women had lower proportions of EPA and docosapentaenoic acid than men, whereas, for DHA, the sex difference was reversed, and women had higher levels than men. This differential pattern of n-3 long-chain PUFA in serum lipids between men and women suggests sex differences in fatty acid metabolism. One would expect that a higher consumption of foods supplying n-3 long-chain PUFA in one sex or the other would be associated with higher proportions of all three n-3 long-chain PUFA not just one or two fatty acids as we found. Intake of individual n-3 long-chain PUFA was not estimated in the NNS97 because the New Zealand food composition database does not have this information. However, fish is the richest source of n-3 long-chain PUFA and the results of the 24 h diet recall showed no differences in fish fat intake between men and women. Meat fat is also a source of n-3 long-chain PUFAReference Quigley, Burlingame, Milligan and Gibson26, Reference Howe, Meyer, Record and Baghurst27, and men consumed more meat fat than women. Thus, intake of all three n-3 long-chain PUFA was probably slightly higher in men than women. The higher intake of n-3 PUFA in men is consistent with a higher proportion of EPA and docosapentaenoic acid in serum phospholipid of men but is not consistent with their lower proportion of DHA if diet was the only determinant of fatty acid status.
Sex differences in n-3 long-chain PUFA composition of tissues have been reported previously in mostReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15–Reference Tavendale, Lee, Smith and Tunstall-Pedoe17 populations. Higher proportions of DHA in women compared with men have been reported in all but oneReference Dewailly, Blanchet, Gingras, Lemieux and Holub18 of four population surveysReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15–Reference Tavendale, Lee, Smith and Tunstall-Pedoe17. Similar differences have been reported in the Norfolk cohort of the European Prospective Investigation into Cancer and NutritionReference Welch, Bingham, Ive, Friesen, Wareham, Riboli and Khaw28 and in another small survey of men and womenReference Bakewell, Burdge and Calder29, although participant recruitment in both these studies was by convenience sampling rather than population-based. The differences in DHA were not explained by diet because fish consumption in women was not higher than in menReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15, Reference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16. Interestingly, in a study of people living in southern Quebec, women consumed less fish than men yet had a higher proportion of DHA in plasma phospholipidsReference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16. With the exception of oneReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15, the proportion of EPA in plasma phospholipids of women was not different from menReference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16, Reference Dewailly, Blanchet, Gingras, Lemieux and Holub18. The present results differ in this regard, because we report a lower proportion of EPA in serum phospholipid of women than men, although the magnitude of difference we report is small.
The higher DHA content of serum phospholipid in women compared with men is consistent with evidence from stable-isotope feeding studies that show women have a higher capacity than men to synthesise DHA from α-linolenic acidReference Burdge, Jones and Wootton11–Reference Pawlosky, Hibbeln, Lin, Goodson, Riggs, Sebring, Brown and Salem14. Other mechanisms may also be in play. For example, it was demonstrated in a stable-isotope feeding trial that DHA can be retroconverted to docosapentaenoic acid and EPAReference Brossard, Croset, Pachiaudi, Riou, Tayot and Lagarde30. It is possible that women have a lower capacity than men to retroconvert DHA, which would explain the sex differences we report. There is evidence to suggest that amongst women of postmenopausal age, hormone replacement therapy reduces the retroconversion of DHA to EPAReference Stark and Holub31. Information regarding menopausal status or use of hormone replacement therapy was not collected from the participants in the NNS97. However, we found no evidence that the difference in DHA between men and women varied with age. This suggests that at a population level, neither menopausal status nor use of hormone replacement therapy has a major effect on serum DHA.
We found that EPA and DHA in serum phospholipid increased with age. Similar changes have been reported in other populationsReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15–Reference Cambien, Warnet, Vernier, Ducimetiere, Jacqueson, Flament, Orssaud, Richard and Claude19, and results from an intervention study suggest that older men have a greater capacity to incorporate dietary EPA into plasma phospholipid than younger menReference Rees, Miles, Banerjee, Wells, Roynette, Wahle and Calder32. We extend these previous reports by showing the age-related increases did not differ between men and women. At all ages, women had lower EPA and higher DHA compared with men. Most evidence, to date, suggests the age-related changes in n-3 long-chain PUFA reflect changes in fish consumptionReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15, Reference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16, Reference Dewailly, Blanchet, Gingras, Lemieux and Holub18. For example, results from a series of surveys in three ethnic groups in Quebec – southern QuebecersReference Dewailly, Blanchet, Gingras, Lemieux, Sauve, Bergeron and Holub16, Inuit of NunavikReference Dewailly, Blanchet, Lemieux, Sauve, Gingras, Ayotte and Holub15 and the Cree of James BayReference Dewailly, Blanchet, Gingras, Lemieux and Holub18 – show the age-related increases in n-3 long-chain PUFA paralleled increasing fish consumption. However, in the two largest surveys, the Scottish Heart Study (n 4114)Reference Tavendale, Lee, Smith and Tunstall-Pedoe17 and the Paris Prospective Study (n 3348)Reference Cambien, Warnet, Vernier, Ducimetiere, Jacqueson, Flament, Orssaud, Richard and Claude19, diet was not reported.
In the present study population, intake of fish fat increased with age. The difference in fish fat intake between 20- and 73-year-olds was 1·1 g/d, equivalent to a DHA intake of 110 mg/d – assuming each gram of fish fat contains approximately 100 mg DHAReference Quigley, Burlingame, Milligan and Gibson26. Based on the results of dietary intervention trials it appears this amount of DHA may account for the age-related changes in serum phospholipid DHA in the present population. Yep et al. (2002)Reference Yep, Li, Mann, Bode and Sinclair33 found that 54 mg DHA/d increased the percentage of this fatty acid in plasma phospholipid by 0·34 %. This increase is larger than the 0·28 mol% increase we found between participants aged 20 and 73 years. The present results suggest the increase in EPA and DHA with age are related to changes in diet, rather than metabolism, because they paralleled increased fish fat intake.
The increase with age in the proportion of docosapentaenoic acid in serum phospholipid in women follows that of the other n-3 long-chain PUFA as well as the intake of fish fat. However, in men docosapentaenoic acid did not increase with age despite the increase in fish fat intake. This suggests that in men, serum phospholipid docosapentaenoic acid may be influenced more by metabolic rather than dietary factors.
The sex and age differences in the n-3 long-chain PUFA composition of serum cholesterol ester were similar to phospholipid, however, the sex differences were not significant for TAG. Thus, the metabolic processes that may influence these sex differences appear to be specific to phospholipid and cholesterol ester but not TAG. Alternatively, the absence of sex-related changes in TAG may be the result of collecting blood samples from participants who were not fasting. At a population level, the fat composition of recent meals will reflect habitual intake, although for the individual there may be a sizeable difference; therefore, any effect of postprandial blood sampling on serum TAG n-3 long-chain PUFA would be on the variability of the estimate and not on the mean fatty acid composition. This increased variability may have reduced the power to detect a statistically significant sex difference in the n-3 long-chain PUFA composition of serum TAG.
The metabolic and physiological effects of EPA and DHA differ. Both fatty acids are important structural components of cell membranes, however, EPA has more pronounced effects on eicosanoid productionReference Calder34 whereas DHA has particular effects on membrane properties and cell signallingReference Salem, Litman, Kim and Gawrisch35. Thus, it is not surprising that the relation between each of the n-3 long-chain PUFA and risk of CVD and some cancers is differentReference Simon, Hodgkins, Browner, Neuhaus, Bernert and Hulley1, Reference Kojima, Wakai and Tokudome36. The present results suggest that there are sex differences in the regulation of n-3 long-chain serum PUFA and that men and women may need to be considered separately when examining the association between disease risk and biomarkers of n-3 long-chain PUFA.
Acknowledgements
This work was supported by a research grant from the National Heart Foundation of New Zealand and a University of Otago Research Committee, by means of a University of Otago Postgraduate Publishing Award (PhD). The New Zealand Ministry of Health funded the 1997 National Nutrition Survey. The Life in New Zealand group was responsible for conducting the 1997 National Nutrition Survey. Christian Thoma, Leanne Hodson, Belinda Hunter and Jody Miller helped with the laboratory analysis. Murray Skeaff conceived the study and obtained the funding for the fatty acid work. Francesca Crowe took overall responsibility for producing the fatty acid dataset. Andrew Gray was the consultant for the statistical analysis. All authors were involved in analysing the dataset, reviewing and interpreting the results and writing the manuscript.






