Dear Editor,
Paraneoplastic sensory neuronopathy (PSN) is a classical paraneoplastic neurological syndrome which is frequently associated with anti-Hu antibody (also called ANNA-1), and the most common underlying malignancy is small-cell lung cancer (SCLC). Reference Dalmau, Graus and Rosenblum1 Gastrointestinal and gynecological malignancies, Hodgkin lymphoma, and neuroendocrine tumors have also been associated with anti-Hu PSN. Reference Kuntzer, Antoine and Steck2 A published report describes Lambert-Eaton myasthenic syndrome and PSN in association with pulmonary large-cell neuroendocrine carcinoma (LCNEC). Reference Di Fabio, Benedetti and Michailidis3 Pulmonary LCNECs are rare tumors with an incidence of 2.1–3.5% in a series of surgically resected cases. Reference Fasano, Della Corte and Papaccio4 They have a poor prognosis even in patients with stage I cancer (5-year survival rates 27–67%), and the recurrence rate is high even after surgery. Reference Fasano, Della Corte and Papaccio4 We describe a case of anti-Hu PSN in a patient with pulmonary LCNEC who is in remission more than 2 years following cancer treatment. The detection of anti-Hu antibodies prior to the cancer led to the early diagnosis and treatment of this aggressive malignancy in our case and likely improved patient’s prognosis. To our knowledge, this is among the few reported cases of association between pulmonary LCNEC and PSN.
A 67-year-old right-handed woman noticed symptoms of numbness, paresthesias, and pain in both hands (right > left) in October 2018 which involved her legs (left > right) 2 weeks later and eventually progressed down to her feet and above to her mid-thighs. She also noticed numbness behind her neck and ear. She developed incoordination and difficulty with balance over the next few months requiring assistance with ambulation and activities-of-daily-living. She was a 25-pack-year-smoker and had lost 25 pounds over a 3-month-period preceding evaluation at our center. Patient did not report sicca syndrome, ptosis, diplopia, dysphagia, or dysarthria. Neurological examination at our center in 2019, 7 months after symptom onset demonstrated an ataxic gait, positive Romberg sign, and right hand pseudo-athetosis indicative of sensory ataxia. Deep tendon reflexes were abolished except both biceps and left brachioradialis. Light touch, pinprick, and temperature sensations were impaired below the knees in a stocking distribution. Vibration was absent in both toes and proprioception was impaired in both toes, left ankle, and right wrist.
Neurophysiological testing demonstrated absent bilateral median and ulnar and right radial sensory responses, diminished left radial sensory response, and preserved right sural sensory response. Motor responses were preserved except for low amplitude peroneal motor study recording extensor digitorum brevis. Electromyography was unremarkable. Electrophysiological findings were consistent with a non-length-dependent asymmetric sensory polyneuropathy or ganglionopathy.
Serum testing was positive for anti-Hu antibody (titer 1:1920; reference <1:240). Cerebrospinal fluid analysis was unremarkable. Brain and spinal cord imaging were unremarkable. Computed tomography scan of the chest, abdomen, and pelvis showed mild right hilar lymphadenopathy but there were no lung masses. Positron-emission computed tomography (PET-CT) scan was done due to high suspicion for malignancy, and it demonstrated mild metabolic activity within mildly enlarged mediastinal and right hilar lymph nodes. The patient underwent ultrasound-guided transbronchial needle aspiration of the enlarged hilar lymph nodes. Cytology revealed the presence of malignant cells that were positive for chromogranin-A and synaptophysin immunostains indicative of a LCNEC (Figure 1). She was evaluated by oncology in 2019 and underwent radiation therapy and chemotherapy (cisplatin and etoposide), which induced remission to date. She also received 12 cycles of intravenous immunoglobulin and steroids for her sensory ganglionopathy with minimal improvement but her symptoms stabilized in late-2019 after cancer treatment. Repeat neurophysiological testing post-platinum based-chemotherapy showed diminished right ulnar and radial sensory responses which were absent on the first study suggestive of interval improvement. She continued to be cancer-free on her last clinic follow-up in mid-2021.
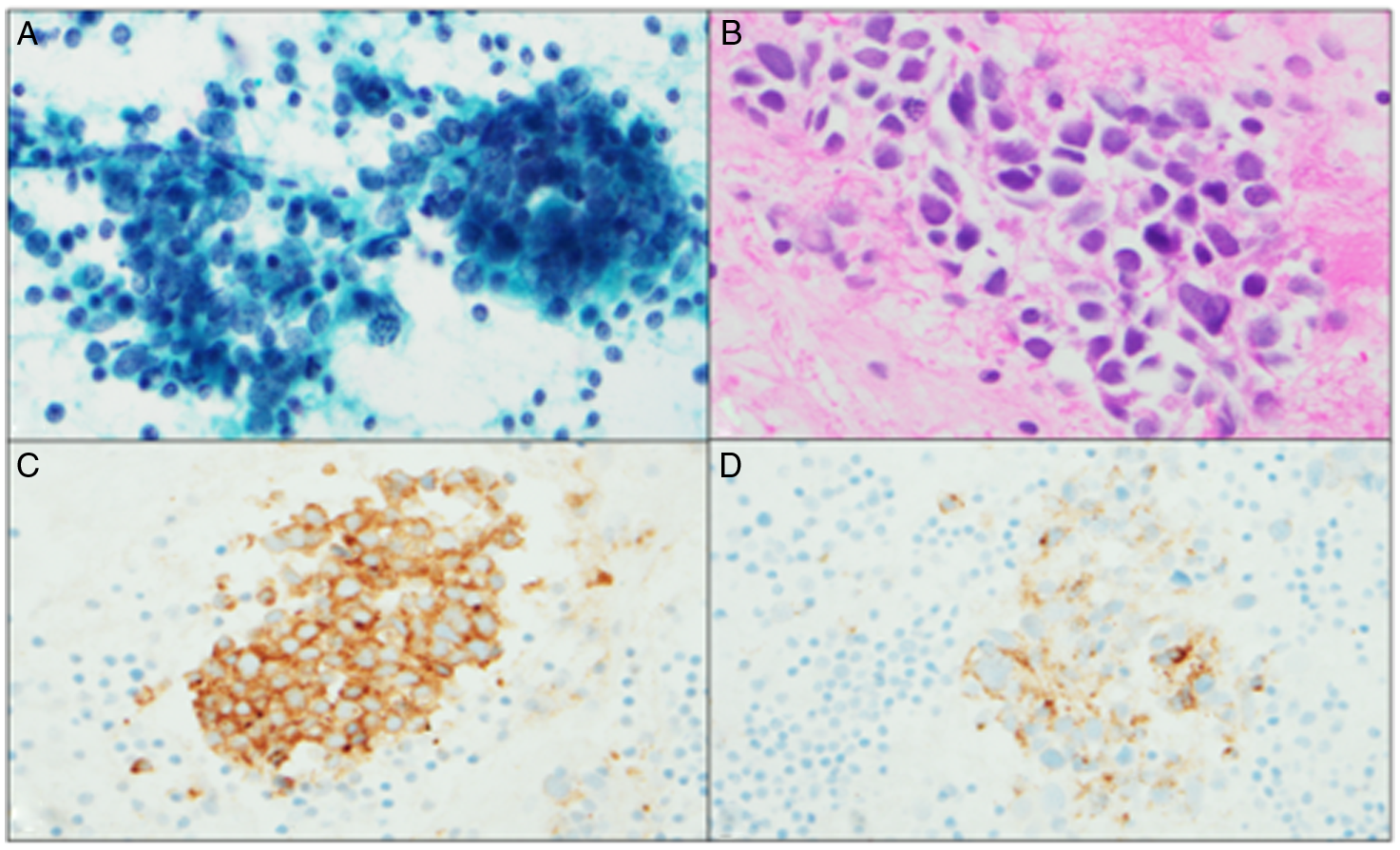
Figure 1: (A & B) Papanicolaou smear and H & E cell block. Groups of malignant cells with high nuclear-cytoplasmic ratio, irregular nuclear membranes, and stippled chromatin. (C) Synaptophysin immunostain (D) Chromogranin-A immunostain.
We describe an uncommon case of PSN in association with pulmonary LCNEC. To our knowledge, only one other report has previously described this association. Reference Di Fabio, Benedetti and Michailidis3 Our patient had typical subacute sensory neuronopathy with non-length-dependent asymmetric and multifocal sensory symptoms, sensory ataxia, and pseudo-athetosis. Electrodiagnostic studies in our case showed reduced or absent upper limb sensory responses with preserved lower limb sural sensory responses favoring a non-length-dependent sensory neuropathy. SN can be due to paraneoplastic phenomenon, HIV infection, Sjögren’s syndrome, chemotherapy (platinum compounds), pyridoxine toxicity, and idiopathic. There was high suspicion for a paraneoplastic etiology since she was an active smoker and had unintentional weight loss over a short period.
Anti-Hu PSN is an autoimmune process where anti-Hu antibodies are directed against the HuD protein which is expressed in the dorsal root ganglion and is also expressed in SCLC tumor cells. The HuD protein is thought to be the initiator of the autoimmune response. Reference Kuntzer, Antoine and Steck2 The anti-Hu antibodies have 99% specificity for the diagnosis of cancer and hence, their presence should raise high suspicion for malignancy. Reference Molinuevo, Graus and Serrano5 If the clinical suspicion for malignancy persists despite negative conventional imaging, a PET-CT should be considered as the tumor can be restricted to the mediastinal lymph nodes, as in our case. Similar findings have been described in other studies. Reference Antoine, Cinotti and Tilikete6
Immunotherapy can sometimes stabilize the PSN but does not improve the sensory symptoms as the pathology lies in the sensory ganglia. Reference Keime-Guibert, Graus and Fleury7 Early cancer diagnosis and treatment has the best chance to stabilize and improve neurological outcomes. Reference Sillevis Smitt, Grefkens and de Leeuw8 Classically, onconeuronal paraneoplastic neurological syndromes respond poorly to immunotherapy. Tumoricidal therapies can halt neurological disease progression but patients are often left with significant irreversible deficits. In contrast, cell surface or synaptic auto-antibody syndromes can have excellent prognosis with malignancy and are less commonly associated with malignancy. Reference Iorio and Lennon9 In our case, tumor treatment stabilized patient’s disease but her gait and ataxia did not improve with immunotherapy, although repeat electrophysiological testing did show improvement in sensory responses compared to the initial study.
There are some differences between our case and the other case reported in the literature. Reference Di Fabio, Benedetti and Michailidis3 In the other case, the patient clinically presented with anti-Hu syndrome with sensory neuronopathy on nerve conduction study, but the serum anti-Hu antibody was negative. Instead, serum P/Q-type-voltage-gated calcium channel antibodies were positive. Our patient’s clinical presentation was suggestive of sensory neuronopathy and her serum anti-Hu antibody titer was high. The other patient had a high-grade tumor that recurred after tumor resection. Our patient’s tumor was diagnosed at a very early stage while it was restricted to the lymph nodes and she has continued to stay in remission with timely treatment. This is anecdotal evidence, but possibly indicates that a better prognosis can be achieved even in cases of highly aggressive tumors, when a paraneoplastic syndrome is recognized and leads to early cancer diagnosis.
Acknowledgements
The authors thank the patient described in this report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
None.



