INTRODUCTION
Chronic hepatitis B (CHB) is a global infectious disease which endangers human health, especially in China. Today, an estimated 130 million persons carry the hepatitis B virus (HBV), with most infected via mother–child transmission. CHB caused by vertical transmission is liable to induce liver cirrhosis and hepatocellular carcinoma [Reference Li1, Reference Yue2], and there is a marked lack of effective therapeutic methods to cure those diseases. As a result, blockage of vertical transmission of HBV is important to reduce HBV transmission. Vertical transmission routes include intrauterine, intrapartum and postnatal infections. In the latter two routes, immunization success can be acquired by injecting hepatitis B (HB) vaccine and immunoglobulin immediately after birth. However, 5–10% of infants fail to immunize [Reference Li3–Reference Xia5], with intrauterine infection being the major cause. The definite mechanism of intrauterine infection of HBV is unclear, and possible transmission routes include haematogenous infection (via the placenta), cellular infection (via peripheral blood mononuclear cells) [Reference Gatta6], and germ line or genetic transmission (via ovum, spermatozoa and fertilized ovum). Another hypothesis is that the ovum is infected with HBV and fertilized with normal spermatozoa, leading to the filial generation being HBV carriers. However, this is a disputed mode of transmission with unclear mechanism.
Transmission to the fetus via germ cells has become a popular research topic worldwide, since Blumberg first raised the hypothesis of HBV DNA integrating into germ cells and infecting the filial generation [Reference Blumberg7]. Many authors have investigated HBV infection of spermatozoa and demonstrated the presence and integration of HBV DNA in spermatozoa of HB patients [Reference Hadchouel8, Reference Davison9]. Animal studies have demonstrated the possibility of spermatozoa and ova as being capable of carrying HBV into embryos [Reference Lang10, Reference Ali, Huang and Xie11]. Due to difficulty in obtaining ova as research material, few studies on ova infected by HBV have been performed. Therefore, the hypothesis of vertical transmission of HBV via the ovum has not been confirmed. Recently, Feng and colleagues [Reference Ye12–Reference Chen, Fan and Gao14] detected HBV in ovaries of women with CHB and found the presence of HBsAg, HBcAg, and HBV DNA in ovaries and ova. However, Lou et al. only found one HBsAg-positive ovary specimen among 68 HBsAg-positive women [Reference Lou15], and all markers of HB were negative in her neonate, which prompted the hypothesis that the transmission of HBV via ova was not the major route of intrauterine infection.
HBV has significant hepatotropic properties, and the ovum is not a specifically targeted cell. Current studies have demonstrated the presence of HBV DNA in the ovary, and this only illustrates the capability of HBV to infect the ovary beyond simply its ability to replicate and circulate within the body. In the cyclogeny of HBV, covalently closed circular DNA (cccDNA) is the primary copying template, which is of great significance for replication of HBV and the establishment of infection [Reference Funk16–Reference Ganem and Prince19]. Methods for the detection of HBV cccDNA are currently in development [Reference Addison20–Reference Takkenberg25]. Sung et al. reported the results of a study which was conducted to detect intrahepatic cccDNA in 47 CHB patients who were treated with lamivudine and indicated that intrahepatic HBV cccDNA was more precise than serum DNA as a predictor of sustained response to therapy [Reference Sung26]. Consequently, cccDNA is a more sensitive and direct marker than HBV DNA for reflecting the status of virus-active replication [Reference Feng27]. No studies for detecting HBV cccDNA in the ovary have been reported so far.
Current studies merely demonstrate the presence of HBsAg, HBcAg, and HBV DNA in the ovary, and transmission to the filial generation has not been confirmed due to lack of mother and infant controls. The objective of our study is to illustrate that HBV can not only infect the ovary but also maintain and replicate in the ovary by detecting both HBV DNA and HBV cccDNA in ovaries from HBV DNA-positive pregnant women. Moreover, the expression of HBV DNA and HBV cccDNA in the ovaries was compared with intrauterine infection in infants to further explore the possibility of HBV transmission to the filial generation via the ovum.
SUBJECTS AND METHODS
Subjects
A total of 33 pairs of pregnant women and their infants were enrolled. These women, aged between 21 and 39 years, underwent caesarean section from 1 January 2008 to 1 January 2010. Before surgery, they had detectable HBsAg and HBeAg as well as HBV DNA that was >1·0×106 copies/ml. In the husbands of these women, HBsAg and HBeAg were both negative, and HBV DNA was <5·0×102 copies/ml. Under preoperative type B ultrasonography or operation research, ovarian cystic, solid or cystic-solid masses were seen and considered as benign or malignant ovarian tumours requiring removal. Patients and their family members provided written informed consent before and during the operation. The study was approved by the Ethics Committee of our hospital.
Methods
The ovarian tissue was divided into two groups: one group was fixed with formaldehyde immediately after isolation for routine pathological examination, and the other was preserved at −70°C. Fluorescent quantitative PCR kits for detection of HBV DNA and HBV cccDNA were purchased from Beijing Suoao Biotechnology Co. Ltd (China) and PCR kits for detection of serum HBV DNA from Shanghai Kehua Bio Engineering Co. Ltd (China). The sensitivities of kits were all 5·0×102–1·0×109 copies/ml. HBV markers (HBVM) were assayed with chemiluminescence, and agents were provided by Abbott Laboratories (USA) and were used according to the manufacturer's instructions. The experimental procedures were operated by one specified person.
Extraction of DNA from ovarian tissue
DNA was extracted from frozen ovarian tissue. The blood on the surface was washed with one cycle of normal saline and then repeated cycles of PBS solution until the washing solution was negative for HBV DNA. Fifty milligrams of sample was collected from each ovary and placed in a 1·5 ml centrifuge tube. Fifty microlitres of DNA extraction solution B was added to the tube, mixed thoroughly (extraction solution was dissolved at room temperature and mixed thoroughly prior to use), and ground thoroughly, followed by 10 min clearance. It was then centrifuged at 18 760 g for 3 min.
Detection of HBV DNA and HBV cccDNA in ovarian tissue
Using PCR kits, we followed the manufacturer's instructions for detection of HBV DNA and HBV cccDNA throughout the study, and the primer sequence of HBV cccDNA detection was as follows. Forward: 5′-CGACCGACCTTGAGGCATAC-3′; reverse: 5′-AGAGTAACTCCACAGAT/AGCTCC-3′; fluorescent probe: FAM 5′-CACCTCTGCCTAATCATCTCTTGTTC-3′ TAMRA. HBV PCR and HBV cPCR mix Taq enzyme systems were thawed and mixed thoroughly at 11 100 g for 10 s. PCR and cPCR mixtures were prepared. Four microlitres of the resulting samples, HBV-positive quantitative quality controls or negative controls, were added to a reaction tube containing the mixtures which were centrifuged at 11 100 g for 30 s, after which the assay was performed. Concurrently, PBS solution was used as the blank control, five ovarian samples of pregnant women with negative HBsAg as negative controls, and five living hepatic tissue samples of non-pregnant women with positive HBsAg as positive controls.
Four millilitres of peripheral venous blood was obtained from each neonate prior to vaccination and was used to detect HBVM and HBV DNA. Subsequently, 20 μg of recombinant yeast genetically engineered HB vaccine (North China Pharmaceutical Group Corporation, China) and 200 IU of HB immunoglobulin (HBIG; Sichuan Chengdu Shuyang Pharmaceutical Co. Ltd, China) were injected immediately as well as at day 15 for HBIG and at months 1 and 6 for HB vaccines. HBVM and HBV DNA were retested at 1, 7 and 12 months.
HBV intrauterine infection was diagnosed as positive when neonates' serum was positive for HBsAg and/or HBV DNA from birth to at least 1 month.
Statistical analysis
SPSS v. 13.0 software (SPSS Inc., USA) was used for statistical analysis. Quantitative data were compared with a t test and categorical data with a χ2 test and Fisher's exact probability method. Variable correlation was analysed with Spearman's rank correlation analysis. P<0·05 was considered significant.
RESULTS
Intrauterine infection in infants
Of 33 infants, six were infected at birth, four of whom sustained an infection at 1, 7 and 12 months. The infection rate was 12·12% (4/33). Four cases had detectable HBsAg and HBeAg as well as HBV DNA >5·0×102 copies/ml at 12 months.
HBV DNA and HBV cccDNA expression in ovarian tissue and correlation with HBV intrauterine infection of infants
A total of 33 cases of diseased ovarian tissue meeting the criteria were obtained. HBV DNA and HBV cccDNA were present in 12 and 14 ovarian samples, respectively (Fig. 1). The expression models included the following. Model A: negative for both HBV DNA and HBV cccDNA; model B: positive for HBV DNA and negative for HBV cccDNA; model C: negative for HBV DNA and positive for HBV cccDNA; model D: positive for both HBV DNA and HBV cccDNA. The correlation between expression models and intrauterine infection of infants is given in Table 1. The overall positive rate of HBV DNA and/or HBV cccDNA was 51·52% (17/33) in ovarian tissue. Intrauterine infection rate of the expression models was A<B<C<D and was positively correlated with the models (Spearman's r=0·431, P=0·012). However, significant differences were only noted between models D and A (χ2=6·061, P=0·014). Both HBV DNA and HBV cccDNA were not detectable in all five samples of ovarian tissue from HBsAg-negative pregnant women and were positive in five living hepatic tissue samples of HBsAg-positive non-pregnant women with.
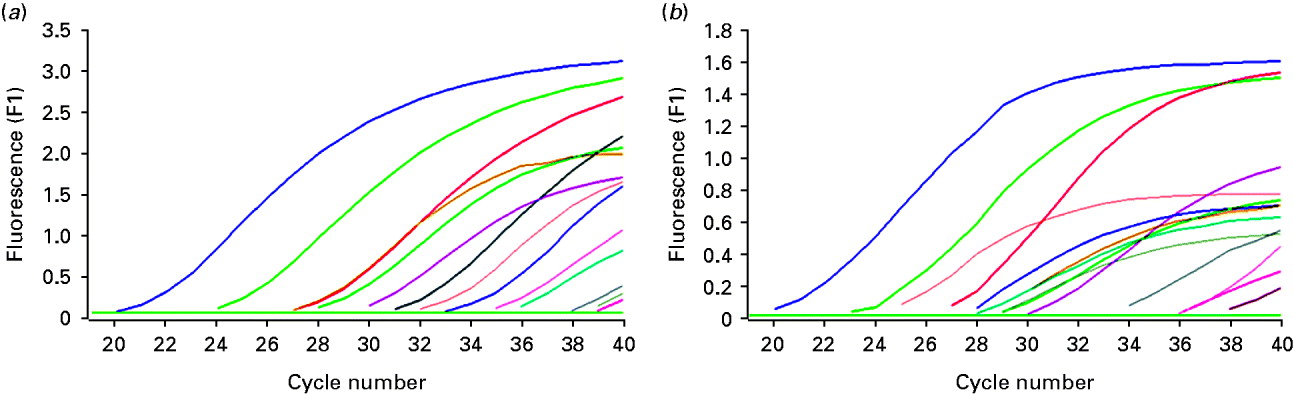
Fig. 1. Detection of HBV DNA and HBV cccDNA in partial ovarian tissue samples by real-time fluorescence quantative PCR. (a) Amplification of HBV DNA; (b) amplification of HBV cccDNA.
Table 1. Comparison of intrauterine infection rate in infants among different expression models of HBV DNA and HBV cccDNA in the ovaries of mothers

Correlation between different expression models and intrauterine infection: Spearman's r=0·431, P=0·012.
χ2 (A, C)=3·36, P=0·067; χ2 (A, D)=6·061, P=0·014; χ2 (B, C)=0·686, P=0·408; χ2 (B, D)=1·333, P=0·248; χ2 (C, D)=0·280, P=0·597.
Expression of HBV DNA and HBV cccDNA in ovarian tissue of mothers with infants with and without intrauterine infection
Expression levels and positive rates of HBV DNA and HBV cccDNA were higher in mothers whose infants were infected in utero than in those whose infants were not infected in utero. (For comparison of positive rates see Table 2.) Differences in HBV DNA were not statistically significant (P>0·05), but levels of HBV cccDNA were significantly different (P<0·01 for expression, P<0·05 for positive rate).
Table 2. The expression levels and positive rates of HBV DNA and HBV cccDNA in ovarian tissue of mothers of infants infected and not infected in the uterus

Comparison of HBV DNA level: t=1·707, P=0·098; comparison of positive rate: χ2=2·936, P=0·087.
Comparison of HBV cccDNA level: t=5·018, P=0·003; comparison of positive rate: χ2=6·177, P=0·013.
Comparison among serum HBV DNA, ovarian HBV DNA and HBV cccDNA in pregnant women
The mean log value of serum HBV DNA of 33 pregnant women was 7·74±0·56. The corresponding values of HBV DNA and HBV cccDNA was 1·39±1·91 and 1·48±1·81, respectively, with no significant differences between the two values (t=−0·207, P=0·837), but both were significantly lower than serum HBV DNA (t=18·324, 18·996, P=0·000).
Comparison between HBV DNA, HBV cccDNA positive rate and intrauterine infection rate between the groups with different serum HBV DNA
The expression rate and intrauterine infection rate in ovarian tissue was higher in pregnant women with HBV DNA ⩾1·0×108 copies/ml than in pregnant women with HBV DNA <1·0×108 copies/ml (Table 3); however, the difference was not significant (P>0·05). Serum HBV DNA in pregnant women and intrauterine infection rate were positively correlated (Spearman's r=0·894, P=0·000).
Table 3. Comparison of HBV DNA, HBV cccDNA, positive rate and intrauterine infection rate between the groups with different serum HBV DNA

Comparison of HBV DNA and positive rate between the two groups: χ2=3·464, P=0·063; comparison of HBV cccDNA and positive rate between the two groups: χ2=0·337, P=0·561; comparison of intrauterine infection between the two groups: χ2=0·836, P=0·361.
Correlation between serum HBV DNA in pregnant women and intrauterine infection rate, Spearman's r=0·894, P=0·000.
DISCUSSION
Evidence for HBV infecting the ovary
HBV has always been believed to be a specific hepatotropic virus. However, in recent years, numerous studies have demonstrated its presence in various extrahepatic tissues and organs. For example, HBV DNA or HB antigens have been detected in monocytes, peripheral blood, central nervous system, stomach, duodenum, pancreas, spleen, and kidney [Reference Chunyan and Xianshi28, Reference Lin29]. These studies demonstrate the affinity of HBV for the above tissues, its replication capability in these tissues, and its pantropic property [Reference Han30–Reference Ursell32]. Amarapurkar & Amarapurkar found the kidney to be the major invasive extrahepatic organ for HBV [Reference Amarapurkar and Amarapurkar31]. The ovary is a genital organ and originates from the parietal mesoderm together with urinary organs. Therefore, the possibility of HBV infecting ovarian tissue and consequent germ cells exists in accordance with the biological properties of HBV and the physical properties of the ovary. In several studies, HBsAg, HBcAg and HBV DNA have been detected in ovarian tissue or follicle cells from women with CHB [Reference Ye12–Reference Lou15], suggesting that HBV can infect the ovary and ovum. In our study, 33 ovarian samples were examined for HBV DNA and HBV cccDNA, of which 12 and 14 samples, respectively, were found to be positive. There were no significant differences between expression levels, but both were significantly lower than serum HBV DNA, possibly because a large number of viruses replicate in the liver, and are then released into the blood and infect the ovary via blood circulation. The ovary is not a specifically targeted organ of HBV, so the chance of it becoming infected and the quantity of virus present are both reduced, suggesting that ovarian infection is uncommonly linked to HBV replication and that serum HBV DNA is not an effective index for evaluating the vertical transmission of HBV via ova. There may be other infection mechanisms and routes, apart from blood transmission. HBV cccDNA is the primary copying template for HBV replication. Chen et al. considered that cccDNA was released from damaged hepatocytes into the blood and is superior to serum HBV DNA for indicating the presence and replication condition of viruses [Reference Chen, Sze and He33]. Therefore, the positive HBV cccDNA in ovarian tissue not only illustrates that the ovary is capable of being infected but also promotes the assembly and replication of HBV in the ovary. This trial provides new evidence for HBV infection and replication in the ovary.
Effect of HBV cccDNA of the ovary in vertical transmission of HBV
In 1977, Blumberg [Reference Blumberg7] hypothesized autosomal recessive inheritance for HBV and transmission via germ cells. The ovary, as the major genital organ, contains follicles in all stages and regularly secretes matured oocytes that may be fertilized with sperm. Therefore, HBV infection in the ovary may cause changes in genetic materials and transmission via germ cells. Animal experiments have demonstrated the possibility of sperm and ova as carriers of the HBV gene into the embryo [Reference Lang10, Reference Ali, Huang and Xie11]. During the fertilization process, there is only one ovum but a great number of sperm. Accordingly, the ovum plays a more important role in HBV vertical transmission than sperm.
Currently, studies on HBV expression in the ovary are rare, and among tissue studied are ovary tissue, predominantly from non-pregnant HB women. Therefore, it has only been established that the ovary can be infected by HBV rather than that HBV can be transmitted to the filial generation via the ovum. Reports concerning the expression of HBV in the ovaries of pregnant women are rare. Lou et al. found no significant correlation between HBV infection in the ovary and intrauterine infection through detection of HBsAg in the ovarian tissue of 68 HBsAg-positive pregnant women [Reference Lou15]. HBsAg is the outer membrane protein of HBV and generally serves as the marker protein of HBV in immunohistochemisty. However, the low expression of HBsAg, low sensitivity for immunohistochemisty, and inability to quantitatively detect HBsAg can easily lead to false-positive results. The sensitivity of PCR is high enough to detect traces of HBV DNA and HBV cccDNA, but localization is impossible to determine. Due to blood contained in ovarian tissue, the specificity is poor, and there is a certain number of false positives. In this study in order to reduce the false-positive rate the sample was repeatedly washed with normal saline and PBS solution until the last washing solution had no HBV DNA present.
The expression of HBV DNA and HBV cccDNA in the ovaries of pregnant women was quantitatively assayed with fluorescent PCR and compared to the level in infants experiencing intrauterine infection. Results indicated that their expression models are positively correlated with HBV intrauterine infection, and the risk of infants experiencing intrauterine infection increases when mothers are positive for HBV DNA and HBV cccDNA in the ovary. Intrauterine infection rate is higher in HBV cccDNA-positive women than in HBV DNA-positive women, but the difference is not significant (P>0·05).
Significant differences were also noted in HBV cccDNA expression level and positive rate in the ovary between mothers having intrauterine-infected infants and infants without intrauterine infections. This suggests that intrauterine infection is closely related to HBV cccDNA expression in the ovary. Therefore, it is inferred that HBV cccDNA plays an important role in the process of ovarian infection by HBV, which is not only the most sensitive marker of HBV infection and replication in the ovary but also the crucial basis for predicting vertical transmission. Moreover, compared to mothers with HBV DNA at <108 copies/ml in serum, intrauterine infection rate increases when HBV DNA is ⩾108 copies/ml in serum, but the difference is not significant (P>0·05), which may be attributed to the small sample size. Serum HBV DNA in pregnant women is positively correlated with intrauterine infection, indicating that serum HBV DNA in the mother is a relatively independent risk factor for intrauterine infection in infants and that blood is still the major route for mother–child transmission [Reference Lou15].
However, due to the small sample size and the capability of detecting HBV cccDNA in ovarian tissue rather than the ova, this detection method also has some disadvantages. Therefore, the effect of HBV cccDNA of ovarian tissue on HBV vertical transmission has not been completely confirmed. However, this study at least illustrates that the possibility of ova infected with HBV increases the genetic risk for HBV if HBV cccDNA is present in the ovary. Mother–child transmission via an ovum is involved in vertical HBV transmission. However, the mechanism of infection of the ovum and the mechanism of vertical transmission need to be further investigated in studies with a larger sample size that use real-time PCR to detect HBV cccDNA in the ovary and the ovum.
ACKNOWLEDGEMENTS
This work was supported by the Science Fund of Natural Science of Jiangsu Province (BK 2008070), and was also supported by Professors Zhao Wei, Zhao Hong and Zhang Hong Mei of Second Affiliated Hospital of Southeast University.
DECLARATION OF INTEREST
None.






