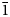Introduction
The Selsurt mountain located in the north-eastern part of the well-known huge Lovozero alkaline complex, Kola Peninsula, Russia is composed mainly of rocks in a layered complex of urtites, foyaites and lujavrites (Bussen and Sakharov, Reference Bussen and Sakharov1972). Various metasomatic assemblages occur at the contacts of the igneous alkaline rocks with metamorphic rocks on the northern spur of the Selsurt mountain.
The new Na-deficient eudialyte-group mineral selsurtite, described in this paper, is named after the discovery locality. The specimens with selsurtite were collected by one of the authors (NVC) in August, 1993.
Selsurtite is the 31st member of the eudialyte group that includes trigonal minerals with the general formula N13N23N33N43N53M16M23–6M3M4Z 3(Si24O72)O′4–6X1X2 (Johnsen et al., Reference Johnsen, Ferraris, Gault, Grice, Kampf and Pekov2003) where N1–5 = Na, K, Н3О+, Ca, Mn2+, Sr, Ba and REE; M1 = Ca, Mn2+, Fe2+, REE, Na and Sr; M2 = Mn2+, Fe2+, Fe3+, Na, Zr, Ta, Ti, K and H3O+; M3 and M4 = Si, S, Nb, Ti, W, Na; Z = Zr, Ti and Nb; O′ = O or OH and H2O; X1 and X2 = F, Cl, H2O, ОН, CO3 and SO4. The complex structures of these minerals are based on a heteropolyhedral framework composed of 9- and 3-membered rings of tetrahedra (Si9O27 and Si3O9), 6-membered rings of octahedra M16O24, ZO6 octahedra and [4–7]M2On polyhedra. Additional M3 and M4 sites located at the centres of the Si9O27 rings have 4- or 6-fold coordination. The framework hosts N1–N5 cations, X1–X2 anions and water molecules. In most eudialyte-group minerals, Na+ dominates among N cations. The only exceptions are three hydronium-rich members of the eudialyte group: aqualite, (H3O)8Na4SrCa6Zr3Si26O66(OH)9Cl (Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2007), ilyukhinite, (H3O,Na)14Ca6Mn2Zr3Si26O72(OH)2⋅3H2O (Chukanov et al., Reference Chukanov, Rastsvetaeva, Rozenberg, Aksenov, Pekov, Belakovsky, Kristiansen and Van2017), and selsurtite, (H3O)12Na3(Ca3Mn3)(Na2Fe)Zr3□Si[Si24O69(OH)3](OH)Cl⋅H2O.
The new mineral and its name (symbol Ssu) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2022-026, Chukanov et al., Reference Chukanov, Aksenov, Kazheva, Pekov, Varlamov, Vigasina, Belakovskiy, Vozchikova and Britvin2022). The holotype specimen is deposited in the collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with the registration number 5843/1.
Experimental methods and data processing
To obtain infrared (IR) absorption spectra, powdered samples were mixed with anhydrous KBr, pelletised, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) in the range of 360–3800 cm–1 at a resolution of 4 cm–1. Sixteen scans were collected for each spectrum. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
The Raman spectrum of randomly oriented samples were obtained using an EnSpectr R532 spectrometer on an OLYMPUS CX 41 microscope, coupled with a diode laser (λ = 532 nm) (Dept. of Mineralogy, Faculty of Geology, Moscow State University). The spectra were recorded at room temperature in the range from 100 to 4000 cm–1 with a diffraction grating (1800 gr mm–1) and spectral resolution of ~6 cm–1. The output power of the laser beam was in the range from 5 to 13 mW. The diameter of the focal spot on the sample was 5–10 μm. The back-scattered Raman signal was collected with a 40× objective; signal acquisition time for a single scan of the spectral range was 1 s, and the signal was averaged over 50 scans. Crystalline silicon was used as a standard.
Ten chemical analyses were carried out using a digital scanning electron microscope Tescan VEGA-II XMU equipped with an energy-dispersive spectrometer (EDS) INCA Energy 450 with semiconducting Si (Li) detector Link INCA Energy at an accelerating voltage of 20 kV, electron current of 190 pA and electron beam diameter of 160–180 nm. Attempts to use wavelength-dispersive spectroscopy (WDS), with a higher beam current, were unsuccessful because of instability of the mineral under the electron beam due to partial dehydration and migration of Na. This phenomenon is typical for high-hydrous sodium minerals with microporous structures.
A good agreement was observed between compositional data obtained under these standard conditions and those obtained under more ‘mild’ conditions (with a current lowered to 90–100 pA and electron beam defocused to an area of 30 × 30 μm).
The L-lines of Ta are not observed in the spectrum, which indicates the absence of detectable amounts of tantalum in selsurtite. Taking into account the overlapping SrLα and SiKα peaks, the SrO content was measured using WDS using the SrLα1 line, at an accelerating voltage of 20 kV and a current of 20 nA. The size of the electronic ‘spot’ on the surface of the sample was 300–320 nm.
The H2O content was determined by means of a modified Penfield method. The CO2 content was not determined because characteristic bands of carbonate groups (in the 1350–1550 cm–1 range) are not observed in the IR spectrum of selsurtite. Analytical data are given in Table 1. Contents of other elements with atomic numbers >8 are below detection limits.
Table 1. Crystal data, data collection information and structure refinement details for selsurtite.

*Note: The weight of the refined formula is close to the empirical formula weight of 2869.8.
Powder X-ray diffraction (XRD) data were collected using a Rigaku R-AXIS Rapid II diffractometer (image plate) using CoKα, 40 kV, 15 mA, a rotating anode with the microfocus optics, Debye-Scherrer geometry, d = 127.4 mm and exposure 15 min. The raw powder XRD data were collected using the program suite designed by Britvin et al. (Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). Calculated intensities were obtained by means of STOE WinXPOW v. 2.08 program suite based on the atomic coordinates and unit-cell parameters.
The single-crystal X-ray diffraction data for selsurtite were collected at room temperature by a Rigaku XtaLAB Synergy diffractometer with graphite monochromatised MoKα radiation and a Hybrid Pixel Array detector using the ω scanning mode. A semi-empirical absorption correction based on intensities of equivalent reflections was applied, and the data were corrected for the Lorentz, polarisation and background effects (Oxford Diffraction, 2009). The analysis of systematic absences of reflections shows R-centring, common for eudialyte-group minerals. Space group R3 was chosen based on cation ordering and lowering of the symmetry similar to other Ca-deficient (i.e. <4.5 Ca atoms per formula unit) members of the oneillite subgroup. The experimental details of the data collection and refinement results are listed in Table 1.
Results
Occurrence, general appearance and physical properties
In the holotype specimen (Figs 1a, 2), selsurtite is a rock-forming mineral which constitutes ~15 vol.% of a metasomatic fenite-like peralkaline rock mainly composed of aegirine, albite and orthoclase. Platy crystals of orthoclase (up to 0.5 × 2 × 2 mm) are embedded in a fine-grained aggregate composed of aegirine and albite. Subordinate minerals are lorenzenite, calciomurmanite, natrolite, Mn- and Ba-rich lamprophyllite, and sergevanite. Sergevanite occurs as small relics in some selsurtite crystals (Fig. 2b). Calciomurmanite forms pseudomorphs after platy lomonosovite crystals (up to 2 × 2 × 0.3 cm) embedded in the rock. The latest-stage mineral is saponite which forms pseudomorphs after grains of an unidentified mineral up to 1 mm across.

Fig. 1. A fragment of the holotype specimen (registration number 5843/1 in the Fersman mineralogical museum) with (a) red to reddish-orange selsurtite grains embedded in the aegirine–feldspar rock and (b) selsurtite crystal (left, brownish-red) in association with lorenzenite (right, dark brown). The FOV widths are 15 mm (a) and 4 cm (b).

Fig. 2. Back-scattered electron image of a polished section of a fragment of selsurtite (Ssu) holotype specimen (specimen No. 5843/1 from the Fersman mineralogical museum) in (a) a rock composed of aegirine (Aeg), orthoclase (Or) and albite (Ab) with accessory lorenzenite (Lrz) and (b) enlarged fragment of this image showing relics of sergevanite (Sgv) with the empirical formula Na9.47(H3O)xK0.16Sr0.47(Ca3.48Mn2.01Fe0.32Ln 0.19)Σ6.00(Na2.05Fe0.56Zr0.39)Σ3.00(Zr2.84Ti0.09Hf0.07)Σ3.00(Si25.58Nb0.42)Σ26.00Cl1.00(SO4)0.04(O,OH)y⋅nH2O in the selsurtite crystal [where Ln = lanthanide series element].
The selsurtite holotype occurs as equant or slightly flattened on (001) crystals up to 2 mm across (Fig. 1a). In some parts of the rock, larger prismatic and rhombohedral selsurtite crystals up to 3 cm long occur together with crystals of lorenzenite and/or calciomurmanite reaching several centimetres across (Fig. 1a). The main crystal forms are the pinacoid {0001}, the hexagonal prism {11$\bar{2}$![]() 0} and the rhombohedron {10$\bar{1}$
0} and the rhombohedron {10$\bar{1}$![]() 1}.
1}.
The colour of selsurtite is brownish-red to reddish-orange. Some small transparent grains demonstrate strong dichroism: cherry red along (001) and orange across (001). The streak of the mineral is white.
Selsurtite is brittle, with a Mohs' hardness of 5. No cleavage is observed. Parting is distinct on (0001). The fracture is uneven. Density measured by flotation in heavy liquids (mixtures of methylene iodide and heptane) is equal to 2.73(2) g⋅cm–3. Density calculated using the empirical formula and unit-cell volume refined from single-crystal XRD data is 2.722 g⋅cm–3.
The new mineral is optically uniaxial (–), with ω = 1.598(2) and ɛ = 1.595(2) (λ = 589 nm). Under the microscope, selsurtite is pleochroic in thick grains (O = pinkish and E = pale yellow-pinkish). The absorption scheme is: O > E.
Infrared spectroscopy
Absorption bands in the IR spectrum of selsurtite (curve a in Fig. 3) and their assignments are (cm–1; s – strong band, w – weak band, sh – shoulder): 3520sh, 3417, 3260sh (O–H stretching vibrations), 1639w (H–O–H bending vibrations), 1150sh (asymmetric stretching vibrations of SO4 tetrahedra), 1003s, 981s, 935sh (Si–O stretching vibrations), 741 (mixed vibrations of rings of SiO4 tetrahedra – ‘ring band’), 667w (mixed vibrations of rings of SiO4 tetrahedra combined with Nb–O stretching vibrations), 520sh [IV(Zr,Fe)–O stretching vibrations], 473s, 451s (lattice mode involving predominantly bending vibrations of rings of SiO4 tetrahedra), and 370 (lattice modes involving VI(Ca,Mn2+)–O stretching vibrations). The shoulder at 1300 cm–1 may correspond to the isolated proton at the N5 site (Chukanov and Chervonnyi, Reference Chukanov and Chervonnyi2016). The assignment of the IR bands was made based on the analysis of IR spectra of many structurally investigated eudialyte-group minerals, in accordance with Rastsvetaeva et al. (Reference Rastsvetaeva, Chukanov and Aksenov2012).

Fig. 3. Powder infrared absorption spectra of (a) selsurtite and (b) sergevanite holotype sample with the crystal-chemical formula N 1–4(Na10.5K0.9REE 0.6)Σ12 N 5[(H3O,H2O)2.25Na0.75]Σ3 M 11Ca3 M 12(Mn1.8Ca1.2)Σ3 M 2(Na2.4Fe2+0.6)Σ3 M 3(Si0.5Ti0.45Nb0.05)Σ1 M 4SiZ(Zr2.7Nb0.3)Σ3[Si3O9]2[Si9O27]2(OH)3(SO4)0.3 X 1[(H2O)0.8Cl0.2]. The spectra are offset for comparison.
The IR spectrum of selsurtite differs from that of its sodium analogue sergevanite (curve b in Fig. 3) with higher intensities of the bands of O–H stretching and H–O–H bending vibrations. A very low intensity of the band at 520 cm–1 observed as a shoulder in the IR spectrum of selsurtite reflects a low content of transitional elements at the M2 site which is in agreement with the structural data (see below).
Raman spectroscopy
A specific feature of the Raman spectrum of selsurtite (Fig. 4), as well as other hydronium-bearing eudialyte-group minerals (the curves a and b in Fig. 5) is a series of bands in the range of 1070–2900 cm–1 corresponding to strong hydrogen bonds formed by hydronium cations in different local situations including Zundel- and Eigen-like ones with short O⋅⋅⋅O distances of ~2.4 and ~2.6 Å (see Discussion section for details). These bands are absent in the Raman spectrum of eudialyte that does not contain H3O+ cations (curve c in Fig. 5).

Fig. 4. Raman spectrum of selsurtite.

Fig. 5. Raman spectra of (a) potassium analogue of aqualite (H3O)8Na5K2Zr3Ca6[Si24O69(OH)3][Si2]Mn(OH)2Cl⋅2H2O from the Kovdor ultrabasic-alkaline-carbonatite complex, Kola Peninsula, Russia (drawn using data from Rastsvetaeva et al., Reference Rastsvetaeva, Chukanov, Pekov and Vigasina2022); (b) ‘potassium-oxonium eudialyte’ with the empirical formula (H3O)x(Na3.3K2.5Sr0.6)Σ6.4Ca6.4Mn0.15Fe1.3Zr2.8Ti0.2Nb0.15Si25.85Cl1.7(O,OH)x⋅nH2O (x ≈ 8) from the Kukisvumchorr mountain, Khibiny alkaline massif, Kola Peninsula (Rastsvetaeva et al., Reference Rastsvetaeva, Chukanov and Aksenov2012); and (c) common accessory eudialyte Na15Ca6Fe2+3Zr3Si26O72(O,OH,H2O)3Cl2 from the Alluaiv mountain, Lovozero alkaline massif, Kola Peninsula.
Other bands in the Raman spectrum of selsurtite are assigned as follows: 3455 and 3550 cm–1 to O–H stretching vibrations of H2O molecules and OH groups; and 900–1100 cm–1 to Si–O stretching modes (probably, except the band at 1073 cm–1 which may correspond to a hydrated proton complex with a Zundel-like configuration – see discussions in text). The band at 792 cm–1 is assigned to mixed vibrations of rings of SiO4 tetrahedra; and 650 and 691 cm–1 to mixed vibrations of rings of SiO4 tetrahedra combined with Nb–O stretching vibrations. Finally 564 cm–1 is assigned to IV(Zr,Fe)–O stretching vibrations and bands ≤410 cm–1 to lattice modes.
Chemical data
Analytical data are given in Table 2. The empirical formula (based on 24.56 Si+Al apfu, Z = 3, in accordance with structural data) is H25.94Na6.03K0.16Mg0.07Ca3.51Sr0.52Ce0.19La0.10Nd0.08Pr0.03Mn1.91 Fe0.47Ti0.22Zr3.16Hf0.06Nb0.24Si24.40 Al0.16S0.10Cl0.82O79.13. Taking into account structural data (see below), the simplified formula can be written as follows: (H3O,Na,□)15[(Mn,Ca)3(Ca,Mn)3] (Na2(Fe,Zr)](□,Nb,Si)(Si,Ti,□)(Si3O9)2[Si9(O,OH)27]2(OH)Cl⋅H2O. The ideal formula is (H3O)12Na3(Mn3Ca3)(Na2Fe)Zr3□Si[Si24O69(OH)3](OH)Cl⋅H2O.
Table 2. Chemical composition of selsurtite.

Notes: bdl – below detection limit; S.D. – standard deviation
*WDS-mode analyses for SrO.
**The H2O content was determined by the modified Penfield method.
The Gladstone–Dale compatibility index 1 – (K p/K c) (Mandarino, Reference Mandarino1981) is equal to –0.011 (rated as superior) with the density calculated using the empirical formula and unit-cell parameters refined from the single-crystal X-ray diffraction data.
X-ray diffraction and crystal structure
Powder X-ray diffraction data of selsurtite are given in Table 3. The unit-cell parameters refined from the powder data are: a = 14.162(2) Å, c = 30.41(1) Å and V = 5282(4) Å3.
Table 3. Powder X-ray diffraction data (d in Å) of selsurtite.

*For the calculated pattern, only reflections with intensities ≥1 are given.
**For the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
The crystal structure was solved and refined on the basis of 2752 independent reflections with I > 3σ(I) using the program package JANA2006 (Petřiček et al., Reference Petřiček, Dušek and Palatinus2006). Extra-framework sites, including split and partially occupied ones, were located in a difference electron-density map. Atomic scattering factors for neutral atoms, together with anomalous dispersion corrections, were taken from International Tables for Crystallography (Prince et al., Reference Prince2004). Illustrations were produced with the JANA2006 program package in combination with the program DIAMOND (Brandenburg and Putz, Reference Brandenburg and Putz2005). Because of the complex chemical composition, the cation distribution on the structural sites was proposed taking into account site-scattering factors, interatomic distances and ionic radii of the cations. At the first step, the number of electrons associated with the atoms at the sites (e calc) was determined. At the second step, for each value of e calc, the most suitable ratio between the atoms with the closest final refined amount of electrons (e ref) was selected and atom coordinates and atomic displacement parameters were refined.
The final refinement cycles converged to R 1 = 4.84%, wR 2 = 7.15% and GOF = 1.14. The highest peak and deepest minimum in the final residual electron density were 1.14 e– Å−3 and −1.11 e– Å−3, respectively. Table 4 lists the fractional atomic coordinates, site multiplicities, atomic displacement parameters and site occupancies. Selected interatomic distances are given in Table 5. Supplementary crystallographic data for this paper has been deposited as CSD 2157470 at the Cambridge Crystallographic Data Center, www.ccdc.cam.ac.uk/ and as Supplementary material with this paper (see below).
Table 4. Atom coordinates (x, y, z), atomic displacement parameters (U, Å2), site multiplicities (Mult.) and site occupancies (s.o.f.) in the structure of selsurtite.

Table 5. Selected interatomic distances (Å) in the structure of selsurtite.

Discussion
Crystal structure
Selsurtite is isostructural with other 12-layered members of the oneillite-type (with the space group R3) representatives of the eudialyte group. On the basis of the refined site-scattering factors, the crystal chemical formula of selsurtite can be written as follows (Z = 3): {N 1[(H3O)2.40Na0.60]N 2[(H3O)2.1Na0.9]N 3[Na1.8(H3O)0.84Sr0.36]N 4[(H3O)0.90Na0.70K0.18]N 5Hx}{Z(Zr2.93Hf0.07)M 1(1)(Mn1.82Ca0.98Ce0.2)M 1(2)(Ca2.7Mn0.19Ce0.11)M 2[Na2.12Fe0.50Zr0.38(H2O)1.28]M 3[□0.44(Nb(OH)3)0.3(SiOH)0.26]M 4[(SiOH)0.30(Ti(OH)3)0.23□0.47][Si3O9]2[Si9O25.59(OH)1.41][Si9O25.68(OH)1.32]X 1(Cl0.81S0.12F0.07)X 2(H2O)0.16.
The main structural features of selsurtite distinguishing it from other eudialyte-group minerals are: (i) cation ordering within the six-membered ring of octahedra resulting in a lowering of symmetry. The six-membered ring is formed by M1(1)O6 and M1(2)O6 octahedra with different occupancies (Fig. 6). The M1(1)O6 octahedron is predominantly occupied by manganese (1.82 apfu), while the M1(2)O6 octahedron is predominantly occupied by calcium (2.7 apfu). (ii) The statistical predominance of sodium (2.12 apfu) at the M2 site. (iii) The predominance of vacancies over NbO3(OH)3 octahedra and SiO3(OH) tetrahedra at the M3 site. (iv) The predominance of tetravalent atoms (with Si > Ti) at the M4 site. (v) The predominance of hydronium at the extra-framework N sites.

Fig. 6. The distribution of Na and Zr over the M2-sites in the crystal structure of selsurtite.
Raman spectroscopy of hydronium and hydrated proton complexes
Numerous ab initio quantum-chemical calculations of hydronium and other hydrated proton complexes, including Zundel (H5O2+) and Eigen (H3O+⋅3H2O) cations have shown that these clusters are characterised by variable configurations and strong hydrogen bonds with O⋅⋅⋅O distances in the range of 2.38–2.8 Å (Vyas, Reference Vyas, Sakore and Biswas1978; Komatsuzaki and Ohmine, Reference Komatsuzaki and Ohmine1994; Corongiu, Reference Corongiu, Kelterbaum and Kochanski1995; Kim et al., Reference Kim, Schmitt, Gruetzmacher, Voth and Scherer2002; Sobolewski and Domcke, Reference Sobolewski and Domcke2002a, Reference Sobolewski and Domcke2002b; Asmis et al., Reference Asmis, Pivonka, Santambrogio, Brümmer, Kaposta, Neumark and Wöste2003; Christie, Reference Christie2004; Headrick et al., Reference Headrick, Bopp and Johnson2004; Laria et al., Reference Laria, Martí and Guàrdia2004; Asthagiri et al., Reference Asthagiri, Pratt and Kress2005; Ortega et al, Reference Ortega, Escribano, Herrero, Maté and Moreno2005; Paddison and Elliot, Reference Paddison and Elliott2005; Vener and Librovich, Reference Vener and Librovich2009; Biswas et al., Reference Biswas, Carpenter, Fournier, Voth and Tokmakoff2017; Carpenter, Reference Carpenter2020). Calculated wavenumbers of vibrational modes corresponding to the hydrated proton complexes are in the range of 1070–3000 cm–1.
There is a negative correlation between the frequency of O–H stretching vibrations and the O⋅⋅⋅O distance between the O atom of the OH group and the O atom – acceptor of the hydrogen bond (McClellan and Pimentel, Reference McClellan and Pimentel1960; see Fig. 7). This correlation is nearly linear in the range of the O⋅⋅⋅O distances from 2.4 to 2.8 Å and deviates significantly from linearity for weaker hydrogen bonds.

Fig. 7. The dependence of the wavenumber shift of O–H stretching vibrations relative to the value of 3750 cm–1 (accepted for non-bonded OH group) on the O⋅⋅⋅O distances for hydrogen bonds in crystals, drawn using data from McClellan and Pimentel (Reference McClellan and Pimentel1960).
The following empirical correlations between O–H stretching frequencies in IR spectra of minerals and O⋅⋅⋅O and H⋅⋅⋅O distances (obtained from structural data) were established by Libowitzky (Reference Libowitzky1999):
In fact, equations 1 and 2 are a very rough approximation and have a restricted applicability. In particular, above 3500 cm–1 substantial deviations from the correlations in equations 1 and 2 are common because O–H stretching frequencies depend not only on O⋅⋅⋅O and H⋅⋅⋅O distances, but also on the nature of cations coordinating O–H groups and H2O molecules, in addition to the O–H⋅⋅⋅O angle, and the influence of these factors becomes most evident in the case of weak hydrogen bonds. Equations 1 and 2 predict that maximum possible values of O–H stretching frequencies for minerals are 3592 and 3632 cm–1, respectively, however in many minerals, including for magnesium serpentines, brucite, kaolinite and amphibole supergroup members, observed frequencies are much higher and can exceed 3700 cm–1. Nevertheless, these correlations can be used for semiquantitative estimations, at least for relatively strong hydrogen bonds. The proposed assignment of Raman bands of O–H stretching vibrations involving H3O+ groups are given in Table 6.
Table 6. The shortest O⋅⋅⋅O distances for hydrogen bonds in selsurtite estimated from the Raman spectrum using ν/d O⋅⋅⋅O correlations [d O⋅⋅⋅O(calc)] and determined as a result of the crystal structure refinement [d O⋅⋅⋅O(ref)].

* Extrapolated values.
Origin of selsurtite
Regardless of the fact that selsurtite is a Na-deficient member of the eudialyte group, its crystal structure is characterised by the presence of the Na-dominant M2 site. In addition to selsurtite, only two 12-layer eudialyte-group minerals: raslakite, N 1–N5Na15M 1[Ca3Fe3]M 2(Na2Zr)ZZr3M 3,M4[(Si,Nb)Si](Si24O72)(OH,H2O,O)4(Cl,OH) (Chukanov et al., Reference Chukanov, Pekov, Zadov, Korovushkin, Ekimenkova and Rastsvetaeva2003), and sergevanite, N 1–N5(Na,H3O)15M 1(Ca3Mn2+3)M 2(Na2Fe2+)ZZr3M 3,M4[Si(Si,Ti)][Si24O72](OH,H2O,SO4)5 (Chukanov et al., Reference Chukanov, Aksenov, Pekov, Belakovskiy, Vozchikova and Britvin2020), have a Na-dominant M2 site. These minerals originate from highly alkaline, hyperagpaitic rocks. In other 12-layer eudialyte-group minerals, the M2 site is predominantly occupied by Fe2+, Fe3+ or Mn2+, or is vacant. The presence of sergevanite relics in some selsurtite crystals (Fig. 2) indicates that selsurtite could be formed as a result of leaching of sodium, protonation and hydration of sergevanite that initially crystallised under highly alkaline conditions. This assumption is in agreement with the association of selsurtite with calciomurmanite forming pseudomorphs after lomonosovite, a mineral considered as a marker of peralkaline conditions. As in the evolution series sergevanite → selsurtite, the evolution series lomonosovite Na10Ti4(Si2O7)2(PO4)2O4 → calciomurmanite (Na,□)2Ca(Ti,Mg,Nb)4[Si2O7]2O2(OH,O)2(H2O)4 (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) is characterised by a significant leaching of Na+ and hydration.
Comparative data for selsurtite and other Na-deficient, hydronium-rich eudialyte-group minerals are given in Table 7.
Table 7. Comparative data for selsurtite and other Na-deficient hydrated eudialyte-group minerals.

Acknowledgements
The authors are grateful to reviewers for the useful discussion. A major part of this work, including chemical analyses, infrared spectroscopy, and identification of associated minerals was carried-out in accordance with the state task of Russian Federation, state registration number ААAА-А19-119092390076-7. The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.136
Competing interests
The authors declare none.