Introduction
Functional disorders are defined by the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders-5 as one or more symptoms that affect a patient's movement or senses and are outside of their conscious control. The patient must not be able to attribute their symptoms to drug use, physical or neurological conditions and may attribute their symptoms to a traumatic or stressful event.1
Functional neurological disorders are documented in the neurology literature and comprise 16 per cent of patients referred to neurology clinics, second only to headaches.Reference Stone, Carson, Duncan, Roberts, Warlow and Hibberd2 A diverse range of functional disorders, including tinnitus, chronic pain and depression,Reference Llinas, Ribary, Jeanmonod, Kronberg and Mitra3 fibromyalgia,Reference Naghdi, Ahonen, Macario and Bartel4 visual snow syndrome,Reference Lauschke, Plant and Fraser5 migraine,Reference de Tommaso, Ambrosini, Brighina, Coppola, Perrotta and Pierelli6 and central hearing lossReference Ban and Jin7 demonstrate similar brain network dysfunction and may provide an aetiological basis for understanding the emergence of certain idiopathic otological symptoms (i.e. functional ear symptoms).
Tinnitus and imbalance often present to the otology clinic as functional symptoms, manifesting because of adaptive brain circuit dysfunction in the absence of active physical disease. Half of dizziness referrals to neurology have been classified as functional.Reference Stone, Carson, Duncan, Roberts, Warlow and Hibberd2 Primary tinnitus, persistent posturo-perceptual dizziness and vestibular migraine are recognised functional disorders.Reference Rauschecker, May, Maudoux and Ploner8–Reference Tedeschi, Russo, Conte, Laura and Tessitore10 Patients with migraine often have audiovestibular symptoms, but the type of migraine has no bearing on audiovestibular abnormality,Reference Dash, Panda, Khandelwal, Lal and Mann11 suggesting a mechanistic overlap in central sensory processing. Otalgia and aural fullness often occur as unexplained presenting symptoms to the otology clinic, together with tinnitus and imbalance, which suggests a common functional mechanism. Aural fullness has been reported to occur in 13.4 per cent of patients as an unexplained or idiopathic symptom.Reference Park, Lee, Kang, Ryu, Lee and Yeo12 These patients experience aural fullness despite normal examination and investigations and can improve with migraine treatment, supporting the idea of a common central mechanistic overlap.Reference Moshtaghi, Ghavami, Mahboubi, Sahyouni, Haidar and Ziai13 Unexplained otalgia has also been associated with migraine mechanisms.Reference Teixido, Seymour, Kung, Lazar and Sabra14 It is also interesting to note that idiopathic aural fullness symptoms have been reported to improve with the administration of an autonomic nerve blocking agent, again indicating a potential central origin of this symptom.Reference Yuasa, Kambayashi, Saijo, Hozawa, Iino and Kaneko15
Various factors have been implicated in the emergence of functional disorders that may be related to neurological adaptation or maladaptation, to challenging experiences, and to perceived threats. These include childhood adversity,Reference Barch, Belden, Tillman, Whalen and Luby16 emotional stress,Reference Clemens, Wagels, Bauchmuller, Bergs, Habel and Kohn17 physical traumaReference Rabellino, Tursich, Frewen, Daniels, Densmore and Theberge18 and sleep deprivation.Reference Feng, Becker, Zheng and Feng19 A recent meta-analysis confirmed that adverse life experiences, especially childhood adversity, are a predisposing factor to the development of functional neurological disorders.Reference Ludwig, Pasman, Nicholson, Aybek, David and Tuck20 The amygdala interacts with the cortical sensory systems in the assessment of threat and modulates reflex responses through projections to the hypothalamus and brainstem.Reference Price21 The ventromedial prefrontal cortex is connected to the amygdala, hypothalamus and periaqueductal grey and allows cortical control over the system in relation to a wider set of emotions.Reference Price21 However, experience can be disproportionately assessed as threatening, leading to adaptive brain circuit dysfunction, loss of cortical control and occurrence of functional symptoms.Reference Sherin and Nemeroff22
We aimed to review the incidence of functional ear symptoms in new referrals to the otology clinic, highlight underlying co-morbidity and present a clinical model outlining the experience-driven brain circuit dysfunction based on neuroscientific research. An understanding of these principles will significantly aid the otologist, neurologist, audiologist and other clinicians managing patients with functional ear symptoms. This is the first research article to specifically address the incidence of functional symptoms in the otology clinic.
Materials and methods
We undertook a retrospective case note review of 1000 consecutive new primary care referrals to a secondary care adult otology clinic. All new patients (aged 16 years and over) were seen by the same consultant otologist, between the dates of 1 January 2017 and 21 August 2019.
The review included data on age, sex, symptoms, examination findings, past medical history, past surgical history, life stressors, audiometry, tympanometry and imaging. Functional disorder was defined by Diagnostic and Statistical Manual of Mental Disorders-5 criteria,1 with functional ear symptoms being identified in the absence of active physical disease, when supported by appropriate examination, audiometry, tympanometry and imaging. The data collection was retrospective, fully anonymised and non-interventional.
Results
Of 1000 patients, 576 (57.6 per cent) were female, and 424 (42.4 per cent) were male. Functional disorder was the most common primary diagnosis (346 of 1000; 34.6 per cent), followed by sensorineural hearing loss (148 of 1000; 14.8 per cent), mucosal chronic otitis media (91 of 1000; 9.1 per cent), otitis externa (70 of 1000; 7 per cent), squamous chronic otitis media (61 of 1000; 6.1 per cent), ear wax accumulation (55 of 1000; 5.5 per cent), otitis media with effusion (35 of 1000; 3.5 per cent) and otosclerosis (33 of 1000; 3.3 per cent) (Figure 1).

Fig. 1. Primary diagnosis for 1000 consecutive new referrals to the adult otology clinic. BPPV = benign paroxysmal positional vertigo; APD = auditory processing disorder
Functional ear symptoms presented more commonly in females (207 of 346; 60 per cent) than males (139 of 346; 40 per cent), occurring across all ages in females (16–85 years) and males (16–92 years; median ages 53 and 49 years, respectively). Symptoms reported were tinnitus (241 of 346; 69.7 per cent), imbalance (82 of 346; 23.7 per cent), otalgia (79 of 346; 22.8 per cent) and aural fullness (66 of 346; 19.1 per cent). Although only 1 ear symptom may be present (259 of 346; 75 per cent), 2 or more functional ear symptoms in the same patient were reported in 87 of 346 patients (25.1 per cent) and 3 or more symptoms were reported in 29 of 346 patients (8.3 per cent) as shown by symptom distribution in Figure 2.

Fig. 2. Distribution of otological symptoms in patients presenting with a functional disorder. Total n = 346.
Aural fullness frequently occurred together with otalgia (30 of 66; 45.5 per cent), tinnitus (39 of 66; 59.1 per cent) and imbalance (14 of 66; 21.2 per cent). Otalgia frequently occurred together with tinnitus (33 of 79; 41.8 per cent), imbalance (16 of 79; 20.3 per cent) and aural fullness (30 of 79; 38.0 per cent). In patients presenting with primary tinnitus, an additional functional ear symptom occurred in 29 per cent (70 of 241), whereas patients presenting with a functional balance disorder had additional functional ear symptoms in 46.3 per cent (38 of 82).
Figure 3 provides details of reported underlying conditions in patients with functional ear symptoms. Commonly encountered underlying conditions included sensorineural hearing loss (135 of 346; 39 per cent), emotional stress (103 of 346; 30 per cent), chronic medical illness (75 of 346; 22 per cent), chronic pain disorder (56 of 346; 16 per cent), anxiety and depression (30 of 346; 8.7 per cent), surgery (22 of 346; 6.4 per cent), noise-induced hearing loss (16 of 346; 4.6 per cent), trauma (15 of 346; 4.3 per cent), migraine (11 of 346; 3.2 per cent), and fibromyalgia (8 of 346; 2.3 per cent). Six cases had significant underlying psychiatric diagnosis, including post-traumatic stress disorder, obsessive compulsive disorder and attention deficit hyperactivity disorder.

Fig. 3. Underlying conditions in 346 patients presenting with a diagnosis of functional ear disorder. PTSD = post-traumatic stress disorder; OCD = obsessive compulsive disorder; ADHD = attention deficit hyperactivity disorder; NIHL = noise-induced hearing loss; SNHL = sensorineural hearing loss
Discussion
Approximately one-third of symptoms reported by patients in primary care and subspecialty settings remain medically unexplained after a complete evaluation.Reference Kroenke23 Similarly, our study showed 34.6 per cent of all new referrals to the otology clinic had a functional disorder. Patients with functional disorders have structurally normal brains that have been shown to exhibit disordered function.Reference Voon, Cavanna, Coburn, Sampson, Reeve and LaFrance24 Patients experience higher rates of psychological and physical co-morbidity along with a higher incidence rate of childhood adversity.Reference Bennett, Diamond, Hoeritzauer, Gardiner, McWhirter and Carson25 In our study, co-morbidities for patients diagnosed with functional ear symptoms included stress (30 per cent), chronic illness (22 per cent), chronic pain disorder (16 per cent) and depression (8.7 per cent).
In somatosensory amplification, abnormal interactions among large-scale neural systems alter visceral-somatic perception, emotional processing and awareness, and cognitive control.Reference Perez, Barsky, Vago, Baslet and Silbersweig26 Amplification of neural signalling within the central nervous system induces functional symptoms and dysregulation of the sensory-motor and immune systems.Reference Monaco, Cattaneo, Marci, Pietropaoli and Ortu27 Deficits in access, engagement and disengagement of large-scale neurocognitive networks play a prominent role in functional disorders.Reference Menon28 The circuitry of fear memory consolidation is also important, being involved in storage and retrieval of information related to experience.Reference Izquierdo, Furini and Myskiw29 Bringing together such concepts, we suggest a clinical model for functional ear symptoms as shown in Figure 4. We will now describe the concepts in our clinical model for functional symptoms.
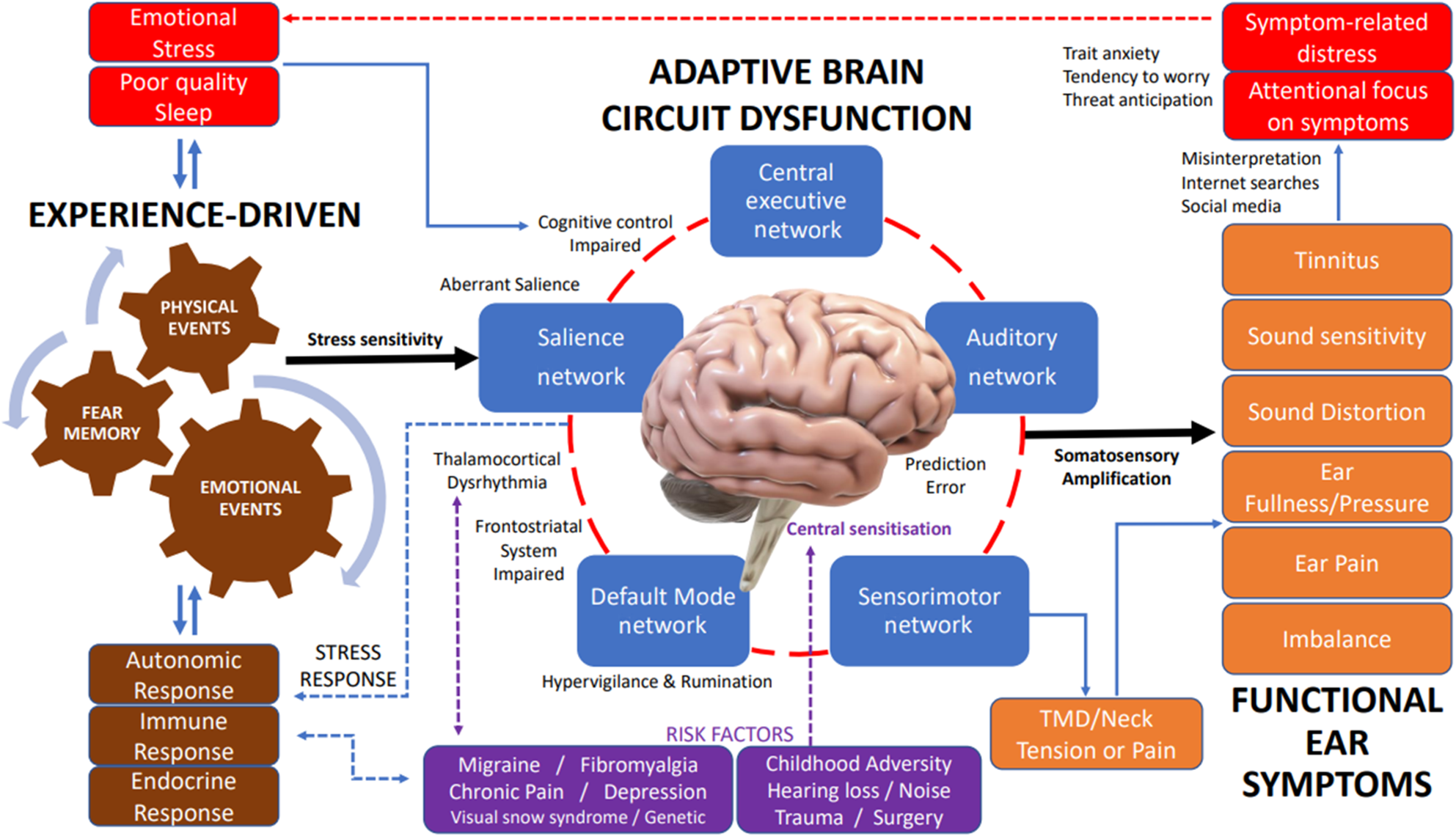
Fig. 4. Model of adaptation of brain circuitry to experience for functional ear symptoms. (1) Risk factors: central sensitisation or aberrant saliency processing can occur after childhood adversity, hearing loss, noise, or physical trauma or surgery. Migraine, fibromyalgia, chronic pain, depression and visual snow syndrome exhibit thalamocortical dysrhythmia, which hampers normal sensory information processing. Trait anxiety increases stress sensitivity. (2) Experience: physical and emotional events, associated with fear memory, generate experience and neural signalling that drives brain circuits. (3) Adaptive brain circuit dysfunction: inappropriate assignment of neural signals (aberrant salience) through the salience network disengages the central executive network (diminished cognitive control) and engages the default mode network (hypervigilance and rumination). Frontostriatal system impairment causes diminished filtering of unwanted signals that are attended and further processed. (4) Subjective perception of functional ear symptoms: aberrant neural activity and functional connectivity with amplified neural signals (somatosensory amplification) manifests as sensory signals and perception of sensory symptoms (functional ear symptoms). Stress mechanisms increase muscle tone leading to temporomandibular disorders and neck tension, which can further impact aural fullness, ear pain and functional imbalance. (5) Responses: symptom misinterpretation and focus cause emotional stress and reduced sleep which further drive the central maladaptive processes. Physiological stress responses can trigger autonomic, immune and hormonal effects. Secondary symptoms can include feeling tense, palpitations, shallow breathing, irritability, sleep disturbance and difficulty concentrating. TMD = temporomandibular disorders
Brain pathophysiology concepts
Intrinsic connectivity networks describe a set of large-scale functionally connected brain networks that can be captured in either resting state or task-based neuroimaging data.Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna30 Intrinsic connectivity network dysfunction forms the neural basis of functional disorders. Three important intrinsic connectivity networks are the default mode network, salience network and the central executive network, which are shown in Figure 4. The default mode network is involved in information monitoring, social cognition and initiating prediction signals. It is active in a range of internally directed cognitive processes including autobiographical memory and thinking of the future.Reference Buckner, Andrews-Hanna and Schacter31 The central executive network has a role in cognitive control. The salience network is important in processing sensory, emotional and cognitive information along with identifying relevant events. The triple network model of psychopathology proposes the salience network acts as a ‘switch’ that engages the default mode network and disengages the central executive network, leading to symptoms.Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna30,Reference Menon and Uddin32
Aberrant salience is the assignment of significance to innocuous stimuliReference Kapur33 and has an adverse effect on how external and internal signals are processed. The salience network plays a crucial role in switching between brain networks involved in externally oriented attention and internally oriented mental processes.Reference Sridharan, Levitin and Menon34 The central experience that defines aberrant salience is when stimuli that ordinarily would not seem important become more significant.Reference Bowers35 Aberrant salience has been implicated in the pathophysiology of functional neurological disorders,Reference Perez, Williams, Matin, LaFrance, Costumero-Ramos and Fricchione36 anxiety and mood disorders.Reference Salviati, Bersani, Valeriani, Minichino, Panico and Romano37
Distress in response to tinnitus has been localised to salience network nodes such as the anterior cingulate cortex, insula and amygdala.Reference Vanneste, Plazier, der Loo, de Heyning, Congedo and De Ridder38 Paralimbic involvement in tinnitus patients has been suggested to indicate tinnitus distress as a state of aberrant salience, similar to other brain disorders.Reference Salviati, Bersani, Valeriani, Minichino, Panico and Romano37 Furthermore, attenuated activation of the central executive network and downregulated baseline connectivity between the central executive network with salience network and autobiographical memory networks has been suggested to maintain chronic tinnitus awareness.Reference Trevis, Tailby, Grayden, McLachlan, Jackson and Wilson39
The intrinsic dynamics of thalamocortical network oscillations are crucial for early sensory processing.Reference Hodkinson, Wilcox, Veggeberg, Noseda, Burstein and Borsook40 Electrophysiological studies have shown altered thalamocortical rhythm, termed thalamocortical dysrhythmia, in a range of disorders including tinnitus, migraine, fibromyalgia and visual snow syndrome.Reference Llinas, Ribary, Jeanmonod, Kronberg and Mitra3,Reference De Ridder, Vanneste, Langguth and Llinas41,Reference Schulman, Cancro, Lowe, Lu, Walton and Llinas42 These disorders share a common temporal pattern of beta-theta and theta-gamma coupling. Self-sustaining generation of low-frequency oscillations can result in long-term cortical dysfunction.Reference Walton, Llinás, Kruger and Light43 The enhancement of low-frequency theta oscillations may be linked to the emergence of negative functional symptoms, while gamma upregulation could serve to induce more positive or excitatory functional symptoms.Reference Llinas and Steriade44
Neurology relationship
Compared with neurology clinics, our study suggests functional disorders are twice as prevalent in otology (34.6 per cent vs 16 per cent).Reference Stone, Carson, Duncan, Roberts, Warlow and Hibberd2 However, functional disorders commonly overlap. In visual snow syndrome, 52–63 per cent of patients experience tinnitus,Reference van Dongen, Waaijer, Onderwater, Ferrari and Terwindt45,Reference Renze46 and the effectiveness of cognitive behavioural therapy in tinnitus has led to its utilisation for visual snow syndrome.Reference van Dongen, Waaijer, Onderwater, Ferrari and Terwindt45 The currently accepted view is that primary headache is a result of abnormal brain function with normal brain structure.Reference May, Ashburner, Büchel, McGonigle, Friston and Frackowiak47 There is a significant relationship between tinnitus and headache laterality and symptom interaction over time, and both disorders may be linked by common pathophysiological mechanisms.Reference Langguth, Hund, Busch, Jürgens, Lainez and Landgrebe48 It has been suggested that clocking tinnitus may be an audiological manifestation of a migraine disorder.Reference Chen, Hsu, Chen and Yin49 In a population-based study, the prevalence of tinnitus has been found to be significantly higher in migraine patients.Reference Hwang, Tsai, Liu, Chen and Lai50 Similarly, isolated prolonged aural fullness has been found to improve with migraine-related lifestyle changes and prophylactic treatment.Reference Moshtaghi, Ghavami, Mahboubi, Sahyouni, Haidar and Ziai13
Stress relationship
Neuroimaging studies suggest amygdala prefrontal circuitry dysfunction in anxiety, creating a bias towards threat-related responses.Reference Bishop51 Stress and anxiety shift the balance of attention away from a task-directed mode, governed by the prefrontal cortex, to a sensory-vigilance mode, governed by the amygdala and other threat-sensitive regions.Reference Arnsten52 In our study, 30 per cent of patients with functional ear symptoms reported increased levels of stress. Stress symptoms have been reported in 65 per cent of tinnitus patients using Lipp's inventory symptoms of stress and are related to increased tinnitus perception.Reference Ciminelli, Machado, Palmeira, Carta, Beirith and Nigri53 Other studies have shown a direct correlation between duration of tinnitus and stress severity.Reference Gomaa, Elmagd, Elbadry and Kader54 Studies provide evidence that stressful life experiences are accompanied by autonomic and neuroendocrine changes capable of influencing immune function, thereby influencing susceptibility to infectious, autoimmune and chronic illness.Reference Ader and Kelley55 Childhood adversity and cumulative adverse life events can prime the stress system into a state of readiness, and even minor subsequent stressors trigger a stress response.Reference Kozlowska56
Mental health relationship
A high incidence of anxiety and depression in primary tinnitus is described in the literature, and co-morbid psychiatric illness increases severity.Reference Pinto, Marcelos, Mezzasalma, Osterne, de Melo Tavares de Lima and Nardi57,Reference Reynolds, Gardner and Lee58 Indeed, the incidence rate of depression in people with tinnitus has been cited as being more than twice that of the national average in the US population (approximately 35 per cent vs approximately 15 per cent, respectively).Reference Folmer, Griest, Meikle and Martin59 Depressive symptoms play a role in triggering and maintaining chronic tinnitus.Reference Trevis, McLachlan and Wilson60 Post-traumatic stress disorder is associated with functional symptoms. A study of 468 Iraq War veterans with post-traumatic stress disorder showed higher levels of dizziness compared with veterans without post-traumatic stress disorder.Reference Hoge, Terhakopian, Castro, Messer and Engel61 Vulnerability to fear and threat is evidenced by increased functional disorders with childhood adversity.Reference Roelofs and Pasman62,Reference Roelofs, Keijsers, Hoogduin, Naring and Moene63 Interestingly, three patients in our study with post-traumatic stress disorder had a clear history of developing a functional disorder after a traumatic event.
Somatosensory amplification
One of the functions of fear is to enhance sensory perception for survival.Reference Susskind, Lee, Cusi, Feiman, Grabski and Anderson64 Somatosensory amplification describes body hypervigilance and the tendency to focus on mild body sensations, which is a key step in leading to functional symptoms (Figure 4). It relates to modern health worries, expectations of symptoms or medication side effects.Reference Koteles and Witthoft65 Somatosensory amplification has been suggested in functional pulsatile tinnitus, where there is no underlying pathology but there is deficient noise-cancelling mechanismReference Song, Vanneste and De Ridder66 and impaired sensory gating.Reference Campbell, Bean and LaBrec67 Somatosensory catastrophising involves false attribution of bodily sensations to serious illness.Reference Seto and Nakao68 In our study, several patients reported increasing anxiety and severity of symptoms after internet searching. The term ‘cyberchondria’ has been coined to describe the unfounded escalation of concerns about common symptomology based on the review of search results and literature online.Reference White and Horvitz69
Functional otalgia and aural fullness
In our study, the symptoms of otalgia and aural fullness were reported in 22.8 per cent and 19.1 per cent of patients, respectively, who exhibited a functional disorder. Tinnitus and chronic pain have been demonstrated to share an overlapping brain network with common activation and connectivity patterns.Reference Vanneste, To and De Ridder70 Vagal nerve stimulation has been employed to neuromodulate brain circuits in a variety of functional disorders, including depression, chronic pain and migraine.Reference Howland71 The role of dysregulation of the autonomic nervous system and central pain pathways in the emergence of temporomandibular disorders has a growing evidence base.Reference Monaco, Cattaneo, Marci, Pietropaoli and Ortu27 The external ear canal has afferent vagal supply, via the auricular branch of the vagus nerve, to brain regions mediating multisensory integration. Autonomic nervous system dysfunction could have mechanistic overlap in aural fullness and otalgia because these symptoms are also associated with temporomandibular disorders.
Patient history
Functional symptoms can arise within the context of stress, pain, fatigue, injury, psychological trauma and sudden intense and overwhelming emotions.Reference Kozlowska56 In our study, 103 patients reported functional symptoms coinciding with increased stress, 14 patients during a bereavement and 23 patients during a period of significant sleep deprivation. Otological negative experience included prolonged or distressing loud noise, painful microsuction or ear syringing, ear trauma, ear surgery, childhood ear problems, and otalgia during flights or diving. Other experiences included physical illnesses, surgery and symptoms coinciding with a negative emotional reaction to starting medication because somatosensory amplification can arise from patient fear of drug side effects.Reference Koteles and Witthoft65
Management of functional ear symptoms
Functional disorders are at the intersection of many disciplines, and treatment options are listed in Table 1. Neurologists, neuro-vestibular physiotherapists and clinical psychologists have substantial experience in managing functional disorders, and working with multidisciplinary teams of otologists and audiologists is warranted for more challenging cases. Sound therapy has been shown to alter limbic and auditory networks,Reference Han, Pengfei, Chunli, Zhaodi, Xindi and Qian72 and neurostimulation has been increasingly explored for neuromodulating brain networks in refractory primary headache.Reference Vukovic Cvetkovic and Jensen73
Table 1. Treatment options for functional ear symptoms

• This is the first research article to address the incidence of functional ear symptoms in an otology clinic
• Functional neurological disorder is well established in neurology; however, it commonly presents to otology clinics
• Important co-morbidities include chronic illness, emotional stresses and adverse life experiences
• Physical or emotional experiences can be disproportionately assessed as threatening, leading to maladaptive neural signalling within brain networks
• Experience-driven neural activity, with disordered central sensory processing, forms the basis for functional symptoms
• Basic understanding of established neuroscientific principles for functional disorders are important for the ENT surgeon and help in patient education
Limitations of our study include single-centre data collection and retrospective review. Future work utilising validated questionnaires could formally assess risk factors including childhood adversity, stress and co-morbid conditions.
Conclusion
Functional disorders commonly present as the primary diagnosis to the otology clinic (34.6 per cent of new referrals). Two or more symptoms, including tinnitus, imbalance, otalgia and aural fullness often coincide (25.1 per cent), demonstrating the concept of multisensory processing at the level of the brain. Associated co-morbidity includes stress (30 per cent), chronic illness (22 per cent), chronic pain disorder (16 per cent) and depression (8.7 per cent). Physical and emotional events create experience, conditioned fear memory and can adversely enhance stress sensitivity (central sensitisation). Otological and non-otological experience drives aberrant neural activity and maladaptive brain connectivity, influencing stress responses (autonomic, endocrine and immune), memory, and cognitive and sensory processing. From this, we propose a brain-centred clinical model of adaptation of brain circuitry to experience for functional ear symptoms (Figure 4).
Stress exacerbates many chronic otological conditions, and this model may also provide insights into the chronic and recurring nature of ear symptoms seen after Ménière's disease, acute vestibular failure, autoimmune ear disease, sudden sensorineural hearing loss and ear surgery. Primary tinnitus also warrants consideration within this clinical model because it often occurs with other functional ear symptoms and has related past adverse experience, stress and co-morbidity that is additional to hearing loss and tinnitus-related distress. It is important to note that the majority of patients who present with clinically significant idiopathic sensorineural hearing loss do not report tinnitus, thus adding weight to the concept that functional factors may play a key role in symptom emergence.
Patient education, acceptance and appropriate support are crucial to avoid an otherwise repetitive cycle of clinic attendance, patient frustration, emotional distress and functional symptoms. Adjunctive use of medication may be warranted in managing associated significant psychiatric conditions, pain and migraine. Neuromodulation modalities to treat maladaptive brain circuit activity warrants further research and development to find effective long-term options.
Competing interests
None declared







