INTRODUCTION
The Rio Grande do Sul Coastal Plain is the geological province that best preserves evidence of climate changes along the Atlantic coast in southernmost Brazil during Quaternary glacial-interglacial cycles. Glacioeustatic sea-level cycles controlled deposition in coastal environments, forming four lagoon-barrier systems (LBS) in southern Brazil (Tomazelli, Reference Tomazelli1985; Villwock et al., Reference Villwock, Tomazelli, Loss, Dehnhardt, Horn, Bachi, Dehnhardt and Rabassa1986). These LBSs are high-frequency depositional sequences controlled by Quaternary glacioeustatic oscillations (Rosa et al., Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017) that were chronologically correlated to the calibrated δ18O curves of Imbrie et al. (Reference Imbrie, Hays, Martinson, McIntyre, Mix, Morley, Pisias, Prell, Shackleton, Berger, Imbrie, Hays, Kukla and Saltzman1984). Numerical ages obtained by thermoluminescence and optically stimulated luminescence in these deposits allowed chronocorrelation of LBS II to Pleistocene interglacial marine isotope stage (MIS) 7, LBS III to MIS 5, and LBS IV to Holocene interglacial MIS 1 (Buchmann et al., Reference Buchmann, Barbosa and Villwock1998; Buchmann and Tomazelli, Reference Buchmann and Tomazelli2003; Lopes et al., Reference Lopes, Dillenburg, Schultz, Ferigolo, Ribeiro, Pereira, Holanda, Pitana and Kerber2014, Reference Lopes, Pereira, Kinoshita, Mollemberg, Barbosa and Baffa2020). The abundant fossil record of mammals from the Lujanian South American land-mammal age in LBS III (ca. 33 ka; Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021) also constrains the timing of deposition to the late Pleistocene. The last glacial maximum (LGM) (e.g., Clark et al., Reference Clark, Dykejeremy, Shakunanders, Carlsonjorie, Wohlfarthjerry, Mitrovicasteven, Hostetlerand and Mccabe2009) peaked at ca. 20 ka, leading to a sea-level fall of ~120 m and temperatures between 9 and 6°C colder than today (e.g., Fairbanks, Reference Fairbanks1989; Peltier and Fairbanks, Reference Peltier and Fairbanks2006; Hansen et al., Reference Hansen, Sato, Russell and Kharecha2013). Although no putative record of this sea-level fall has been claimed yet for Rio Grande do Sul coastal plain, the best record resides in the loess deposits of the Cordão Formation (29–11.7 ka; Lopes et al., Reference Lopes, Dillenburg and Schultz2016). The Cordao Formation overlies LBS II (Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021) at the southern of PCRS and probably LBS III, considering its age (ca. 120 ka to ca. 33 ka; Rosa et al., Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017; Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021), its adjacent lateral distribution to the east of LBS II (Fig. 1B), and the ages of the Cordão Formation, deposited during the MIS 2.

Figure 1. Location map of studied area. (A) Position of studied area in South America. (B) Position of studied outcrops in Rio Grande do Sul Coastal Plain. (C) Distribution of geologic units in the area of the studied outcrops. (D) Detail of studied outcrops close to Osório city. Modified from Lopes et al. (Reference Lopes, Dillenburg, Schultz, Ferigolo, Ribeiro, Pereira, Holanda, Pitana and Kerber2014) and Ramos et al. (Reference Ramos, Netto and Sedorko2021).
Pleistocene deposits of the Rio Grande do Sul Coastal Plain have been extensively studied from sedimentological, stratigraphical, and paleontological approaches (e.g., Tomazelli and Villwock, Reference Tomazelli, Villwock, Holz and De Ros2000; Lopes et al., Reference Lopes, Dillenburg, Schultz, Ferigolo, Ribeiro, Pereira, Holanda, Pitana and Kerber2014, Reference Lopes, Dillenburg and Schultz2016, Reference Lopes, Pereira, Kinoshita, Mollemberg, Barbosa and Baffa2020, Reference Lopes, Dillenburg, Savian and Pereira2021; Rosa et al., Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017). In terms of ichnology, several studies highlighted the presence of trace fossils produced by marine and non-marine organisms on the deposits of LBSs II and III (Berqvist and Maciel, Reference Bergqvist and Maciel1994; Gibert et al., Reference Gibert, Netto, Tognoli and Grangeiro2006; Netto et al., Reference Netto, Tognoli, Gibert and Oliveira2007, Reference Netto, Curran, Belaustegui and Tognoli2017; Lopes and Pereira, Reference Lopes and Pereira2019; Freitas et al., Reference Freitas, Francischini, Tâmega, Spotorno-Oliveira and Dentzien-Dias2020; Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021; Ramos et al., Reference Ramos, Netto and Sedorko2021). Trace fossils are chiefly abundant on the LBS III deposits exposed in sand quarries in the Osório region (northern littoral of the Rio Grande do Sul State, Fig. 1). The most outstanding burrows in the marine deposits of LBS III correspond to the ichnogenus Ophiomorpha (Gibert et al., Reference Gibert, Netto, Tognoli and Grangeiro2006; Netto et al., Reference Netto, Curran, Belaustegui and Tognoli2017; Freitas et al., Reference Freitas, Francischini, Tâmega, Spotorno-Oliveira and Dentzien-Dias2020), a callianassid crustacean burrow commonly referred to as “Callichirus sp.” (e.g., Tomazelli, Reference Tomazelli1985; Tomazelli and Villwock, Reference Tomazelli, Villwock, Holz and De Ros2000). Cylindrichnus helix, Diplocraterion parallelum, Macaronichnus isp., and Rosselia socialis also occur (Gibert et al., Reference Gibert, Netto, Tognoli and Grangeiro2006; Netto et al., Reference Netto, Curran, Belaustegui and Tognoli2017; Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021).
Gibert et al. (Reference Gibert, Netto, Tognoli and Grangeiro2006) also reported an insect-dominated trace fossil assemblage in the overlying eolian facies composed mostly of the ichnogenera Krausichnus, ?Vondrichnus, cf. Celliforma, and root traces. Preliminary studies led by Netto et al. (Reference Netto, Tognoli, Gibert and Oliveira2007) on this assemblage recognized nests attributed to ants, termites, beetles, solitary wasps, and cicadas. Among the abovementioned insects, termites are the unique group that depends on cellulose and humidity in the substrate to construct the nest. Due to this dependence, termite nests are usually interpreted as an indicator of perennially vegetated paleosols, such as those of grasslands and dense forests (Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000). This led Netto et al. (Reference Netto, Tognoli, Gibert and Oliveira2007) to preliminarily infer more mature soil colonization in back-barrier alluvial settings developed over the eolian dunes, which would imply an abrupt sea-level fall and might represent a forced regression linked to the sea-level fall of the last glacial epoch. However, Ramos et al. (Reference Ramos, Netto and Sedorko2021) demonstrated that termite nests occur in the present-day eolian frontal dunes (LBS IV, Holocene to present) in different latitudes throughout the Rio Grande do Sul Coastal Plain. Thus, termite nests might be present in immature soils formed in the coastal eolian deposits of LBS III, not necessarily representing a signature of continental settings.
Insect burrows usually exhibit specific morphologies due to the high speciation capacity of the group, which facilitates identification of the edaphic paleofauna preserved in the geological record, especially after the Cretaceous (Genise, Reference Genise1995). Climate and environmental conditions play an important role in the biogeographic distribution of many insect groups, particularly termites, bees, and wasps, making their nests good indicators of paleoenvironmental and paleoclimatic conditions (e.g., Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000; Genise, Reference Genise2017; Sánchez et al., Reference Sánchez, Bellosi, Genise, Kramarz and Sarzetti2021). Considering the remarkable presence of insect trace fossils and rhizoliths in the eolian deposits of LBS III in the Osório region, this study aims to (1) characterize these trace fossil suites, (2) use them as relative proxies to infer the climate regimes that controlled LBS III sedimentation, and (3) better understand the climate changes that controlled the study area coastal dynamics that culminated in the LGM.
GEOLOGICAL SETTING
The Rio Grande do Sul Coastal Plain extends for ~620 km and covers an area of ~33,000 km2 (Fig. 1A, B). It consists of two types of depositional siliciclastic systems—alluvial fan systems and lagoon-barrier systems (Tomazelli and Villwock, Reference Tomazelli, Villwock, Holz and De Ros2000). The lagoon-barrier systems characterize ancient coastlines and represent fourth-order depositional sequences formed by successive glacioeustatic-controlled sea-level oscillations during the Pleistocene and Holocene (Rosa et al., Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017). The oldest sequence characterizes lagoon-barrier system (LBS) I, and the youngest characterizes LBS IV, which comprises the present-day coastline (Tomazelli and Villwock, Reference Tomazelli, Villwock, Holz and De Ros2000). Except for the last, all others represent the maximums of the postglacial marine transgressions that occurred during the Pleistocene (Villwock and Tomazelli, Reference Villwock and Tomazelli1995; Rosa et al., Reference Rosa, Barboza, Dillenburg, Tomazelli and Ayup-Zouain2011, Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017).
LBS I was established ca. 400 ka and configured the oldest coastal deposits in the Rio Grande do Sul Coastal Plain. Three main transgressive-regressive events that took place on the South Atlantic coast formed LBS II–IV. The timing of LBS III was correlated to Marine Isotope Stage (MIS) 5 of Imbrie et al. (Reference Imbrie, Hays, Martinson, McIntyre, Mix, Morley, Pisias, Prell, Shackleton, Berger, Imbrie, Hays, Kukla and Saltzman1984) and dated as 120 ka (Villwock and Tomazelli, Reference Villwock and Tomazelli1995; Rosa et al., Reference Rosa, Barboza, Dillenburg, Tomazelli and Ayup-Zouain2011). Thus, this system has been linked to the last transgressive peak of the Pleistocene (Cananéia Transgression; Suguio and Martin, Reference Suguio and Martin1978). LBS IV developed during the Holocene, ca. 7 ka due to the last great post-glacial transgression when sea level rose 4–5 m above the current level (Dillenburg et al., Reference Dillenburg, Roy, Cowell and Tomazelli2000; Angulo et al., Reference Angulo, Lessa and de Souza2006; Rosa et al., Reference Rosa, Barboza, Dillenburg, Tomazelli and Ayup-Zouain2011; Santos et al., Reference Santos, Lavina, Paim, Tatumic, Yeec, Santos and Kern2022). Regarding modern climatic patterns in the Rio Grande do Sul Coastal Plain, temperatures vary during the year from a minimum average of 7.2°C in July (Southern Hemisphere winter) to a maximum average of 31.7°C in January (Southern Hemisphere summer). Rain is well distributed, varying between 74 mm in the driest month and 164 mm in the rainiest month (see Ramos et al., Reference Ramos, Netto and Sedorko2021). Wind speeds are constant throughout the year in the entire coastal plain, with average annual winds between 7.0 and 8.0 m/s at 50 m height (Amarante and Silva, Reference Amarante and Silva2002). Tide is semi-diurnal and has a mean range of 0.5 m (Dillenburg et al., Reference Dillenburg, Barboza, Tomazelli, Hesp, Clerot, Ayup-Zouain, Dillenburg and Hesp2009). Meteorological tides derived by storms can reach up to 1.9 m for short periods (Parise et al., Reference Parise, Calliari and Krusche2009).
Deposits of LBS III can be subdivided into a shallow marine and an eolian facies association (e.g., Tomazelli et al., Reference Tomazelli, Dillenburg and Villwock2000; Gibert et al., Reference Gibert, Netto, Tognoli and Grangeiro2006, Tomazelli and Dillenburg, Reference Tomazelli and Dillenburg2007). The shallow marine deposits characterize the lower strata in the studied quarries. They are composed of fine- to medium-grained quartz sand with low-angle cross-lamination, wave-ripple cross-lamination, and planar cross-stratification (interval 0–3 m at Transareia Quarry and interval 0–5.5 m at Gomes Quarry; Fig. 2A, B). Arenicolites isp., Cylindrichnus helix, Diplocraterion isp., Macaronichnus segregatis, Ophiomorpha nodosa, Ophiomorpha puerilis, and Rosselia socialis occur in these lower beds, suggesting deposition in shoreface settings (Gibert et al., Reference Gibert, Netto, Tognoli and Grangeiro2006; Netto et al., Reference Netto, Curran, Belaustegui and Tognoli2017). The eolian facies association comprises ~5 m of well-sorted fine-grained quartz sand with parallel lamination interbedded with planar cross-stratification intercalated with several muddy sand horizons each of which is a few centimeters thick. Two strongly weathered horizons consisting of reddish fine- to medium-grained beds characterize ortsteins and occur in the eolian deposits (Fig. 2A, B), from which rhizoliths and predominantly insect nest burrows descend. This eolian ichnoassemblage is the focus of this work and will be discussed here in detail. Due to the proximity among the prospected quarries (Fig. 1), we assume that these pedogenic horizons are correlative and used them to understand the vertical stacking of trace fossils.

Figure 2. Geologic sections and trace fossils of studied outcrops. (A, B) Geologic sections of Transareia (A) and Gomes (B) quarries. (C) General view of Gomes Quarry with eolian facies overlying marine facies (contact between facies indicated by the yellow line; dashed yellow line indicates sand-covered contact between facies). O1 and O2 surfaces correspond to the pedogenic horizons found in the eolian deposits. Adapted from Gibert et al. (Reference Gibert, Netto, Tognoli and Grangeiro2006).
MATERIAL AND METHODS
The study locale is located in the municipality of Osório (the Rio Grande do Sul State, southernmost Brazil), ~100 km east of Porto Alegre, on two adjacent outcrops in sand quarries, Gomes (29°90′51″S, 50°23′97″W) and Transareia (29°90′06″S, 50°23′06″W) (Figs. 1, 2A, B). The sedimentological analysis considered texture, composition, bed geometry, and primary sedimentary structures and follows Gibert et al. (Reference Gibert, Netto, Tognoli and Grangeiro2006). The morphology of paleosol profiles was described in the field following the nomenclature and procedures from Schoeneberger et al. (Reference Schoeneberger, Wysocki, Benham and Broderson2001) and Retallack (Reference Retallack2001). The following morphological attributes were described: soil horizon (types and boundaries), soil structure, soil color, bioturbation features (types, patterns, and abundance), and density of trace fossils.
Insect trace fossils are good indicators of climate conditions and are useful for local microclimate determination (e.g., Ekdale et al., Reference Ekdale, Bromley, Loope and Miller2007; Buatois and Mángano, Reference Buatois and Mángano2011; Genise, Reference Genise2017). The body temperature of insects fluctuates with the temperature of their surrounding environment. Thus, insect nesting activity is controlled by regional climate and local microclimate (e.g., Grassé, Reference Grassé1986, Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000, Reference Genise, Bellosi, Gonzalez and McIlroy2004; Duringer et al., Reference Duringer, Schuster, Genise, Mackaye, Vignaud and Brunet2007). The trace fossil analysis considered structures preserved in the eolian facies of LBS III only and followed the ichnotaxobases approach (Bromley, Reference Bromley1996, Bertling et al., Reference Bertling, Braddy, Bromley, Demathieu, Genise, Mikuláš and Nielsen2006). The ichnotaxonomical identification followed Genise (Reference Genise2017). However, many structures were loose on the substrate due to the present-day wind action that erodes the unlithified deposits of the LBS III. Thus, the original orientation of many burrows with respect to the original soil surface could not be determined.
All the studied trace fossils occur exclusively in the Upper Pleistocene eolian deposits of LBS III that are exposed in the Transareia and Gomes quarries. The 178 collected specimens are housed in the paleontological collection of the Museu da História Natural do Rio Grande do Sul (MHGEO) at Unisinos University, under the numbers ULVG-13129 to ULVG-13349.
RESULTS
The ichnotaxonomy of the trace fossils present in the LBS III eolian deposits is characterized in the Supplemental Material. The ichnoassemblage shows a moderate to high ichnodiversity and a high abundance of burrows. Two trace fossil suites, Vondrichnus and Celliforma, were identified in the eolian facies.
The Vondrichnus suite is characterized by the dominance of spherical to ovoid structures, locally with interconnections and generally presenting walls with reddish color compared to the host rock, which are attributed to Vondrichnus obovatus (Fig. 3A–C). Dense galleries with primary and secondary order, identified as Termitichnus isp. (Fig. 3D), also occur in this suite. These structures are interbedded to locally dense intervals with rhizoliths. Isolated cells attributed to Celliforma isp. (Fig. 3E) and simple tunnels (Fig. 3F) that may be part of the V. obovatus connections occur subordinately. This suite is characterized by moderate burrow density and, although ichnodiversity is low, the tiering structure is complete, revealing the varied depth of colonization by different groups of insects. The burrows in this suite descend from the pedogenic horizon O1 (Fig. 2). Abundant rhizoconcretions and root molds occur mainly in Bhm horizons, with circular to subcircular forms varying from 0.5 to 2 cm in diameter. They are cemented by iron and show an average length of 20 cm, with dichotomous and herringbone patterns.
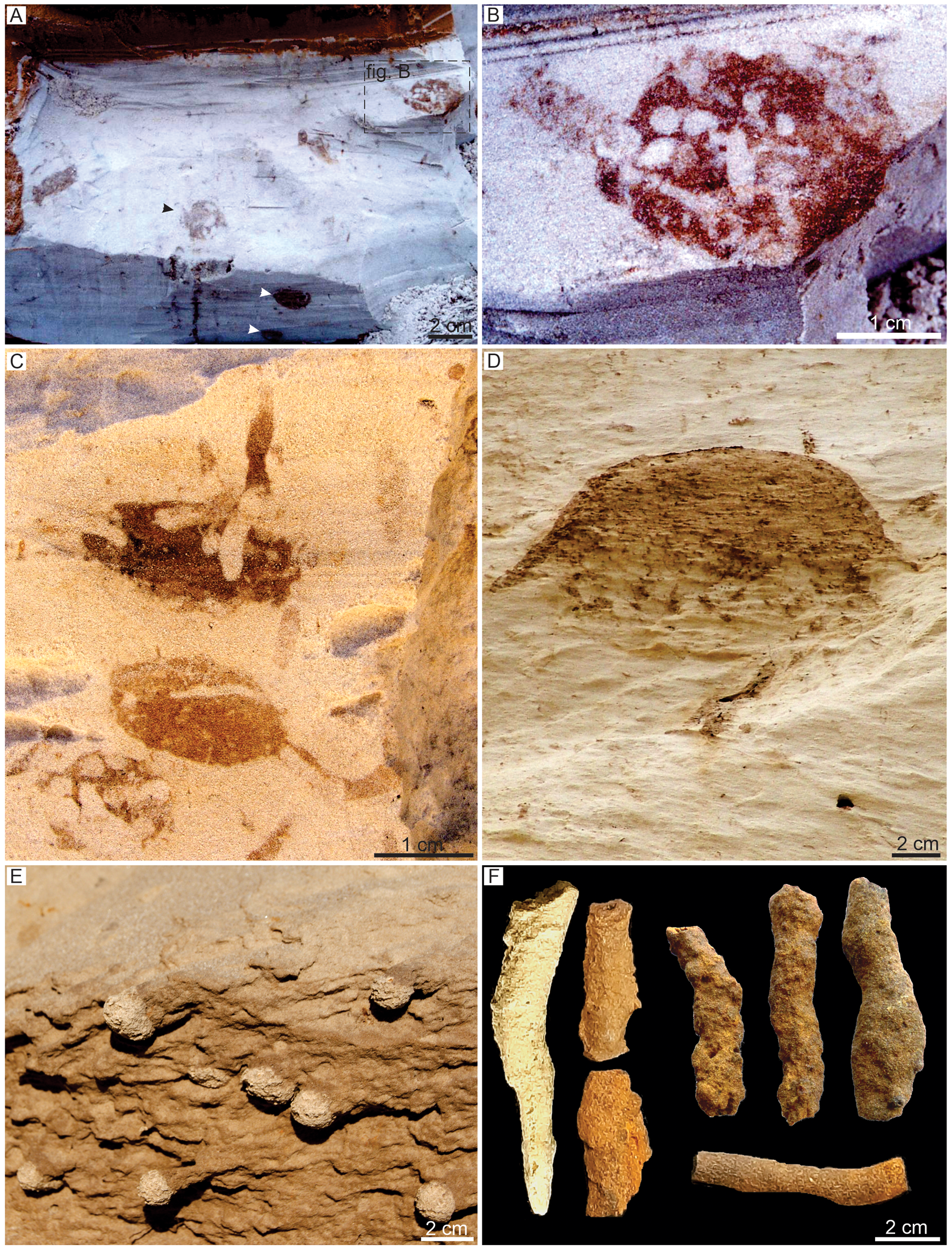
Figure 3. In situ trace fossils from studied sections. (A–C) Krausichnidae: Vondrichnus obovatus; note the wall lined by oxides with sharp contact with surround substrate, the disposition in different planes (black and white arrows in A), and the alveolar filling of the structure (B, C) (D) Termitichnus isp. with a wall lined by oxides with sharp contact with surround substrate. (E) Celliformidae: Celliforma isp. showing the isolated cells. (F) Simple tunnels with walls lined by oxides.
The Celliforma suite is dominated by several morphologies of isolated cells, identified as Celliforma isp. (Fig. 4A), C. curvata (Fig. 4B, C), C. rosellii (Fig. 4D, E), and C. spirifer (Fig. 4F–H), and large, condensed burrows attributed to Krausichnus isp. (Fig. 5A, B). Rows of cells identified as Uruguay isp. (Fig. 4I–K), chambers attributed to Rebuffoichnus casamiquelai (Fig. 4L, M) and Teisseirei isp. (Fig. 4N), and less-common spherical to ovoid structures attributed to V. obovatus occur as secondary structures. Trace fossils occur in moderate to high density, forming pavements of Celliforma and simple tunnels. This suite is more diverse than the Vondrichnus suite and has a complete tiering structure, revealing the varied depth of colonization by different insect groups. These insect burrow-rich intervals are interbedded with locally dense intervals with rhizoliths (Fig. 5) below the pedogenic horizon O2 (Fig. 5C) from where the trace fossils descend.

Figure 4. Morphological variation of Celliformidae and Coprinisphaeridae specimens. (A–K) Celliformidae: (A) Celliforma isp. with a rounded base and a flat top with a constriction near the top. (B, C) Elongated specimens with curved necks, attributed to C. curvata. (D, E) Celliforma rosellii with rounded bottom end and rough microstructure walls. (F–H) Celliforma spirifer cells with a central stem resembling an eroded spiral closure. (I–K) Clustered cells attributed to Uruguay isp. (L–N’) Coprinisphaeridae: (L–M) Morphological aspects of Rebuffoichnus casamiquelai, characterized by oblate chambers with rough external wall, that may preserve a terminal circular aperture (L, L’). (N, N’) Morphological aspects of Teisseirei. Scale bars = 5 mm.

Figure 5. In situ trace fossils from studied sections. (A, B) Krausichnidae: Krausichnus isp. exhibiting ovoid (A) and conical (B) morphologies. (C) Rhizoliths lined by oxides. (D) General view of O1 surface with rhizoliths.
DISCUSSION
Trace fossil suites
The trace fossil suites represent insect activity, which implies subaerial exposure and substrate quiescence that allowed colonization by this biologic group (Genise, Reference Genise2017). These insect populations colonized unlithified, fine-grained sandy soils formed in stabilized coastal eolian dunes. The lower eolian deposits in LBS III (below OS1) were colonized chiefly by termites, while bee and wasp nests dominate in the upper eolian deposits (above OS1 and below OS2).
Insect trace fossils are ecologically controlled, with climate, soil, and vegetation playing a more relevant role in substrate colonization than depositional processes (Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000). Climate also influences the biogeographical distribution of ectotherm organisms, such as insects. Termites and hymenopteran insects (bees and wasps) have been used in the ichnological literature as key indicators of substrate moisture, the former suggesting more humid conditions and the latter drier conditions (e.g., Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000, Reference Genise, Bellosi, Gonzalez and McIlroy2004, Reference Genise, Alonso-Zarza, Krause, Sánchez, Sarzetti, Farina, González, Cosarinsky and Bellosi2010; Genise, Reference Genise2017; Sánchez et al., Reference Sánchez, Bellosi, Genise, Kramarz and Sarzetti2021). The dominance of termite structures has been interpreted as colonization in more humid conditions than those that characterize the Celliforma-dominated suites (Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000; Duringer et al., Reference Duringer, Schuster, Genise, Mackaye, Vignaud and Brunet2007). Thus, the shift of these two trace fossil suites in paleosols might be a good indicator of climatic dynamics.
It has been postulated that trace fossil suites dominated by termites indicate forested, often humid substrates and warm climates in the scope of the Termitichnus ichnofacies (e.g., Grassé, Reference Grassé1986; Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000, Reference Genise, Bellosi, Gonzalez and McIlroy2004, Reference Genise, Alonso-Zarza, Krause, Sánchez, Sarzetti, Farina, González, Cosarinsky and Bellosi2010; Buatois and Mángano, Reference Buatois and Mángano2011). However, termite nests are also abundant in settings other than forests in tropical latitudes, including wet and arid lowlands such as the Pantanal wetlands (central-west Brazil), the border of the Namib Desert (Namibia), and the sub-tropical Pampa grasslands in the Rio Grande do Sul State. Although not common, they occur in the restinga-vegetated frontal dunes in the active backshore zone of LBS IV where water sources are available (Ramos et al., Reference Ramos, Netto and Sedorko2021). Termites depend on humidity and vegetation to survive; thus, nesting in arid settings requires ecological adaptations such as the excavation of horizontal foraging tunnel systems close to the soil surface to collect plant fragments and vertical tunnels connecting the nest to moist soil at great depths, in addition to nocturnality (Tschinkel, Reference Tschinkel2010). However, the architecture of these nests differs completely from those found in the eolian beds of LBS III.
While termite nests shelter a colony that cares for their larvae, most ground-nesting bees and wasps do not care for their offspring after sealing the provision-rich brood cells (Antoine and Forrest, Reference Antoine and Forrest2021). Thus, the females must find a high-quality substrate for nest construction to guarantee larval survival. Protection against harsh weather conditions and excessive humidity is imperative for larvae development (Roulston and Goodell, Reference Roulston and Goodell2011). Unlike termites, adult bees can thermoregulate, allowing some ground-nesting species (e.g., Bombus polaris) to adapt to low temperatures.
Ants are adapted to various habitat types and can rapidly excavate a new nest to move their colonies according to soil moisture changes (e.g., Lamb et al., Reference Lamb, McCombe, Lawrence, Macwan, Mayer and Jandt2020). Ant mounds were found in Holocene outlet glaciers in southern Norway (Hill et al., Reference Hill, Vater, Geary and Matthews2018), proving they can survive in cold climates and adverse soil conditions. Ground-nesting beetles are also widely distributed throughout the globe (Genise, Reference Genise2017) and are mainly controlled by temperature (Ernst and Buddle, Reference Ernst and Buddle2015). Although their richness decreases towards high latitudes, they occur in high arctic locations (Ernst and Buddle, Reference Ernst and Buddle2015). Thus, ant and beetle structures are not good indicators of climate-related changes in soil abiotic conditions.
The predominance of termite nests in the Vondrichnus suite, together with the restricted presence of bee cells (e.g., Celliforma) and the absence of ant structures, suggests the prevalence of more humid and warmer conditions during colonization of the paleosols capped by OS1. In contrast, the abundance of hymenopteran (mostly bee structures, mainly represented by C. curvata) in the Celliforma suite indicates drier climate conditions during colonization of the podzols below OS2 (e.g., Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000; Duringer et al., Reference Duringer, Schuster, Genise, Mackaye, Vignaud and Brunet2007).
The local abundance of rhizoliths in these paleosols suggests periods of relatively high water tables (Hesp, Reference Hesp1991; Bonilha et al., Reference Bonilha, Casagrande, Soares and Reis-Duarte2012; Marques et al., Reference Marques, Silva and Liebsch2015). The root decay provides organic matter for soil development and is an important food source for soil macrofauna. The thick, cemented horizon observed on the O2 surface represents a longer time hiatus for weathering (compared to the thin horizon observed on the O1 surface), allowing establishment of a Celliforma suite with a higher ichnodiversity than the Vondrichnus suite.
The subordinate presence of termite and ant nests in the Celliforma suite and the absence of calcareous soils preclude a precise attribution of this suite to the Celliforma ichnofacies (e.g., Buatois and Mángano, Reference Buatois and Mángano2011; Genise, Reference Genise2017). The Vondrichnus suite might be an expression of Coprinisphaera ichnofacies (e.g., Genise et al., Reference Genise, Mángano, Buatois, Laza and Verde2000), but the restricted occurrence of coprinisphaerid structures also precludes this attribution.
Paleoclimatic and stratigraphic implications
Netto et al. (Reference Netto, Tognoli, Gibert and Oliveira2007) preliminarily inferred that the insect trace fossil assemblage preserved in LBS III aeolian strata represents soil colonization in alluvial plains formed by global sea-level lowering of ~120 m during the last glacial period. This inference considered the abundance of termite nests, chiefly Vondrichnus, in these beds and the environmental distribution of the insect-dominated ichnofaunas, as expressed by Genise et al. (Reference Genise, Mángano, Buatois, Laza and Verde2000). However, Termitichnus-like termite nests occur in eolian dunes that formed very close to the marine shoreline in LBS IV (the present-day active beach; Fig. 1), indicating that termites can colonize incipient soils in backshore settings (Ramos et al., Reference Ramos, Netto and Sedorko2021). Although not abundant, several closely spaced nests may occur in sparsely vegetated areas with nearby water sources. Thus, termite nests are not exclusive of continental settings and can be found in frontal eolian dunes in the Rio Grande do Sul Coastal Plain.
Nonetheless, the abundance and diversity of termite nests were higher in the LBS III eolian deposits than in those of LBS IV, suggesting a warmer and humid climate than during colder and drier intervals of the last glacial epoch, and more hospitable ecological conditions during soil colonization than today. Palynological and isotopic data indicate that climate conditions during this interglacial interval were wetter than today in southern Brazil (e.g., Clapperton, Reference Clapperton1993; Ledru et al., Reference Ledru, Braga, Soubiès, Fournier, Martin, Suguio and Turcq1996; Behling, Reference Behling2002; Leite et al., Reference Leite, Costa, Loss, Rocha, Batalha-Filho, Bastos and Quaresma2016). Thus, the Vondrichnus suite may be a good indicator of soil colonization by termites during periods of humid conditions.
On the other hand, the Celliforma suite indicates the dominance of drier conditions than represented by the Vondrichnus suite. Flooding events or rainfall periods are not suitable for emplacement of bees and dung beetles in the substrates because excessive moisture can generate poor oxygen diffusion, resulting in death; flooding events or rainfall periods also increase liquefaction of the substrate and/or decay by fungal attack (Genise, Reference Genise2017). Thus, these tracemakers generally prefer well-drained substrates for colonization; in fact, the diversity and abundance of bees are better registered in warm-temperate xeric regions worldwide (Stephen et al., Reference Stephen, Bohart and Torchio1969; Michener, Reference Michener2007). Additionally, bee cells are lined with water-repellent lipids to maintain the moisture conditions, allowing short-term survival from occasional floods (Michener, Reference Michener2007). However, the termite structure Vondrichnus is a subordinate trace in the Celliforma suite, occurring below the O2 surface (Fig. 5C), suggesting seasonal climate oscillation during the colonization window.
Deposits of LBS III were formed during the global transgressive event that reached a maximum ca. 120 ka and represent active coastal sedimentation during the interglacial epoch that preceded the last glacial maximum (LGM) (e.g., Tomazelli and Villwock, Reference Tomazelli, Villwock, Holz and De Ros2000; Rosa et al., Reference Rosa, Barboza, Dillenburg, Tomazelli and Ayup-Zouain2011). The climate during the late Pleistocene was marked by a glacial cycle; almost 30% of the globe was covered by ice, forming ice sheets that could extend up to 40°N latitude (e.g., Ehlers and Gibbard, Reference Ehlers and Gibbard2008). Due to the smaller extent of continental masses compared to the Northern Hemisphere, the record of ice sheets in South America is restricted to the Andes and the southernmost areas of Patagonia (e.g., Hulton et al., Reference Hulton, Purves, McCulloch, Sugden and Bentley2002; Ehlers and Gibbard, Reference Ehlers and Gibbard2008).
The drier conditions that preceded the LGM allowed development of extensive eolian dune fields in the Pampa and Chaco regions (mid-latitudinal South America), spreading out to the southeast of the Rio Grande do Sul, forming vast loess deposits (Clapperton, Reference Clapperton1993). These deposits characterize the Cordão Formation, which overlies LBS II deposits in the southern portion of the Rio Grande do Sul Coastal Plain and correlates with Marine Isotope Stage (MIS) 2 (Lopes et al., Reference Lopes, Dillenburg, Savian and Pereira2021). Although the Cordão Formation does not occur in the study area, its age and position in the Rio Grande do Sul Coastal Plain stratigraphic framework indicate that they also overlay LBS III deposits (Fig. 1), being removed by weathering or erosion. Thus, the orsteins formed in the eolian deposits of LBS III might represent phreatic oscillation due to a shift in humid-dry climate conditions during the last Pleistocene interglacial stage, with a prevalence of drier conditions towards the LGM.
Diets inferred from δ13C in fossil teeth from extinct mammalian herbivores in chrono-correlated deposits (late Pleistocene) indicate that they fed on a mixture of C3 (herbs, shrubs, and/or trees) and C4 (grasses) plants, which suggests open grasslands with sparse trees and shrubs, very similar to the modern local subenvironments (e.g., Oliveira, Reference Oliveira, Rabassa and Salemme1999; Lopes et al., Reference Lopes, Ribeiro, Dillenburg and Schultz2013). Based on late Quaternary palynological data, Behling (Reference Behling2002) concluded that the areas occupied by the Atlantic rainforest were significantly reduced in southernmost Brazil during the glacial periods and replaced by cold-adapted forest taxa or grassland. Grasslands dominated the landscape during the late Pleistocene in southern Brazil, even in the highlands; climates were markedly drier and temperatures 5–7°C cooler than today (Behling, Reference Behling2002). According to Tomazelli et al. (Reference Tomazelli, Dillenburg and Villwock2000), the cyclic alternation of humid and arid conditions, and possibly shorter oscillations, controlled sedimentation during the Quaternary in the Rio Grande do Sul Coastal Plain (e.g., Rodrigues et al., Reference Rodrigues, Giannini, Fornari and Sawakuchi2020; Leal et al., Reference Leal, Barboza and Bitencourt2022). Calcareous and ferruginous pedogenic concretions occur in the lagoon deposits of LBS I, II, and III, locally in high concentration, forming Ca-rich layers up to 1 m thick. These deposits led Tomazelli et al. (Reference Tomazelli, Dillenburg and Villwock2000) to conclude that alternations between wet and dry periods prevailed in the region during the Pleistocene.
Quaternary eolian deposits from the western Mediterranean, for example, also accumulated during lower sea-level periods characterized by colder and arid conditions, whereas pedogenic processes are inferred to be more significant during warm-wet climates linked to interglacial periods (Fornós et al., Reference Fornós, Clemmensen, Gómez-Pujol and Murray2009; Muhs et al., Reference Muhs, Budahn, Avila, Skipp, Freeman and Patterson2010; del Valle et al., Reference del Valle, Genise, Pons, Pomar, Vicens and Fornós2020). In this sense, the recurrence of horizons colonized by insect trace fossils observed in the studied area, limited by paleosol surfaces and the replacement of a Vondrichnus suite by a Celliforma suite, signals wet and dry periods, common in the late Pleistocene, but with increasing drier conditions towards the top.
In terms of stratigraphy, the LBSs represent high-frequency (fourth-order) depositional sequences generated by sea-level highstands, controlled by Pleistocene–Holocene glacioeustatic oscillations (e.g., Rosa et al., Reference Rosa, Barboza, Dillenburg, Tomazelli and Ayup-Zouain2011, Reference Rosa, Barboza, Abreu, Tomazelli and Dillenburg2017), forming a prograding trend. Trace fossils also reflect this trend in the LBS III deposits exposed in the Transareia and Gomes quarries. According to Gibert et al. (Reference Gibert, Netto, Tognoli and Grangeiro2006), trace fossils indicative of shoreface (e.g., Diplocraterion, Cylindrichnus, Ophiomorpha, Rosselia) shift upward to monotypic colonization in foreshore (Macaronichnus) in the marine facies association. The marine paleoichnocoenosis is abruptly replaced by continental suites in the eolian facies association, and above, the Vondrichnus suite gives way upward to the Celliforma suite. The establishment of the Celliforma suite at the top of these deposits may indicate a regressive phase in the LBS III, linked to drier climate conditions during the late Upper Pleistocene.
Thus, the presence of termite nests overlying marine deposits does not imply a significant sea-level fall for the LBS III eolian deposits because this suite can occur in coastal environments, as reported from the Rio Grande do Sul Coastal Plain in the present day (e.g., Ramos et al., Reference Ramos, Netto and Sedorko2021). In terms of colonization pattern, the lower eolian interval was only colonized during more humid conditions, resulting in the dominance of termite structures (Vondrichnus suite) and rhizoliths descending from the O1 surface (Figs. 5D, 6). The second eolian interval also indicates dominance of drier conditions during deposition (Fig. 6). However, the O2 surface (Figs. 2, 6) represents less humid conditions than the lower period, expressed by the dominance of the Celliforma suite and very rare specimens of Vondrichnus. Therefore, the O1 surface associated with the Vondrichnus suite can be attributed to a humid period during the late Pleistocene (e.g., Clapperton, Reference Clapperton1993). Similarly, humid and warmer conditions were assumed for the Pliocene–Pleistocene strata of the Buenos Aires Province coastal plain (latitudes 38°16′17″–38°00′08″S), based on the occurrence of termite nests (Genise, Reference Genise1997; Laza, Reference Laza2006). In this sense, the O1 surface with termite and other insect nests suggests a seasonal climate, with marked rainy and dry seasons (Fig. 7). The O2 surface associated with the Celliforma suite is linked to drier conditions in the late Pleistocene, possibly because of the reduction in temperature and precipitation (Fig. 7). Stepped warming starting at ca. 17 ka led to deglaciation, causing an abrupt sea-level rise between 15–14 ka (Hulton et al., Reference Hulton, Purves, McCulloch, Sugden and Bentley2002).

Figure 6. Inferred colonization sequence of trace fossils in LBS III deposits. Note that the sequence of climatic variation was similar in both suites (i.e., increase in moisture and organic matter followed by subsequent decreases of both), but the Celliforma suite indicates drier conditions than the Vondrichnus suite.

Figure 7. Distribution of trace fossils in a composite section from LBS III deposits. The regressive trend of these deposits evinces a shallow marine setting (Skolithos ichnofacies) overlapped by a Vondrichnus suite representing more humid conditions (O1 surface), and finally superimposed by a Celliforma suite representing drier conditions (O2 surface). This replacement in trace fossil suite is concomitant with the general reduction in average temperature (red line). *Temperature deviation adapted from Ellis and Palmer (Reference Ellis and Palmer2016).
CONCLUSIONS
The paleoichnocoenosis present in the eolian deposits of LBS III associated with laterally extensive horizons with root traces and forming ortsteins are diagnostic of soil formation in eolian dunes in the Rio Grande do Sul Coastal Plain during the late Pleistocene. The prevalence of callichnia burrows indicates substrate stabilization by vegetation and immature soil formation, possibly triggered by periods of higher water table in wetter climate conditions. The presence of the Vondrichnus and Celliforma suites indicates a variation between humid and dry climates throughout LBS III sedimentation. These suites reinforces the hypothesis that a relatively dry climate controlled the coastal dynamics in the Rio Grande do Sul Coastal Plain during the last glacial cycle. The prevalence of the Celliforma suite at the top of the eolian deposits indicates the intensification of a drier climate, possibly due to late Pleistocene cooling.
Thus, the Celliforma suite indicates drier conditions, while the Vondrichnus suite indicates humid conditions for the studied sections. The Vondrichnus suite is interpreted to be a common assemblage in coastal settings of the study area, with its distribution controlled by biologic aspects of pioneer colonizers.
Acknowledgments
The authors thank the Brazilian Council for Scientific and Technological Development (CNPq) for the research grant 424237/2018-0 that supported this research. KSR thanks CNPq for the Master of Science fellowship (grant 130470/2019-8) and FAPERGS/CAPES Consortium (Edital 03/2018 - PRÓ-EQUIPAMENTOS; grant 18/2551-0000429-4), which provided logistical support for this work. RGN thanks CNPq for research fellow grants 303863/2016-1 and 310377/2019-6. DS thanks CNPq for the post-doctoral fellowship (grant 159548/2018-7). DLN thanks CNPq for the Doctoral fellowship (grant 140807/2017-9). This paper is a contribution to the BRASILEX Project from Unisinos University.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2022.55










