Glycaemic index (GI) is an approach to classify carbohydrate foods by comparing the glycaemic effect of carbohydrate weight in individual foods(Reference Jenkins, Wolever and Taylor1). In a meta-analysis of randomised controlled trials, diets with a lower GI were associated with a modest improvement in HbA1c in individuals with diabetes(Reference Brand-Miller, Hayne and Petocz2).
Postprandial hyperglycaemia is not only associated with impaired glucose tolerance (IGT) but also with hyperlipidaemia and oxidative stress, both of which increase the risk of CVD(3, Reference Nakagami, Qiao and Tuomilehto4). There is, however, considerable debate regarding the optimum and specific diet composition for preventing postprandial hyperglycaemia(5).
In Japan, many naturally viscous foods such as some types of potatoes, vegetables, mushrooms and seaweeds are cooked and eaten. They contain viscous polysaccharides such as mannan, pectin, alginic acid and galactan. Natto, a traditional and popular Japanese food made by fermenting boiled soyabeans with Bacillus natto (Reference Sumi, Hamada and Tsushima6), has viscous properties. We have previously demonstrated that the consumption of natto and viscous vegetables combined with white rice (WR) suppressed postprandial hyperglycaemia and hyperinsulinaemia in healthy young subjects(Reference Taniguchi, Yamanaka-Okumura and Nishida7).
It is known that guar suppresses postprandial glucose and insulin. Ebeling et al. (Reference Ebeling, Yki-Järvinen and Aro8) found that this effect is amplified with repeated dosing for 4 weeks in type 1 diabetes. Moreover, as higher insulin secretion occurs in the morning because of which insulin sensitivity is lower than that in the afternoon(Reference Zimmet, Wall and Rome9, Reference Lindgren, Mari and Deacon10), we decided to assess the repeated effect of a breakfast of natto and viscous vegetables on glycaemic control and insulin sensitivity. We chose to study a single-meal intervention over multiple meals in order to ensure higher compliance, and thus increase the accuracy of the findings.
Although IGT is a major risk factor for type 2 diabetes mellitus, adjustments in lifestyle are likely to be the most effective way to prevent or delay the onset of type 2 diabetes mellitus. However, to date, most studies examining the effect of anti-hyperglycaemic diet on blood glucose, lipids and body composition have been conducted in diabetic subjects(Reference Rizkalla, Taghrid and Laromiguiere11, Reference Jenkins, Kendall and McKeown-Eyssen12).
Therefore, the aim of the present study was to investigate whether natto and viscous vegetables in addition to WR suppress postprandial glucose and insulin responses in overweight subjects with IGT, and whether incorporating natto and viscous vegetables into a Japanese breakfast for 2 weeks would have a favourable effect on glucose utilisation, insulin sensitivity, lipid metabolism and oxidative stress.
Subjects and methods
Subjects
A total of eleven free-living overweight subjects with IGT and hyperinsulinaemia were recruited for the present study (Table 1). Diagnosis of IGT was made on the basis of the results of the 75 g oral glucose tolerance test (OGTT) during screening. The WHO criteria of 2 h postprandial blood glucose levels between 7·8 and 11·0 mmol/l was used to define IGT. A total of fourteen volunteers were recruited. On screening using OGTT, three were excluded because their insulin secretion was insufficient, and thus they did not have hyperinsulinaemia. Subjects were not treated with dietary or exercise therapy or with any type of medication.
Table 1 Characteristics of the subjects
(Mean values with their standard errors, seven males and four females)
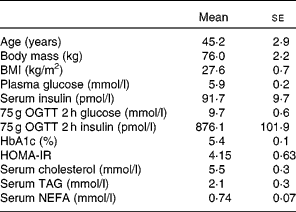
OGTT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the Faculty of Medicine, Tokushima University Hospital, Tokushima, Japan. Written informed consent was obtained from all subjects.
Meal
Reference food (white rice)
Aseptically packed Satou WR (200 g) was prepared as the reference food in the acute study. A measure of 200 g was chosen as it is the mean of the recommended amount suggested for the Japanese population.
Test meal
The test meal consisted of combined WR with viscous natto, yams and okra. The viscous substance of natto is a polyglutamic acid associated with polysaccharide. Japanese yams (Dioscorea japonica Thunb) and okra (Abelmoschus esculentus (L.) moench) are popular vegetables with a slimy consistency and also contain viscous polysaccharides such as mannan, pectin and galactan. The viscosity of natto, Japanese yams and okra increases like a slice of gooey cheese pizza when they are grated, cut and stirred.
Control meal
The control meal included combined WR with non-viscous boiled soyabeans, potatoes and broccoli. We adopted these non-viscous foods because they were classed within the same food group as the foods in the test meal.
Both the test and control meals were designed to resemble a typical Japanese breakfast meal and contained comparable amounts of carbohydrate, fat, protein and fibre (Table 2). Meals were freshly prepared in a microwave oven, and the cooking time and preparation methods were kept the same. While the acute study was conducted in the laboratory, the longer-term study was conducted at the subjects' homes. Water (200 ml) was served with each test meal. The effect of these meals was compared in both the acute and longer-term study, and both studies followed a crossover design.
Table 2 Composition of the test meals
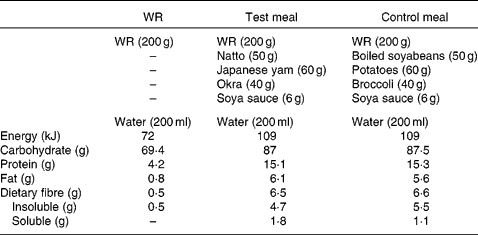
WR, white rice.
Standardised meal
The standardised meal consumed by all participants on the evening before each experimental day consisted of retort-pouched curry with WR, boiled egg, cheese and vegetable juice. The energy content of the evening meal amounted to 38 % of the daily energy needs of each individual.
Acute study design
The present randomised crossover study was conducted on three different days separated by weekly intervals. On the day before the test days, each subject consumed a standardised meal for dinner and was asked to standardise their exercise and to refrain from consuming alcohol. After an overnight fast, venous blood samples were drawn before (0 min) and after (30, 45, 60, 90, 120 and 180 min) the test meal in order to analyse glucose, insulin and NEFA levels. Subjects were instructed to consume the meal within 18 min and chew for the same number of times during both meals in order to try and standardise digestion amongst subjects. The incremental areas under the curve (AUC) for glucose and insulin were calculated using the trapezoidal rule(Reference Wolever13).
Longer-term study design
In a randomised crossover study, subjects with IGT were given either the test meal or the control meal during two consecutive 2-week periods (Fig. 1). The dietary periods were separated by a 2-week washout interval. The subjects were free-living. They were instructed to cook their breakfasts according to written instructions and eat the test meal or the control meal for breakfast only and not for any other meal. They were also instructed to maintain their normal diet except for breakfast throughout the study and were asked to continue with their normal daily activities without varying the amount of exercise they normally performed.

Fig. 1 Protocol of the (a) acute and (b) longer-term study. 75 g oral glucose tolerance test (OGTT). ▲, Blood collection for plasma glucose, serum insulin and serum NEFA.
Measurements and calculations
The 75 g OGTT were performed at baseline and during two post-meal periods (Fig. 1). Blood samples were collected to determine plasma glucose and serum insulin levels at time 0, 30, 45, 60, 90, 120 and 180 min after the glucose load. Chronic glycaemic control indices (including HbA1c, fructosamine and 1,5-anhydroglucitol), fasting serum lipid (including TAG, total cholesterol, HDL-cholesterol and LDL-cholesterol), adipocytokine (leptin and adiponectin) and oxidative stress indices (malondialdehyde-modified LDL and N ɛ-carboxymethyllysine(Reference Yamagishi and Matsui14)) were also measured. All participants consumed the standardised meal on the evening before the 75 g OGTT and the collection of the fasting blood sample.
Insulin sensitivity was evaluated using the homeostasis model assessment of insulin resistance index(Reference Matthews, Hosker and Rudenski15) and the composite insulin sensitivity index (CISI) determined by the formula ![]() during the OGTT(Reference Matsuda and DeFronzo16); values at six points (0, 30, 60, 90, 120 and 180 min) were computed.
during the OGTT(Reference Matsuda and DeFronzo16); values at six points (0, 30, 60, 90, 120 and 180 min) were computed.
Statistical methods
All numerical values were expressed as means with their standard errors. For the acute study, group differences were evaluated with a repeated-measure ANOVA, followed by Fisher's protected least significant difference test. For the longer-term study, the Student's t test was also used to compare serum lipid and oxidative stress indices between the periods.
All statistical calculations were performed with StatView 5.0 (SAS Institute, Inc., Cary, NC, USA). Only P values < 0·05 were considered as significant.
Results
Acute study
There was no significant difference in fasting values in subjects taking any of the three types of test meals. However, the plasma glucose responses at 30, 45 and 60 min and serum insulin responses at 30, 45, 60 and 90 min after the test meal were significantly lower than those after the control meal (Fig. 2(a) and (b)). In addition, the glucose AUC after the test meal were significantly lower ( − 34 and − 21 %) than those after the control meal for the periods 0–60 and 0–120 min (Fig. 2(d)). The insulin AUC after the test meal were also significantly lower ( − 38, − 31 and − 27 %) compared to those after the control meal for the time periods 0–60, 0–120 and 0–180 min (Fig. 2(e)). Interestingly, glucose AUC after the WR meal was larger than that after the test meal for the period 0–60 min, despite the WR meal having lower carbohydrate content than the test meal. However, the insulin AUC after the WR meal was not significantly different to that after the test meal for the period 0–60 min (Fig. 2(e)). The NEFA level after the test meal was significantly higher than that after the control meal at all the time points (Fig. 2(c)).

Fig. 2 Acute response to the test and the control meal in subjects with impaired glucose tolerance. Values are means, with their standard errors represented by vertical bars of (a) plasma glucose, (b) serum insulin and (c) NEFA concentrations after mixed meals, n 11. ![]() , White rice (WR);
, White rice (WR); ![]() , test meal;
, test meal; ![]() , control meal. Values are means, with their standard errors represented by vertical bars of areas under the curve (AUC) for (d) glucose and (e) insulin calculated over 60, 120 and 180 min time periods after mixed meals, n 11. □, WR; ■, test meal;
, control meal. Values are means, with their standard errors represented by vertical bars of areas under the curve (AUC) for (d) glucose and (e) insulin calculated over 60, 120 and 180 min time periods after mixed meals, n 11. □, WR; ■, test meal; ![]() , control meal. Group differences were tested with a repeated-measures ANOVA followed by Fisher's protected least significant difference post hoc test. * Mean values were significantly different from the response to control meal (P < 0·05). † Mean values were significantly different from the response to WR meal (P < 0·05). IAUC, incremental AUC.
, control meal. Group differences were tested with a repeated-measures ANOVA followed by Fisher's protected least significant difference post hoc test. * Mean values were significantly different from the response to control meal (P < 0·05). † Mean values were significantly different from the response to WR meal (P < 0·05). IAUC, incremental AUC.
Longer-term study
All of the eleven subjects completed the study. Individual compliance in the present study was evaluated using a record of daily activities and a food diary, which revealed that subjects compliantly adhered to the prescribed diet. On the basis of the food diary, total dietary energy consumption comprising all ingested food was not significantly different between each period (data not shown). Body weight of the subjects remained stable throughout the study (Table 3).
Table 3 Body weight and blood glucose control indices in the longer-term study*
(Mean values with their standard errors, n 11)
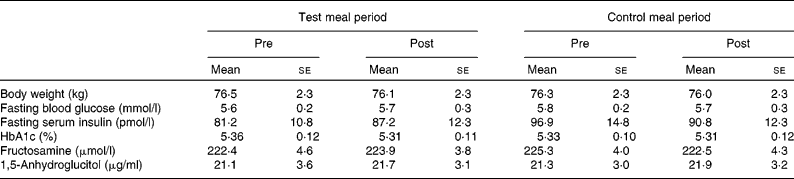
* Group differences were tested with a repeated-measures ANOVA followed by Fisher's protected least significant difference test. No significant differences were observed.
During the study, no adverse events were experienced and therefore there was no reason to discontinue the intervention, although symptoms of fatigue and a slight cold were observed. This dietary intervention was not associated with gastrointestinal symptoms such as diarrhoea, abdominal pain, hypophagia, vomiting, constipation or hyperphagia.
Glucose metabolism
HbA1c, fructosamine and 1,5-anhydroglucitol, all indices of chronic glycaemic control, did not significantly change as a result of dietary intervention (Table 3).
Serum insulin levels at 180 min obtained from 75 g OGTT after both the test and control meal periods decreased compared to the baseline. CISI after the test meal period increased compared to the baseline, whereas there was no significant change after the control meal period. However, there was no significant difference in homeostasis model assessment of insulin resistance between baseline and post-meal periods. Similarly, leptin and adiponectin levels also did not change (Table 4).
Table 4 The 75 g oral glucose tolerance test (OGTT), insulin sensitivity indices and adipocytokine in the longer-term study†
(Mean values with their standard errors, n 11)

HOMA-IR, homeostasis model assessment of insulin resistance; CISI, composite insulin sensitivity index.
* Mean values were significantly different from the baseline (P < 0·05).
† Group differences were tested with a repeated-measures ANOVA followed by Fisher's protected least significant difference test.
Lipid metabolism
Serum total cholesterol and LDL-cholesterol after the test meal period markedly decreased compared to the pre-test meal period, although no significant changes were observed after the control meal period (Fig. 3).

Fig. 3 Changes in serum lipid levels after the test (■) and control (![]() ) period in the longer-term study. Values are means, with their standard errors represented by vertical bars, n 11. The differences from zero were tested with the paired t test. *Mean values were significantly different from zero (P < 0·05). CHO, cholesterol; T-CHO, total CHO.
) period in the longer-term study. Values are means, with their standard errors represented by vertical bars, n 11. The differences from zero were tested with the paired t test. *Mean values were significantly different from zero (P < 0·05). CHO, cholesterol; T-CHO, total CHO.
Oxidative stresses
Malondialdehyde-modified LDL after both the test and control meal periods significantly decreased compared to the baseline level, and malondialdehyde-modified LDL:LDL-cholesterol ratio significantly decreased after the test meal period but not after control meal periods. N ɛ-carboxymethyllysine level after the test meal period was lower than that after the control meal period (Fig. 4).

Fig. 4 Changes in oxidative stress markers from baseline to the post-meal period in the longer-term study. (a) Malondialdehyde-modified LDL (MDA-LDL), (b) MDA-LDL:LDL-cholesterol and (c) N ɛ-carboxymethyllysine. Values are means, with their standard errors, n 11. ■ shows the change in test meal period and ○ shows the change in the control meal. * Mean values were significantly different from baseline (P < 0·05). † Mean values were significantly different from baseline (P < 0·01). ‡ Mean values were significantly different from control meal (P < 0·05; paired t test).
Discussion
The result of the present study confirmed that the consumption of natto and viscous vegetables combined with WR improved the postprandial blood glucose and insulin profiles in subjects with IGT. We were also able to demonstrate that consumption of natto and viscous vegetables in a Japanese-style breakfast for 2 weeks improved insulin sensitivity, lipid profile and oxidative stress in subjects.
Although postprandial glucose and insulin responses to the test meal were greater in subjects with IGT rather than in healthy subjects(Reference Taniguchi, Yamanaka-Okumura and Nishida7), the responses were markedly suppressed when compared to the control meal. Soluble viscous fibres generally have a greater effect on carbohydrate metabolism in the small intestine by delaying gastric emptying, although a slower rate of absorption may also play a role. Here, natural vegetables with viscosity might affect carbohydrate metabolism by a similar mechanism. For example, the prolonged satiety after the viscous meal, as assessed by visual analog scales, may reflect delayed gastric emptying (data not shown). Unlike milk products that stimulate insulin secretion and lower blood glucose(Reference Liljeberg Elmstahl and Bjorck17), consumption of the viscous meal significantly reduced insulinaemia, owing to alterations in the dynamics of glucose absorption and insulin secretion. This is potentially beneficial, as even short durations of hyperinsulinaemia induce insulin resistance in healthy subjects(Reference DeFronzo and Ferrannini18). Furthermore, the fact that postprandial NEFA levels after ingestion of the test meal were higher than the control meal might lead to lower NEFA levels at a subsequent fasting state, which is a favourable effect on glucose utilisation and insulin profile(Reference Roden, Price and Perseghin19).
Improvement of insulin sensitivity after intake of natto and viscous vegetables for 2 weeks was demonstrated by CISI, which is a more sensitive marker than homeostasis model assessment of insulin resistance. Homeostasis model assessment of insulin resistance is computed by fasting values of plasma glucose and serum insulin only, whereas CISI is calculated using fasting values and the mean of the plasma glucose and serum insulin readings taken during the OGTT. Decreased plasma glucose and serum insulin during OGTT after the test meal period derived lower CISI than after the control meal periods. Improvement of insulin sensitivity may be due to the repeated suppression of postprandial hyperglycaemia and hyperlipidaemia as a result of consuming viscous vegetables(Reference Weyer, Bogardus and Mott20), and the decreased insulin secretion result in reduced synthesis of cholesterol in the liver(Reference Adeli, Taghibiglou and Van Iderstine21). Several studies have shown that postprandial hyperglycaemia causes oxidative stress, inflammation and endothelial dysfunction(Reference Monnier, Mas and Ginet22). Controlling postprandial hyperglycaemia by viscous vegetable consumption may serve to prevent or improve a number of symptoms that are associated with insulin resistance syndrome and CVD(Reference Hanefeld, Cagatay and Petrowitsch23). Overall, low-GI foods have a high fibre content, making it difficult to independently define the effect of GI and fibre(Reference Rave, Roggen and Dellweg24). The test meal and control meal within the present study, however, have a comparable amount of fibre; hence, the favourable results observed are likely to be a consequence of suppression of postprandial hyperglycaemia and hyperinsulinaemia.
Several types of low-GI foods served as breakfast meals have the ability to lower glucose tolerance during the subsequent meal, i.e. lunch(Reference Jenkins, Wolever and Taylor25–Reference Arai, Mizuno and Sakuma27). The mechanism for the ‘second-meal effect’ is that a prolonged absorptive phase following breakfast will favour more efficient suppression of NEFA, thus improving insulin sensitivity at the time of the next meal(Reference Wolever, Bentum-Williams and Jenkins28). The effect of viscous vegetables as the only meal intervention of the day in the present study can be explained by the second-meal effect. As total dietary GI modification of all meals is difficult, positive effects from a single-meal replacement with a low-GI diet is of particular interest, and suggests that such a diet should be strongly recommended to promote good health and disease prevention. However, total dietary intervention, not only at breakfast but also at lunch, dinner and in-between meal snacks, although difficult to implement and continue, is likely to have greater potential benefits than single-meal intervention.
The effect of diet is very complex and multiphasic. This is evident when looking at GI. It has been demonstrated that the GI of foods can be reduced by adding fat(Reference Frost, Brynes and Dhillo29). However, a high-fat diet induces hypertriacylglycerolaemia, resulting in insulin resistance(Reference Bloomgarden30) and has damaging effects on endothelial function, producing oxidative stress and inflammation(Reference Ceriello, Quagliaro and Piconi31). The test meal in the present study has low fat because WR contains little fat and goes well with low-fat side dishes. Actually, the addition of a low-fat viscous side dish to WR resulted in no increase in plasma glucose, serum insulin or postprandial serum TAG (data not shown); this combination may be effective for glycaemic control, insulin resistance and CVD.
Limitations of the present study include its small sample size and short duration. Studying more subjects for a longer period of time is clearly needed to determine the sustainability and long-term health effects of natto and viscous vegetables. The strengths of the present study include good subject compliance and effective preparation of subjects for both OGTT and blood sample collection. This was done by standardising the final meal before the beginning and ending of the test periods, ensuring that accurate evaluation was possible on dietary intervention.
In summary, the breakfast of natto and viscous vegetables combined with WR consumed for 2 weeks improved insulin sensitivity, serum lipid concentration and oxidative stress by decreasing postprandial glucose and insulin responses in overweight subjects with IGT, suggesting metabolic benefits in relation to the insulin resistance syndrome.
Acknowledgements
The authors thank the study subjects for their dedication and interest and also to Takafumi Katayama, Makiko Fukaya, Kazusa Sato, Emi Shuto and numerous other staff at the Institute of Health Biosciences at the University of Tokushima Graduate School for the contributions and technical assistance. The present study was supported by Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (H. Y.-O., H. Y., Y. T. and E. T.) and the grant from the Ministry of Agriculture, Forestry, and Fisheries for a food research project titled ‘Integrated Research on Safety and Physiological Function of Food’. All authors contributed to the design of the study. E. T., H. Y.-O., Y. T. and H. Y. recruited the subjects. A. T.-F., H. Y.-O., Y. N. and Y. N.-K. managed the testing sessions. A. T.-F. performed the statistical analysis and drafted the manuscript. All authors reviewed and approved the final version of the manuscript. The authors declare that they have no conflicts of interest.










