Multiple myeloma is a primary bone neoplasm with monoclonal proliferation of plasma cells that produce monoclonal immunoglobulin in blood and urine. Meningeal myelomatosis is a rare finding of this malignancy and occurs when malignant cells infiltrate brain and/or spinal meninges.Reference Laribi, Mellerio and Baugier1–Reference Leifer, Grabowski, Simonian and Demirjian5
We report two cases of meningeal myelomatosis with different clinical and radiological presentations. There are no reports in the literature of meningeal myelomatosis lesions characterized on FLAIR post-contrast magnetic resonance imaging (MRI).
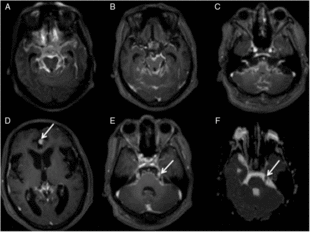
Case 1: A 62-year-old man diagnosed with multiple myeloma and IgG Kappa expression 18 years ago and who had been treated with two hematopoietic stem cell transplantations presented with a recent relapse. He showed aggressive behavior and progressive impairment of attention, and his symptoms had worsened over the last year, with visual hallucinations, speech disorder, and gait disturbance. Figure 1 shows the MRI image. Cerebral spinal fluid (CSF) analysis showed elevated protein (696 mg/ml; reference <45) and pleocytosis (51 cells/mm3; reference <3) with 83% of abnormal plasma cells. Immunophenotyping and immunoelectrophoresis confirmed his diagnosis.
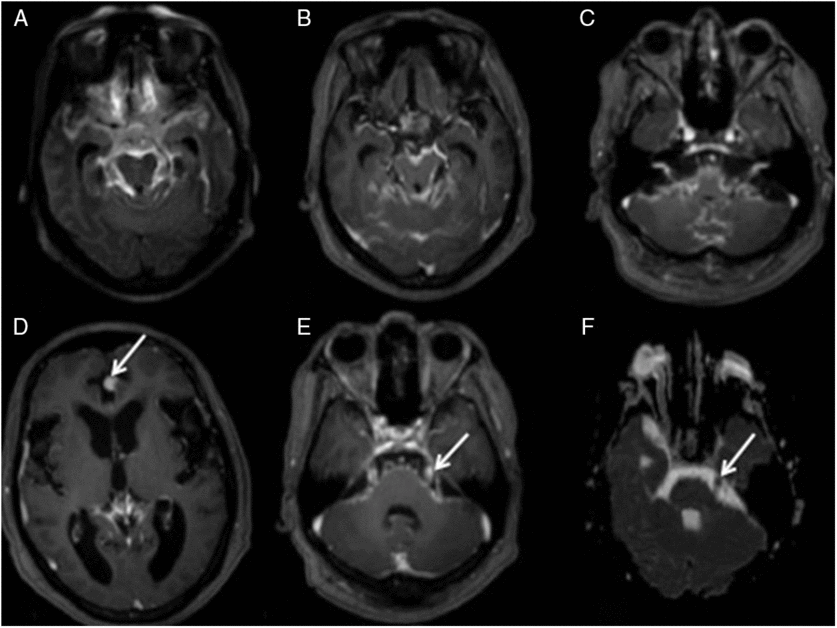
Figure 1: (A) Axial FLAIR – showing diffuse leptomeningeal hyperintensity in the interpeduncular, ambiens, and suprasellar cisterns, and adjacent to temporal and frontal lobes. (B) Axial T1Gd – demonstrating diffuse foci of irregular leptomeningeal enhancement including the III cranial nerves. (C) Axial T1 Gd – illustrating enhancement in the VI, VII, and VIII nerves. (D) Axial T1 Gd – areas of nodular enhancement by the contrast adjacent to the anterior aspect of the inter-hemispheric fissure (arrow) and (E) in the V-cranial nerve (arrow). (F) Axial ADC-restricted diffusion in the left V nerve (arrow). Note that there is more conspicuity of the alterations on FLAIR post-contrast (A) than in (B) – T1 after contrast.
The patient underwent intrathecal chemotherapy and radiotherapy, with a poor outcome.
Case 2: A 69-year-old man was diagnosed with multiple myeloma and IgA lambda expression after a pathological diaphyseal femur fracture. A few months later, during his chemotherapy treatment, he presented an acute behavioral disturbance, speech disorder, and weakness of the left lower extremity. Symptoms rapidly progressed to a decreased level of consciousness, without fever. CSF analysis showed elevated protein (90 mg/ml; reference <45) and pleocytosis (138 cells/mm3; reference <3) with 91% of abnormal plasma cells. Immunophenotyping and immunoelectrophoresis confirmed his diagnosis. Figures 2A, B, and D shows the brain MRI and Figure 2C shows the computed tomography (CT). The patient underwent intrathecal chemotherapy and autologous hematopoietic stem cell transplantation. He died of urinary sepsis a month after transplantation.
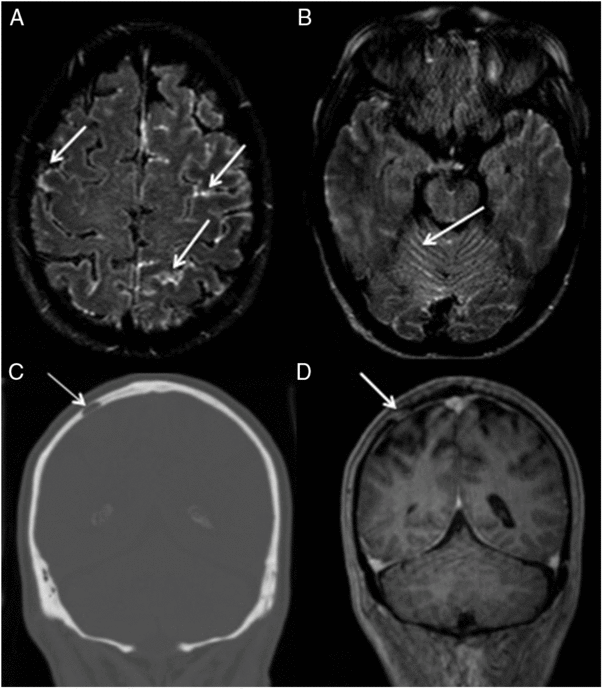
Figure 2: (A and B) Axial FLAIR Gd – demonstrating diffuse enhancement by gadolinium contrast in the cerebellar and cerebral sulci (arrows). (C) Coronal CT – a lytic lesion (arrow) in the right parietal bone. (D) Coronal T1 with gadolinium demonstrating a lytic lesion with homogeneous enhancement by contrast (arrow). Note that there is no evidence of leptomeningeal enhancement in D, as demonstrated on FLAIR post-contrasted (A and B).
Meningeal myelomatosis is a rare presentation that occurs in <1% of patients with multiple myeloma and has a poor prognosis. Men are more affected than women. The rarest tumor subtypes (IgA and IgD) are those that most frequently develop meningeal myelomatosis (23% and 13%, respectively), with a greater propensity to infiltrate the meninges.Reference Laribi, Mellerio and Baugier1–Reference Leifer, Grabowski, Simonian and Demirjian5 The other subtypes include IgG (23%), light chains only (8%), and IgM (3%).Reference Moran, Anderson, Caldemeyer and Smith2, Reference Leifer, Grabowski, Simonian and Demirjian5 Leptomeningeal infiltration is a consequence of a hematogenous spread. Central nervous system (CNS) involvement can also occur by dissemination from contiguous structures (skull lytic lesions) and, in such cases, dural and bone masses are observed.Reference Laribi, Mellerio and Baugier1, Reference Gascón, Pérez-Montero, Guardado, D’Ambrosi, Cabeza and Pérez-Regadera6
Meningeal myelomatosis generally develops as a terminal event in patients with stage III of the disease, although in some cases it can be the first presentation; relapse manifestations have been reported in a few cases. The clinical findings are variable and multifocal. Changes in mental status are the first neurological manifestation in 30% of patients.Reference Leifer, Grabowski, Simonian and Demirjian5 Other neurological findings include headache, seizures, cranial nerve palsy, lower extremity weakness, hemiparesis, gait disturbance, speech disorders, vertigo, and radiculopathy.Reference Laribi, Mellerio and Baugier1–Reference Leifer, Grabowski, Simonian and Demirjian5
CT and MRI are important tools in the diagnosis of meningeal myelomatosis. CT can demonstrate dural mass with pachymeningeal involvement. Iso- or hyperdensity with enhancement by contrast, a mass effect, and parenchymal edema can also be detected. In MRI, the dural mass is generally isointense on T1-weighted images and iso- or hypointense on T2-weighted images (some authors have described marked hypointensity on T2) and can be enhanced homogeneously by contrast. Frequently, there is diffuse pachymeningeal thickening. MRI studies can demonstrate leptomeningeal nodular thickening and enhancement in the cerebral and cerebellar sulci, and abnormal enhancement of the cerebral nerves on T1 and FLAIR post-contrasted images.Reference Laribi, Mellerio and Baugier1, Reference Moran, Anderson, Caldemeyer and Smith2, Reference Gascón, Pérez-Montero, Guardado, D’Ambrosi, Cabeza and Pérez-Regadera6 This nodular thickening may show restricted diffusion, on DWI images, a finding that can be observed in inflammatory process (with the presence of pus), but, also, when there is high cellularity of the infiltrate, as in these two neoplastic cases. No other studies have discussed or showed restricted diffusion in meningeal myelomatosis. In a study by Bozzao et al.,Reference Bozzao, Floris, Fasoli, Fantozzi, Colonnese and Simonetti7 FLAIR sequences confirmed an enhanced CSF signal in most of the patients when obtained after contrast injection. The paramagnetic contrast can change the relaxation of CSF. The conspicuity of FLAIR post-contrasted sequence is related to detection of lesions causing T2 prolongation with nulling of normal CSF background, leading to high lesion contrast. In this paper,Reference Bozzao, Floris, Fasoli, Fantozzi, Colonnese and Simonetti7 in pathologic conditions such as acute stroke, multiple sclerosis, and high-grade gliomas, intravenous injection of gadolinium chelates resulted in increased signal inside the cerebrospinal fluid; however, it did not include any cases in multiple myeloma, as we illustrate.
In a recent multi-institutional retrospective study of 167 patients with confirmed CNS involvement in multiple myeloma who underwent MRI and/or CT, Jurczyszyn et al.Reference Jurczyszyn, Grzasko and Gozzetti4 reported leptomeningeal involvement in 57% of the patients and a cerebral mass lesion in 53%; leptomeningeal involvement alone was detected in 38%, mass alone in 34%, mass and leptomeningeal in 19%, and no mass or leptomeningeal involvement in 9%. This study indicated that post-contrasted MRI is the most sensitive imaging tool for diagnosing CNS involvement, although it has a false-negative rate of 10% and does not specify whether this is achieved with T1 or FLAIR contrast-enhanced sequences.Reference Jurczyszyn, Grzasko and Gozzetti4 Prospective studies have demonstrated that contrast-enhanced FLAIR sequences can detect subtle superficial brain abnormalities and are more precise when compared with T1-weighted images. When there is a suspicion of meningeal myelomatosis, FLAIR contrast-enhanced images should be included.Reference Bozzao, Floris, Fasoli, Fantozzi, Colonnese and Simonetti7, Reference Jackson and Hayman8 To our knowledge, no previous reports have characterized meningeal myelomatosis based on FLAIR post-contrast MRI.
Differential diagnosis for leptomeningeal enhancement includes pyogenic meningitis, and other infections (such as viral, fungal, or micobacterial), leptomeningeal carcinomatosis, meningeal myelomatosis, lymphoma, rheumatoid meningitis, and sarcoidosis.
The definitive diagnosis of meningeal myelomatosis is made by demonstrating neoplastic monoclonal plasma cells in CSF based on immunophenotyping or immunoelectrophoresis. There is no standard treatment. Radiotherapy and systemic and intrathecal chemotherapy are used in different combinations, but the patients still have a poor prognosis.Reference Laribi, Mellerio and Baugier1, Reference Jurczyszyn, Grzasko and Gozzetti4
Disclosure
None of the authors has any disclosures related to this work.
Statement of Authorship
NS – manuscript writing and editing and image selection.
MD – manuscript review.
GD – manuscript review.
FR – manuscript writing, manuscript review, and image selection.