Electroconvulsive therapy (ECT) remains one of the most effective treatments for refractory depression. Ketamine has gained increasing attention for use during ECT in the light of evidence supporting subanaesthetic ketamine's antidepressant properties. Ketamine is a prototype rapid-acting antidepressant for which preclinical data suggest both N-methyl-d-aspartate (NMDA) receptor and non-NMDA receptor-dependent mechanisms. Reference Autry, Adachi, Nosyreva, Na, Los and Cheng1,Reference Zanos, Moaddel, Morris, Georgiou, Fischell and Elmer2 In depressed humans, meta-analyses of randomised placebo-controlled trials employing intravenous ketamine provide strong evidence of antidepressant efficacy, Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam3,Reference Xu, Hackett, Carter, Loo, Galvez and Glozier4 and other routes of administration have also garnered randomised controlled evidence of efficacy. Reference Loo, Galvez, O'Keefe, Mitchell, Hadzi-Pavlovic and Leyden5 Moreover, ketamine results in widespread increases in brain-derived neurotrophic factor, Reference Autry, Adachi, Nosyreva, Na, Los and Cheng1 which is a putative mechanism underlying ECT's effects, despite conflicting evidence. Reference Polyakova, Schroeter, Elzinga, Holiga, Schoenknecht and de Kloet6 We previously reported a preliminary systematic review and meta-analysis of randomised controlled trials (RCTs) using ketamine in ECT, which found increased seizure duration, but not efficacy for ketamine as an adjunct. Reference McGirr, Berlim, Bond, Neufeld, Chan and Yatham7 However, our report involved a small number of trials and patients, and furthermore considered trials employing either a course of ECT or a single session. Few trials examined cognitive function. Moreover, a clinically important source of heterogeneity was concomitant anaesthetic administration, an important consideration as barbiturate anaesthetic compounds may counteract ketamine's antidepressant effects. Reference Homayoun and Moghaddam8 In light of these limitations, and given that several studies have since been published, we sought to update our systematic review and meta-analysis, focusing on RCTs involving an index course of ECT.
Method
The study was conducted following the Preferred Reporting Items for Systematic Meta-Analyses (PRISMA) guidelines, and the protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42016043909).
Search strategy
We searched Medline, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception until 30 July 2016. The search procedures are described in online supplement DS1. We also reviewed the references of included studies for unidentified RCTs.
Study selection
Inclusion criteria were as follows:
-
(a) study validity: random allocation, double-blind, controlled, parallel arm design;
-
(b) sample characteristics: a diagnosis of primary major depressive episode according to DSM-IV or ICD criteria;
-
(c) treatment characteristics: ketamine given as an adjunct to ECT.
Studies were excluded if they comprised a single ECT session, or if summary data were not reported and raw data or summary data could not be obtained from the investigators.
Data extraction
Data were recorded by two independent observers, with subsequent review and consensus. The primary outcome was change in clinician-rated depressive symptoms pre- and post-intervention, assessed with the Hamilton Rating Scale for Depression (HRSD) or the Montgomery–Åsberg Depression Rating Scale (MADRS). Secondary outcomes were clinical response (>50% reduction in depressive symptoms) and cognitive testing. Adverse events were extracted as described in the trials.
Statistical analysis
Analyses were performed using Comprehensive Meta-Analysis version 2.0. Given that true treatment effects were likely to vary between studies given different methodological characteristics, we used a random effects model. Intention-to-treat data were analysed. We used standardised mean differences (SMD) to quantify changes in depression scores and conservatively assumed a pre–post correlation of 0.7. We used risk difference for primary analyses, an absolute measure of the difference in proportions of individuals achieving the pre-specified outcome, to quantify clinical response and remission. Although absolute measures are less consistent than relative effect measures, our preliminary meta-analysis indicated consistent effects. Moreover, risk difference was chosen over relative effect measures such as odds ratios not only for its ease of clinical interpretability, but also because our preliminary meta-analyses revealed the absence of clinical response or remission in some studies, in which case relative statistics are incalculable. We did, however, use odds ratios for adverse events, given the low incidence of such events. Pre-planned subgroup analyses were performed for studies involving co-administration of ketamine with a barbiturate anaesthetic agent (thiopental), and studies that co-administered ketamine with propofol or in isolation.
Heterogeneity was assessed using Q statistics and I 2. Values of P<0.1 for the former and P>35% for the latter were deemed as indicative of study heterogeneity. Finally, we used funnel plots and Egger's regression intercept to test for publication bias. Funnel plots are a graphical representation of effect size and study precision (standard error) with an inverted funnel boundary depicted representing the 95% confidence region expected in the absence of publication bias and heterogeneity. This permits qualitative evaluation of publication bias, with a symmetric relationship implying dissociation of study precision and effect size, whereas an asymmetric relationship suggests possible publication bias. Egger's regression intercept employs a quantitative estimation of the relationship between effect size and study precision using regression, with an intercept statistically different from zero suggesting publication bias. Study quality was assessed using the Cochrane Collaboration's tool for assessing risk of bias concomitant to eligibility.
Results
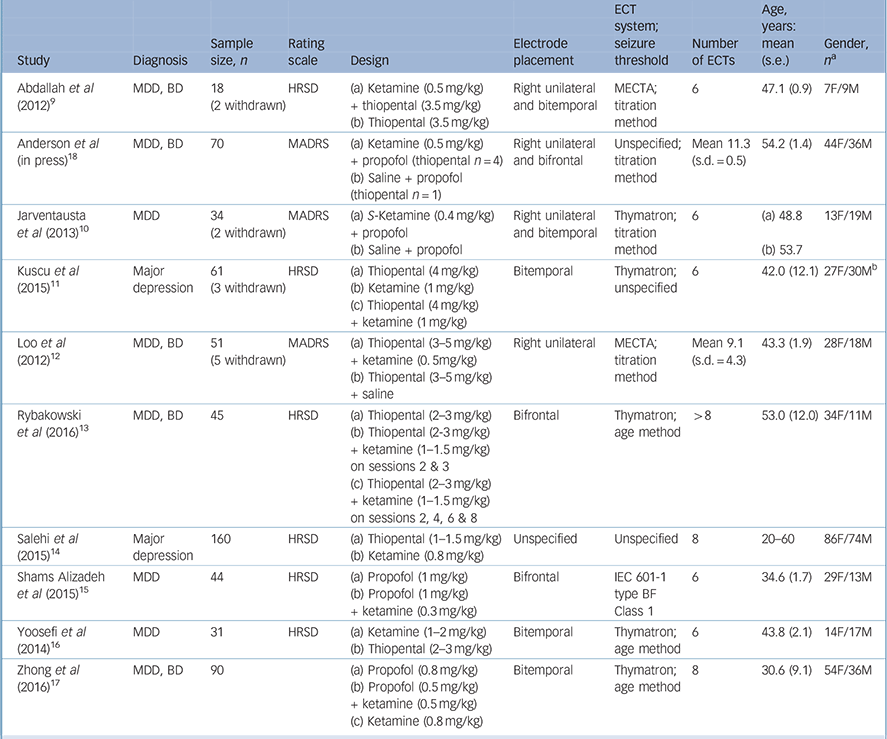
Our literature search resulted in ten RCTs considered suitable for our meta-analysis (Fig. 1, Table 1). Reference Abdallah, Fasula, Kelmendi, Sanacora and Ostroff9–Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 These trials comprised 602 participants with a major depressive episode (ketamine group n = 333, comparator group n = 269). One study was retained despite important methodological concerns, including contradictory statements as to whether the trial was a single- or double-blind RCT, Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 and therefore analyses were repeated excluding this trial. Other excluded studies are listed in online Table DS1 and study quality is summarised in online Table DS2. Five studies had diagnostically mixed samples comprising people with bipolar disorder as well as those with major depressive disorder. Reference Abdallah, Fasula, Kelmendi, Sanacora and Ostroff9,Reference Loo, Katalinic, Garfield, Sainsbury, Hadzi-Pavlovic and MacPherson12,Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13,Reference Zhong, He, Zhang, Wang, Jiang and Li17,Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 None of the trials focused on patients who did not respond to ECT, and no trial compared ketamine with another augmentation strategy. One study involved two subgroups in which ketamine was co-administered with thiopental: Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 in the first subgroup patients received ketamine on the second and third ECT, whereas the second subgroup received ketamine on the second, fourth, eighth and tenth sessions. Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 As ketamine exposure was uneven after five ECT sessions in this study, we extracted data after the fifth session. In all studies ketamine was administered intravenously. Three studies used ketamine at a dose of 0.5 mg/kg, Reference Abdallah, Fasula, Kelmendi, Sanacora and Ostroff9,Reference Loo, Katalinic, Garfield, Sainsbury, Hadzi-Pavlovic and MacPherson12,Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 one study gave subgroups doses of 0.5 mg/kg or 0.8 mg/kg, Reference Zhong, He, Zhang, Wang, Jiang and Li17 whereas the remaining studies used doses of 0.3 mg/kg, Reference Shams Alizadeh, Maroufi, Nasseri, Sadeghi Najafabadi, Mousavi Taghiabad and Gharibi15 0.4 mg/kg, Reference Jarventausta, Chrapek, Kampman, Tuohimaa, Bjorkqvist and Hakkinen10 0.8 mg/kg, Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 1 mg/kg, Reference Kuscu, Karacaer, Biricik, Gulec, Tamam and Gunes11 1.5 mg/kg, Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 and 1–2 mg/kg. Reference Yoosefi, Sepehri, Kargar, Akhondzadeh, Sadeghi and Rafei16
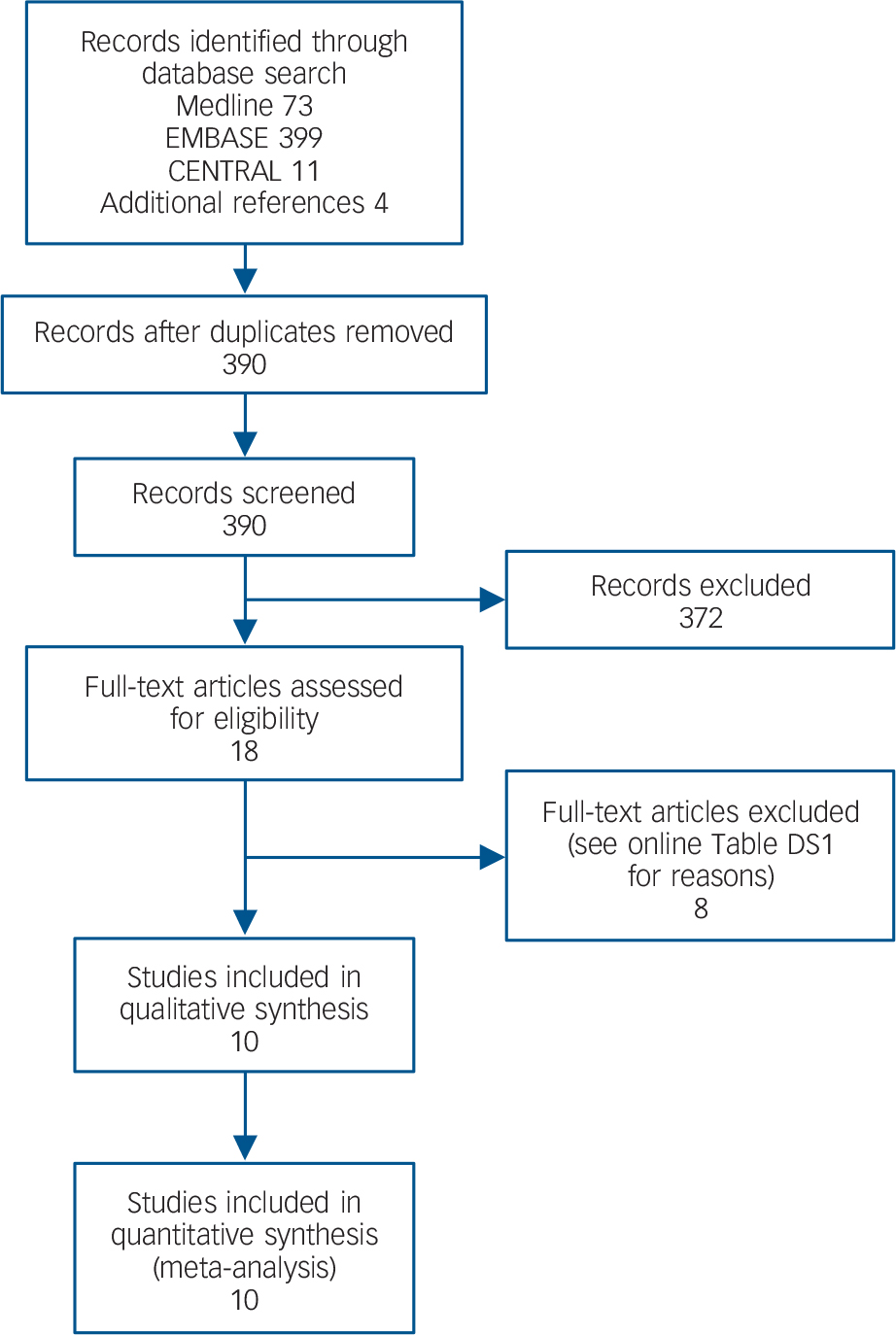
Fig. 1 Study selection.
Table 1 Characteristics of included studies
| Study | Diagnosis | Sample size, n |
Rating scale |
Design | Electrode placement |
ECT system; seizure threshold |
Number of ECTs |
Age, years: mean (s.e.) |
Gender, n a |
| Abdallah et al
(2012) Reference Abdallah, Fasula, Kelmendi, Sanacora and Ostroff9 |
MDD, BD | 18 (2 withdrawn) |
HRSD | (a) Ketamine (0.5 mg/kg) + thiopental (3.5 mg/kg) (b) Thiopental (3.5 mg/kg) |
Right unilateral and bitemporal |
MECTA; titration method |
6 | 47.1 (0.9) | 7F/9M |
| Anderson et al
(in press) Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 |
MDD, BD | 70 | MADRS | (a) Ketamine (0.5 mg/kg) + propofol (thiopental n = 4) (b) Saline + propofol (thiopental n = 1) |
Right unilateral and bifrontal |
Unspecified; titration method |
Mean 11.3 (s.d. = 0.5) |
54.2 (1.4) | 44F/36M |
| Jarventausta et al (2013) Reference Jarventausta, Chrapek, Kampman, Tuohimaa, Bjorkqvist and Hakkinen10 |
MDD | 34 (2 withdrawn) |
MADRS | (a) S-Ketamine (0.4 mg/kg) + propofol (b) Saline + propofol |
Right unilateral and bitemporal |
Thymatron; titration method |
6 | (a) 48.8 (b) 53.7 |
13F/19M |
| Kuscu et al
(2015) Reference Kuscu, Karacaer, Biricik, Gulec, Tamam and Gunes11 |
Major depression |
61 (3 withdrawn) |
HRSD | (a) Thiopental (4 mg/kg) (b) Ketamine (1 mg/kg) (c) Thiopental (4 mg/kg) + ketamine (1 mg/kg) |
Bitemporal | Thymatron; unspecified |
6 | 42.0 (12.1) | 27F/30M b |
| Loo et al
(2012) Reference Loo, Katalinic, Garfield, Sainsbury, Hadzi-Pavlovic and MacPherson12 |
MDD, BD | 51 (5 withdrawn) |
MADRS | (a) Thiopental (3–5 mg/kg) + ketamine (0.5mg/kg) (b) Thiopental (3–5 mg/kg) + saline |
Right unilateral | MECTA; titration method |
Mean 9.1 (s.d. = 4.3) |
43.3 (1.9) | 28F/18M |
| Rybakowski et al (2016) Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 |
MDD, BD | 45 | HRSD | (a) Thiopental (2–3 mg/kg) (b) Thiopental (2–3 mg/kg) + ketamine (1–1.5 mg/kg) on sessions 2 & 3 (c) Thiopental (2–3 mg/kg) + ketamine (1–1.5 mg/kg) on sessions 2, 4, 6 & 8 |
Bifrontal | Thymatron; age method |
>8 | 53.0 (12.0) | 34F/11M |
| Salehi et al
(2015) Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 |
Major depression |
160 | HRSD | (a) Thiopental (1–1.5 mg/kg) (b) Ketamine (0.8 mg/kg) |
Unspecified | Unspecified | 8 | 20–60 | 86F/74M |
| Shams Alizadeh et al (2015) Reference Shams Alizadeh, Maroufi, Nasseri, Sadeghi Najafabadi, Mousavi Taghiabad and Gharibi15 |
MDD | 44 | HRSD | (a) Propofol (1 mg/kg) (b) Propofol (1 mg/kg) + ketamine (0.3 mg/kg) |
Bifrontal | IEC 601-1 type BF Class 1 |
6 | 34.6 (1.7) | 29F/13M |
| Yoosefi et al
(2014) Reference Yoosefi, Sepehri, Kargar, Akhondzadeh, Sadeghi and Rafei16 |
MDD | 31 | HRSD | (a) Ketamine (1–2 mg/kg) (b) Thiopental (2–3 mg/kg) |
Bitemporal | Thymatron; age method |
6 | 43.8 (2.1) | 14F/17M |
| Zhong et al
(2016) Reference Zhong, He, Zhang, Wang, Jiang and Li17 |
MDD, BD | 90 | (a) Propofol (0.8 mg/kg) (b) Propofol (0.5 mg/kg) + ketamine (0.5 mg/kg) (c) Ketamine (0.8 mg/kg) |
Bitemporal | Thymatron; age method |
8 | 30.6 (9.1) | 54F/36M | |
BD, bipolar disorder; ECT, electroconvulsive therapy; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; MDD, major depressive disorder.
a. F female, M male.
b. Not fully reported.
Depressive symptoms
Clinician-rated depressive symptoms were available for all ten RCTs. A non-significant SMD of 0.18 in favour of ketamine (95% CI −0.25 to 0.61, P = 0.41; Fig. 2) was observed, with significant statistical heterogeneity (Q = 55.9, I 2 = 83.9, P<0.001). Sensitivity analyses revealed that excluding the trial by Zhong et al Reference Zhong, He, Zhang, Wang, Jiang and Li17 decreased heterogeneity (Q = 24.8, I 2 = 67.7, P<0.01); however, exclusion of no other trial reduced I 2 by 5% or more. Examination of the funnel plot revealed a symmetrical distribution with outliers (online Fig. DS1). Egger's intercept was not statistically significant (t(8) = 0.87, P = 0.40). We repeated the analysis excluding the trial with major methodological issues, Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 which similarly revealed a non-significant SMD of 0.14 in favour of ketamine (95% CI −0.37 to 0.66, P = 0.59). A second sensitivity analysis was performed excluding the trial in which ketamine was alternately applied with thiopental. Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 This again revealed a non-significant SMD in favour of ketamine (SMD = 0.17, 95% CI −0.30 to 0.65, P = 0.47). A leading hypothesis for ketamine's ineffectiveness in ECT relates to concomitant barbiturate use. Therefore, we performed a separate analysis excluding trials (or subgroups within trials) in which ketamine was co-administered with a barbiturate anaesthetic agent (7 RCTs; ketamine group n = 247, comparator group n = 219). Again, we observed a non-significant SMD in favour of ketamine (SMD = 0.24, 95% CI −0.33 to 0.83, P = 0.40) and significant statistical heterogeneity (Q = 51.71, I 2 = 88.39, P<0.001). Egger's intercept did not reveal statistical evidence of publication bias (t(5) = 0.42, P = 0.68).
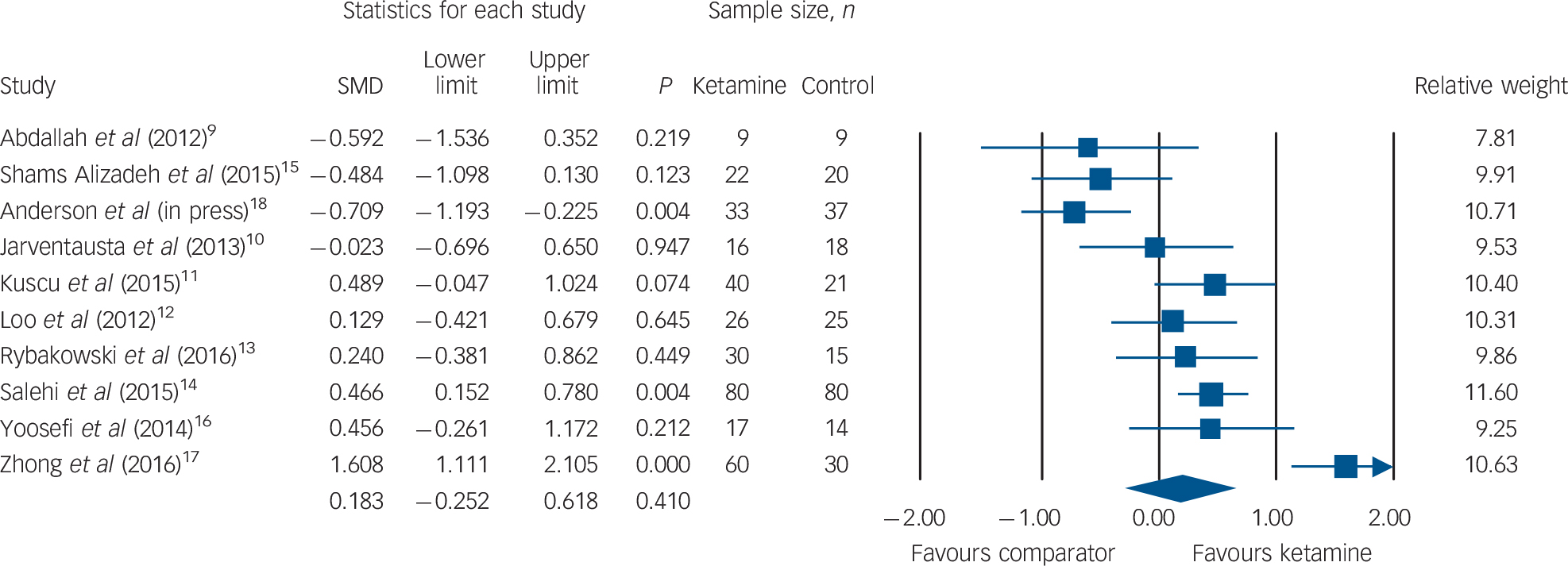
Fig. 2 Change in clinician-administered depression rating scores. SMD, standardised mean difference.
Clinical response
Clinical response rates were available for seven RCTs (ketamine group n = 191, comparator group n = 148). No difference in clinical response was observed between patients receiving ketamine v. comparator (ketamine n = 103, 53.9%; comparator n = 79, 53.4%; risk difference (RD) = 0.010, 95% CI −0.06 to 0.08, P = 0.78; Fig. 3). Qualitative inspection of the funnel plot revealed a broadly symmetrical distribution with some asymmetry at lower precision (online Fig. DS2); however, Egger's intercept did not reveal statistical evidence of publication bias (t(5) = 1.00, P = 0.35).
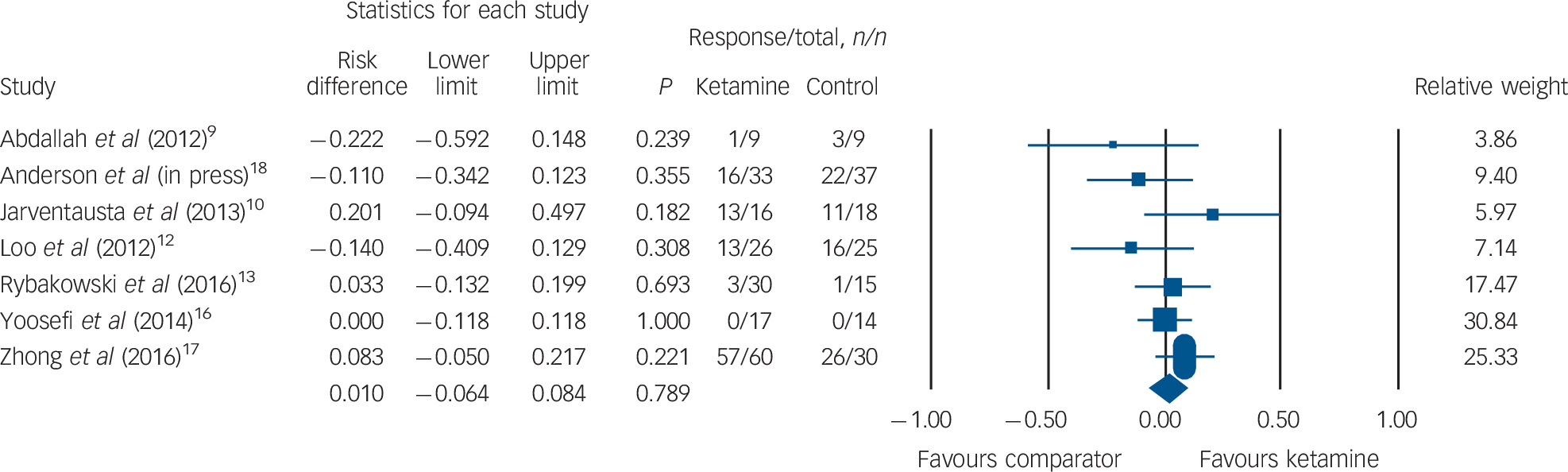
Fig. 3 Rates of clinical response.
Clinical remission
Rates of clinical remission were available for seven RCTs (ketamine group n = 191, comparator group n = 148). No difference in remission was observed between patients receiving ketamine v. comparator (ketamine n = 32, 16.7%; comparator n = 28, 18.9%; RD = 0.026, 95% CI −0.02 to 0.07, P = 0.30; Fig. 4). The funnel plot revealed minimal spread around a risk difference of zero (online Fig. DS3) and Egger's intercept did not reveal statistical evidence of publication bias (t(5) = 0.56, P = 0.59).
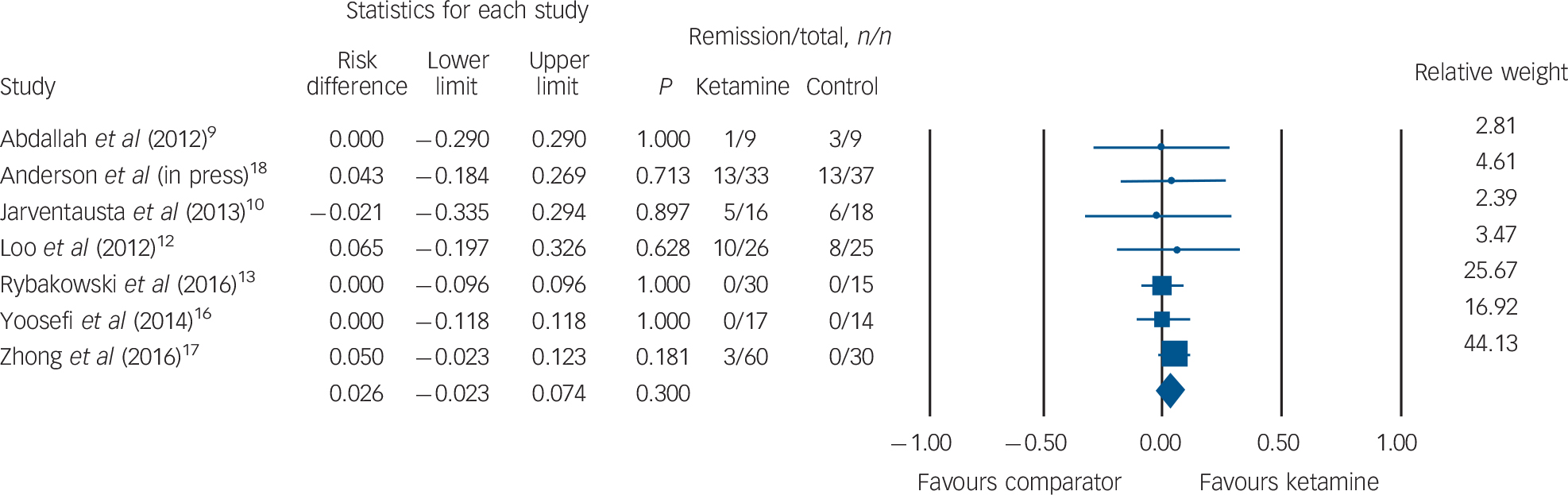
Fig. 4 Rates of clinical remission.
Cognitive testing
Several studies examined cognitive indices; however, few had overlapping instruments, preventing quantitative synthesis. Two studies with extensive neuropsychological batteries, including the autobiographical memory interview, found no benefit associated with ketamine. Reference Loo, Katalinic, Garfield, Sainsbury, Hadzi-Pavlovic and MacPherson12,Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 Rybakowski et al reported poorer performance associated with ketamine on the Rey Auditory Verbal Learning Task and the Verbal Fluency Test, but not on the Stroop test or Digit Span Test. Reference Rybakowski, Bodnar, Krzywotulski, Chlopocka-Wozniak, Michalak and Rosada-Kurasinska13 Zhong et al reported no difference on the Digit Span Test, Digit Symbol Test, Word Fluency Test and Visual Recognition Test; however, they did find evidence for poorer performance on the Wisconsin Card Sorting Task and the Tower of Hanoi test with ketamine, and better performance on the Trail Making Test. Reference Zhong, He, Zhang, Wang, Jiang and Li17 Time to reorientation was slower in ketamine-treated patients in one RCT, Reference Shams Alizadeh, Maroufi, Nasseri, Sadeghi Najafabadi, Mousavi Taghiabad and Gharibi15 but similar in another. Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18 One RCT used the Mini-Mental State Examination and found benefit in favour of ketamine. Reference Yoosefi, Sepehri, Kargar, Akhondzadeh, Sadeghi and Rafei16
Adverse events
Adverse events, as defined and reported by the trials, are presented in online Fig. DS4. We excluded one trial owing to unusually high rates (>60%) of ‘prolonged delirium’ in both ketamine and comparator groups. Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 We found an increased risk of confusion (OR = 6.01, 95% CI 1.03 to 94.30, P = 0.046). One trial could not be pooled as adverse events were presented by affected organ system; however, it reported an increased rate of mild and moderate adverse events among ketamine-treated patients. Reference Anderson, Blamire, Branton, Brigadoi, Clark and Downey18
Discussion
This updated systematic review and meta-analysis confirms that ketamine is not more efficacious than other induction agents in ECT, even without co-administered barbiturate anaesthetic compounds. Moreover, the literature does not support procognitive effects, and adverse events – notably confusion – are more common with ketamine. The potential for temporising relief should not be discounted, Reference McGirr, Berlim, Bond, Neufeld, Chan and Yatham7,Reference Zhong, He, Zhang, Wang, Jiang and Li17 especially among those who are highly suicidal or medically compromised by poor fluid and/or nutrient intake. Although three trials supported early efficacy, Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14,Reference Yoosefi, Sepehri, Kargar, Akhondzadeh, Sadeghi and Rafei16,Reference Zhong, He, Zhang, Wang, Jiang and Li17 several features of these trials overshadow this conclusion: one trial had minimal benefit in either condition; Reference Yoosefi, Sepehri, Kargar, Akhondzadeh, Sadeghi and Rafei16 another had a 100% response rate within eight sessions, Reference Zhong, He, Zhang, Wang, Jiang and Li17 in a treatment-resistant sample where a 60% response rate would be expected; Reference Prudic, Haskett, Mulsant, Malone, Pettinati and Stephens19 and the third had quality concerns. Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 Overall, the randomised, controlled research does not support accelerated clinical improvement with ketamine.
Limitations
Several sources of potential bias were identified in the included trials, but there was no relationship between these methodological issues and outcomes reported. Moreover, high-quality trials consistently report null results, supporting the interpretation of results from our quantitative synthesis of data. Nevertheless, explicit quality issues were identified in one trial, Reference Salehi, Mohammadbeigi, Kamali, Taheri-Nejad and Moshiri14 and repeated analyses excluding these data also supported our conclusions. It is important to note that there was tremendous variability in the rates of response identified in the included trials. Although the cause of this variability is unclear, possibilities include an overly brief course of ECT. Although a short index course is the preferred methodological approach to avoid ceiling effects when testing an augmenting agent, in the absence of treatment response in the comparison group, a larger issue with trial design or patient selection cannot be excluded. Clinical heterogeneity was evident, with differences in ketamine dose, comparison induction agent, co-administered agents and stimulation parameters, as well as in rating scales. We also observed statistical heterogeneity. As with our preliminary report, a major limitation is the applicability to patients for whom therapeutic seizures cannot be obtained in the absence of adjunctive agents. Clinical equipoise in that context might justify the use of ketamine in those for whom adequate seizures cannot otherwise be obtained. Additional questions deserving of investigation relate to potential dose response and long-term safety.
Clinical implications
Our meta-analysis of randomised controlled trials does not support the use of ketamine over other induction agents in ECT.








eLetters
No eLetters have been published for this article.