INTRODUCTION
Infections of the central nervous system (CNSI) are among the leading causes of death and severe long-term disability in children in developing countries [Reference Chong and Tan1–Reference Wang3]. The clinical presentations, treatment regimens, and outcomes of CNSI are heterogeneous and highly dependent on a multitude of variables including the neurovirulence of the pathogen, host susceptibility, epidemiological factors and variation in environmental exposures. The etiological agents (including bacteria and viruses) and the modes of transmission (such as vector-borne or person-to-person) of CNSI are additionally diverse, and in many cases unidentified [Reference Chong and Tan1, Reference Newton2].
In a low-resource country like Vietnam, the specific pathogen associated with an individual CNSI is often undetermined. Studies designed to identify the etiological agents of CNSI in Vietnam fail to identify a pathogen in approximately half of the CNSI patients. Among those identified, the most common bacterial pathogens of paediatric CNSI in Vietnam are Streptococcus pneumoniae and Haemophilus influenzae type b (Hib), whilst the most common viral pathogens are Japanese encephalitis (JE), Dengue virus, and a range of enteroviruses [Reference Ho Dang Trung4–Reference Anh6]. Although a vaccine against JE virus was introduced in the Expanded Programme on Immunization (EPI) in Vietnam in 2006, mosquito-borne viral encephalitis is still considered to be a major public health concern in Vietnam [Reference Jit7]. Vaccines against S. pneumoniae and Neisseria meningitidis (meningococcus) are available in Vietnam but are expensive and not yet incorporated into EPI schedule. With support from the Global Alliance for Vaccine and Immunization (GAVI), Vietnam introduced a Hib vaccine in 2010, which was incorporated into a pentavalent formulation (diphtheria, tetanus, pertussis, Hepatitis B, and Hib). The World Health Organization (WHO) estimated that the national coverage of Hib vaccine in Vietnam increased from 63% in 2010 to 97% in 2015 [8]. However, GAVI will not continue their funding for this initiative after 2016 and as the results, the Vietnamese government will face a sixfold increase in the cost of Hib vaccines [Reference Le9].
There is evidence to suggest that various spatial, climatic and socio-demographic risk factors may be associated with the incidence of CNSI in low-middle income countries such as those located in the African sub-Saharan meningitis belt and Asia (e.g. vector-born encephalitis is associated with proximity to rice cultivation and pig rearing and bacterial meningitis is associated with seasonality (the winter months) and high population densities) [Reference Agier10–Reference Erlanger17]. Vietnam faces continuing lack of access to costly vaccines against bacterial and viral etiologies of CNSI. Therefore, we aimed to provide a better understanding of sub-populations at risk of CNSI so they can be targeted for improved immunization strategies. We conducted an analysis of the spatial distribution and climatic risk factors of paediatric CNSI in Ho Chi Minh City (HCMC), the major economic centre in the south of Vietnam, which may assist in efficiently targeting prevention and control methods for CNSI, such as vector control measures and immunization programmes.
METHODS
Data sources
Data for this study were derived from the electronic hospital databases of all paediatric (aged <16 years) Vietnamese inpatients admitted to three large main hospitals located in central HCMC: Children's Hospital one (CH1), Children's Hospital two (CH2), and the Hospital for Tropical Diseases (HTD). These hospitals receive most of the paediatric patients especially those requiring hospital care from HCMC, an economic centre with around 8 million population in the South of Vietnam. Data from the two paediatric hospitals (CH1 and CH2) were obtained between 2005 and 2015, while the data from HTD were obtained between 2008 and 2015. All the three study hospitals employed the International Classification of Disease version 10 (ICD10) for coding disease diagnoses. Some routine electronic hospital data were available from these hospitals before the study period; however, we only utilized data generated in years that used ICD10 coding to ensure the consistency in database quality. We only included patients residing in HCMC, excluding patients from surrounding provinces, in this analysis anticipating the data were representative of HCMC population.
We classified CNSI into two major groups based on the ICD10 codes outlined by the WHO: (1) Presumed bacterial CNSI (BI), which were amalgamated under the ICD10 codes G00-G01 and G03 for bacterial meningitis, not elsewhere classified, meningitis in bacterial diseases classified elsewhere and for meningitis due to other and unspecified causes respectively. (2) Presumed non-bacterial CNSI (non-BI), which incorporated the ICD10 codes A80-A89 for viral and prion infections of the central nervous system, G02 for meningitis in other infectious and parasitic diseases classified elsewhere, G04-G05 for encephalitis, myelitis and encephalomyelitis (online Supplementary Table S1). As most of the paediatric patients with tuberculous meningitis (TBM) are treated at another hospital specialized for tuberculous diseases in HCMC, ICD10 codes for TBM (A17·0) was not included in our analysis. Data from patients with the selected ICD10 codes who resided in HCMC were extracted and analyzed. Each patient record included age, sex, date of admission, date of discharge, ICD10 code at discharge, and residential district.
HCMC is subdivided into 24 districts (land areas ranging from 4·18 to 704·22 km2). There are five rural districts (population density ranging from 100 to 3326 people/km2) and 19 urban districts (population density ranging from 2360 to 45 582 people/km2) [18]. There are two distinct seasons in HCMC: a dry season (from December to April) and a rainy season (from May to November). Various characteristics of the districts, including population density (1000/km2), average number of pig herds with pigs aged >2 months, land usage for rice cultivation, and population data were obtained from the HCMC statistical office [18]. As disaggregated population figures for those aged <16 years per district were unavailable, the total population was used to estimate disease incidence. Patient addresses in HCMC were geocoded to district centroids. City level monthly average climate data during 2005–2015 were obtained from the Ministry of Natural Resources and Environment of Vietnam. These data included monthly average temperature (median = 28·3°C, range = 25·9–31·3°C), relative humidity (median = 74·4%, range = 60·4% to 83·2%), rainfall (median = 4·9 mm, range = 0–17 mm), and water level of the Don Dien River (median = 10·6 cm, range = −19·3 to 39·1 cm) [Reference Loc19]. District elevation data were obtained from the CGIAR Consortium for Spatial Information (CGIAR-CSI) [Reference Jarvis20].
Spatial mapping
To reduce the variation of raw incidence estimates due to the variation in population sizes, district incidence rates (number of CNSI cases per 100 000 district total population for the entire study period) were smoothed using local empirical Bayes estimates for rates reduced to a neighborhood mean. The neighborhoods were identified by a neighborhood list based on districts with contiguous boundaries (R package spdep version 0·5–88) [Reference Bivand, Hauke and Kossowski21]. Kulldorff and Nagarwalla's method over district centroids was used to detect clusters of disease [Reference Kulldorff and Nagarwalla22].
A Poisson generalized linear model was used to explore the relationship between district CNSI incidence and district characteristics (the logarithm of the distance from district centroids to the nearest study hospital, district population density, number of pigs in herds with pigs aged >2 months by district, % area of district used for rice cultivation, and district elevation). Confidence intervals (CI) and statistical tests were based on robust standard errors to control for mild violations of the distributional assumption that the variance equals the mean [Reference Cameron and Trivedi23].
Time series analysis
The time series of citywide monthly incidence of CNSI (number of monthly cases per 100 000 total population of that month) were examined for seasonality, time trends, and associations with climatic covariates by Poisson generalized additive mixed models (GAMM) implemented in the R package mgcv version 1·8–7 [Reference Lin and Zhang24, Reference Wood25]. Seasonal cycles and time trends of monthly CNSI incidence were evaluated by the basic GAMM models comprising the month of year (Ms = 1–12) as cyclic cubic regression splines and the month of the whole study period (Mt = 1–96) as cubic regression spline. As monthly CNSI incidence may be associated with the CNSI incidence and climatic conditions of the previous months, in addition to the elements in the basic model, the full GAMM model also contained lags of CNSI monthly incidence of up to 4 months, concurrent climatic condition and lags of climatic condition up to 4 months. Adjusted R 2 and model diagnostic plots were used to evaluate the performance of the GAMM models. Temporal correlation of the model residuals was evaluated by autocorrelation function (ACF) and partial ACF.
Notably, the CNSI data prior to 2008 may contain ICD10 codes for neurological complications associated with hand, foot, and mouth diseases (HFMD) (potentially coded as A85·0 for enteroviral encephalitis). From 2008, patients with HFMD were coded with separate ICD10 codes (B08·4). HFMD is currently epidemic in Vietnam and may have different spatiotemporal patterns and climatic risk factors in comparison with other CNSI. Therefore, to avoid the bias induced by the inclusion of HFMD in these data, only CNSI data from 2008 to 2015 were used for the analysis of temporal patterns and climatic risk factors. Spatial mapping and statistical analyses were stratified by BI and non-BI and all subsequent analyses were performed in R version 3·2·2 [26].
RESULTS
Features of paediatric CNSI in HCMC
From 2005 to 2015, there were 9469 paediatric (<16 years old) patients residing in HCMC admitted to the three study hospitals for CNSI (1·5% of all paediatric admissions). There were 5182 patients (55% of all CNSI admissions) with ICD10 codes synonymous with presumed BI and 4287 patients (45%) with ICD10 codes synonymous with presumed non-BI. The BI patients were significantly younger than the non-BI patients (median (interquartile-IQR) = 1·1 years (0·1, 5·0 years) vs. 3·8 years (1·6, 6·9 years), P < 0·0001). CNSI patients aged ⩽1 year at admission accounted for 33% (3110/9469) and the vast majority (2469/3110; 79%) were diagnosed as having presumed BI. Whereas, the majority of CNSI patients aged >1 year were diagnosed as presumed non-BI (3597/5872; 59%). BI patients were hospitalized for a significantly longer period of time than non-BI patients (median (IQR) = 13·0 days (8·0, 20·0 days) vs. 4·0 days (3·0, 7·0 days), P < 0·0001). Mortality of BI patients was significantly lower than the non-BI patients (1% vs. 4%, P < 0·0001) (Tables 1 and 2).
Table 1. The characteristics of paediatric patients with presumed bacterial and non-bacterial central nervous system infections (CNSI) in Ho Chi Minh from 2005 to 2015

N: total number of patients in the group; n: number of patients with available information.
* P-value from Wilcoxon's rank sum test comparing presumed bacterial infections vs. presumed non-bacterial infections.
† P-value from Fisher's exact test comparing presumed bacterial infections vs. presumed non-bacterial infections.
Table 2. The diagnostic groups and ICD10 codes of paediatric patients with central nervous system infections (CNSI) by age at admission in Ho Chi Minh from 2005 to 2015
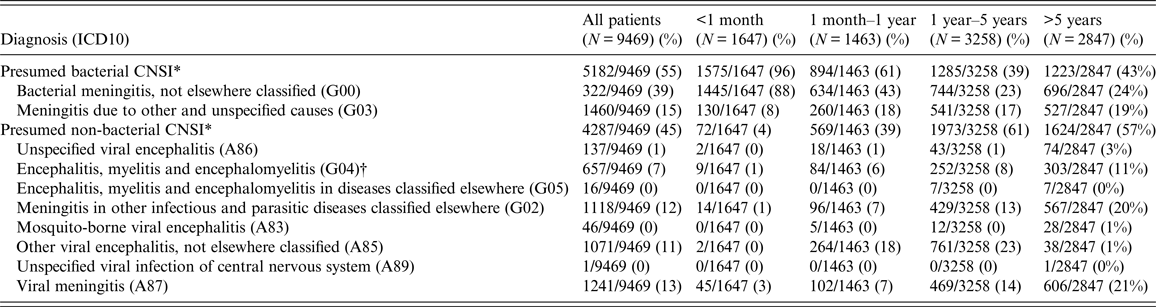
ICD10, International Classification of Disease version 10.
* Fisher's exact test comparing the proportion of presumed bacterial CNSI and presumed non-bacterial CNSI across age groups P-value <0·0001.
† ICD10 code G04 may include both bacterial and viral CNSI. Most of the ICD codes in our databases only contained general ICD codes like G04 therefore it was not straightforward how to classify G04 codes into BI or non-BI group based on the data in our databases. However, we learned that, clinically, most of the CNSI cases coded with G04 in our three study hospitals were non-BI. Therefore, for the purpose of our analysis, all CNSI cases with ICD10 code G04 were grouped as non-BI.
More detailed diagnoses and ICD10 codes for the whole study period are in online supplementary Table S1.
Overall, the most commonly diagnosed groups were bacterial meningitis, not elsewhere classified (ICD10 code = G00) (3722/9469; 39%), meningitis due to other and unspecified causes (G03) (1460/9469; 15%), viral meningitis (A87) (1241/9469; 13%), meningitis in other infectious and parasitic diseases classified elsewhere (G02) (1118/9469; 12%), other viral encephalitis, not elsewhere classified (A85) (1071/9469; 11%) and encephalitis, myelitis, and encephalomyelitis (G04) (657/9469; 7%) (Table 2). A more detailed outline of the diagnoses, ICD10 codes and explanation for categorizing ICD10 codes from our data into BI and non-BI groups are shown in online Supplementary Table S1.
The spatial distribution of paediatric CNSI in HCMC
Over the entire study period (2005–2015), the median district incidence of BI (number of BI cases per 100 000 district population) was 64·5 (range = 40·3, 105·1); the median district incidence of non-BI was 51·4 per 100 000 (range = 32·1, 84·2). The highest incidences of BI and non-BI were identified in the urban districts of HCMC that were in the closest proximity to CH1 and CH2 (districts 3, 10, 11, 5, 6, Tan Binh, and Tan Phu) and some rural districts located significant distances from the study hospitals (Hoc Mon and Binh Chanh) (P < 0·0001) (Fig. 1). There were two periods in which there were notable peaks in non-BI and BI, these occurred in 2007 and 2009, respectively. The increase in non-BI and BI incidence during these periods occurred concurrently in the same nine districts identified above (online supplementary Figs S1, S2, S3 and S4). We also observed an overall decrease in BI incidence in these nine districts between 2013 and 2015 (online supplementary Fig. S1).

Fig. 1. The empirical Bayesian estimated incidence rates (EBR) of paediatric CNSI of presumed bacterial and non-bacterial origin in Ho Chi Minh City from 2005 to 2015. (a) District level EBR per 100 000 district total population of presumed bacterial CNS infections for the whole study period. (b) District level EBR per 100 000 district total population of presumed non-bacterial CNS infections for the whole study period. The green points show the locations of the study hospitals, CH1; Children hospital 1, CH2; Children hospital 2, and HTD; the Hospital for Tropical Diseases. An asterisk highlights rural districts.
The district level incidence of BI over the study period was significantly associated with the logarithm of Euclidean distance from district centroids to the nearest study hospital (rate ratio (RR) for two times increase in distance = 0·915, 95% confidence interval (CI) 0·875–0·957) and district population density (RR for 1 unit (1000/km2) increase in population density = 1·006, 95% CI 1·001–1·011). However, in a multivariate model containing both the logarithm of distance to hospital and population density, only the logarithm of distance to hospital remained statistically significant (RR = 0·911, 95% CI 0·850–0·977). The district incidence of non-BI was not significantly associated with any of the district variables examined (online supplementary Fig. S5).
Temporal patterns and climatic risk factors of paediatric CNSI
The monthly incidence of BI (number of monthly cases per 100 000 population) ranged from 0·19 to 1·61 (median = 0·48) and the monthly incidence of non-BI ranged from 0·07 to 1·28 (median = 0·29) per 100 000. There was one major peak of BI, this occurred in the dry season of 2009 and was associated with the ICD10 code of bacterial meningitis not elsewhere classified (G00) (68% of BI patients (41% of all CNSI patients) during that period). Generally, most periods with a high incidence of BI were associated with the dry season and were preceded by a smaller peak in the late months of rainy season. There was a single exceptionally large peak of non-BI in the wet season of 2007; this was related to enteroviral encephalitis (A85·0) (88% of non-BI patients (70% of all CNSI patients) during that period). After this potential outbreak, the incidence of non-BI exhibited sporadic peaks in 2009 and 2010, which were mainly associated with the ICD10 code of meningitis in viral diseases classified elsewhere (G02·0) (74% and 47% of non-BI patients during those periods, respectively). Again, a peak in non-BI in 2012 and 2015 was generally associated with viral meningitis (A87) (59% and 63%, respectively). With the exception of the large peak in non-BI during the rainy season of 2007, the highest incidences of non-BI displayed a similar pattern of peaking mostly in the dry season. Overall, the climatic data displayed exhibited similar seasonal patterns over the 11-year study period (Fig. 2).

Fig. 2. The monthly incidence of paediatric presumed bacterial and non-bacterial CNSI vs. climate covariates and maps for the major outbreaks. Plots showing time series data for (from top to bottom); BI (presumed bacterial CNSI), non-BI (presumed non-bacterial (CNSI)), temperature (°C), humidity (%), rainfall (mm), and river water level (cm). Adjacent maps show the district incidence (number of cases per 100 000 district total population) of non-BI and BI during the two major outbreaks and most commonly recorded ICD10 codes at these time points. The green points show the locations of the study hospitals. The districts are labelled and an asterisk highlights rural districts. 1–12: district 1–12; 13–24: district Binh Chanh, Binh Tan, Binh Thanh, Can Gio, Cu Chi, Go Vap, Hoc Mon, Nha Be, Phu Nhuan, Tan Binh, Tan Phu, Thu Duc, respectively.
A basic GAMM model indicated that BI exhibited significant seasonality (P < 0·0001). Overall, the monthly incidence of BI significantly decreased over time (incidence rate ratio (RR) for 1 month increase in time = 0·61, 95% CI 0·38–0·98, P = 0·0441) and the monthly incidence of non-BI significantly increased over time (RR = 2·04, 95% CI 1·19–3·5, P = 0·0113) (Table 3).
Table 3. Results of the full generalized additive mixed models (GAMM) for paediatric central nervous system (CNS) infections in Ho Chi Minh from 2008 to 2015
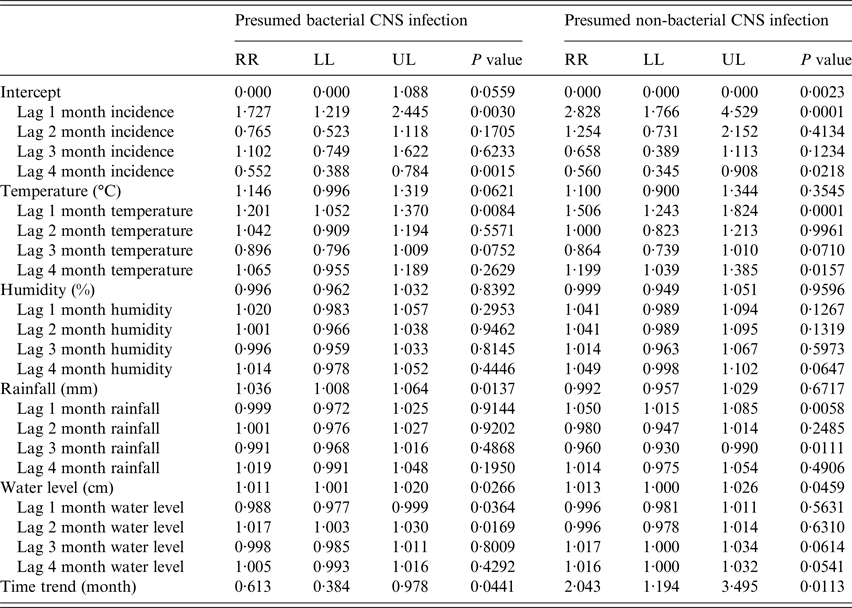
Results of fixed effects are shown in the table.
P values for seasonality for presumed bacterial and non-bacterial CNS infection = 0·1990 and 0·5268, respectively.
Adjusted R 2 for presumed bacterial and non-bacterial CNS infection = 0·78 and 0·74, respectively.
RR: (incidence) rate ratio for one unit increase of covariates. LL and UL: lower and upper limits of 95% confidence interval.
The monthly incidence of BI was significantly associated with BI incidence 1 and 4 month previously (i.e. 1 and 4 month lags of monthly BI incidence) (RR = 1·73, 95% CI 1·22–2·45, P = 0·003 and RR = 0·55, 95% CI 0·39–0·78, P = 0·0015, respectively). Additionally, the monthly incidence of BI was significantly associated with a 1 month lag in monthly average temperature (RR for 1°C increase in temperature = 1·2, 95% CI 1·05–1·37, P = 0·0084), average monthly rainfall (RR for 1 mm increase in rainfall = 1·04, 95% CI 1·01–1·06, P = 0·0137), and was concurrent (and 1 and 2 month lags) with the average monthly river water level (RR for 1 cm increase in river water level = 1·01, 95% CI 1·0–1·02, P = 0·0266, and RR = 0·99, 95% CI 0·98–0·99, P = 0·0364 and RR = 1·02, 95% CI 1·0–1·03, P = 0·0169, respectively) (Table 3).
The monthly incidence of non-BI was significantly associated with non-BI incidence 1 and 4 months previously (i.e. 1 and 4 month lags of monthly non-BI incidence) (RR = 2·83, 95% CI 1·77–4·53, P = 0·0001 and RR = 0·56, 95% CI 0·35–0·91, P = 0·0218, respectively), 1 and 4 month lags in temperature (RR = 1·51, 95% CI 1·24–1·82, P = 0·0001 and RR = 1·2, 95% CI 1·04–1·39, P = 0·0157, respectively), 1 and 3 month lags in rainfall (RR = 1·05, 95% CI 1·02–1·09, P = 0·0058 and RR = 0·96, 95% CI 0·93–0·99, P = 0·0111, respectively), and water level (RR = 1·01, 95% CI 1·0–1·03, P = 0·0459) (Table 3). The full GAMM models fitted the data well for both BI (adjusted R 2 = 0·77) and non-BI (adjusted R 2 = 0·74) (Fig. 3 and online supplementary Fig. S6). Lastly, a temporal correlation of monthly incidence was well controlled for both non-BI and BI in the full GAMM models (online supplementary Fig. S7).

Fig. 3. The monthly incidence time series of paediatric presumed bacterial and non-bacterial CNSIs. (a) Plot showing the smoothed seasonal cycle (red line), the citywide monthly incidence (number of monthly cases per 100 000 total population of that month) time trend (blue line) of the presumed bacterial CNSI (BI) from the basic Poisson generalized additive mixed model (GAMM) and the predicted monthly BI incidence (green line) from the full Poisson GAMM with observed monthly BI incidence (black line). (b) Plot showing the smoothed seasonal cycle (red line), the citywide monthly incidence (number of monthly cases per 100 000 total population of that month) time trend (blue line) of the presumed non-bacterial CNSI (non-BI) from the basic GAMM and the predicted monthly non-BI incidence (green line) from the full Poisson GAMM with observed monthly non-BI incidence (black line).
DISCUSSION
Our study examined the spatial distribution, temporal patterns, and climatic risk factors for paediatric CNSI in southern Vietnam over an 11-year period. Our data show that, over the study period, presumed BI were the major contributor to CNSI in neonates and infants, while presumed non-BI were more common in older children. These data are consistent with the findings from other countries [Reference Dash27, Reference Abucejo-Ladesma28]. The longer hospitalization periods associated with BI, in comparison with non-BI patients, may be attributed to the longer intravenous treatment of bacterial CNSI in neonates and infants [Reference Caserta29]. We additionally identified a higher mortality rate in the non-BI group. This maybe due to the fact that although some non-BI diseases may be self-limiting, there is currently no specific treatment for the majority of viral-associated CNSI, and many agents of viral CNSI, such as JE are fatal or can cause severe complications.
Given the hospital referral patterns and population structures of HCMC, it is unsurprising that the incidences of CNSI were higher in the districts in close proximity to the hospitals. However, a high incidence of CNSI, specifically during the major disease peaks, was also identified in the rural districts of Hoc Mon and Binh Chanh, which are located in the outer part of the city and some distance from the study hospitals. It is likely that a high incidence of CNSI in these districts may truly reflect higher incidences of CNSI in these districts in comparison with other HCMC districts, as the majority of paediatric patients with CNSI require hospital care.
The exceptional disease peak observed for enteroviral encephalitis (ICD10 = A85·0) in 2007 probably includes patients with CNS complications associated with HFMD. There were no patients entered into the databases with the ICD10 A85·0 (HFMD) after 2008, because all patients with HFMD were coded with a different ICD10 code (B08·4) from 2008 onwards. Our time series analysis was conducted on data from 2008 to 2015; therefore our findings are not likely to be biased by the inclusion of patients with HFMD prior to 2008.
A high incidence of BI in the dry season (winter and spring months) is consistent with a high incidence in the dry season of bacterial meningitis in many countries [Reference García-Pando11, Reference Paireau12]. The relatively strong association between BI and non-BI incidence with 1-month-lag of temperature may explain the stronger peaks of BI and non-BI mostly in the dry season. The slightly significant association of BI incidence with concurrent or lags of rainfall and river water level may explain the smaller peaks in BI incidence in the late months of rainy season. This may also reflect the heterogeneity of the BI and non-BI groups in our data in term of microbiological sub-classes as well as corresponding temporal pattern and association with climatic risk factors. The significant association of both BI and non-BI incidence with concurrent or lags of temperature, rainfall and river water level suggests that climatic factors do play a critical role in the fluctuations of CNSI incidence. Climatic factors have also found to be associated with seasonality and temporal patterns of bacterial meningitis in Africa; dust in dry season could play a role in facilitating the entry of bacterial pathogens into the host [Reference Agier10, Reference Paireau13]. We additionally observed a dramatic decrease in the incidence of BI associated with CNSI through time. The overall decreasing trend in BI may be due to the effectiveness of Hib vaccine as part of the current EPI schedule [Reference Le9], and may additionally be influenced by the usage of vaccines against pneumococcus and meningococcus in the private sector. This is consistent with the downward trend of bacterial CNSI in the countries where Hib immunization has been implemented [Reference Dash27]. The incidence of viral encephalitis has been reported as being higher in summer-fall (rainy) months in the North of Vietnam [Reference Lee16], while a higher incidence of non-BI in our data was identified in the winter and spring (dry) months. This disparity may be associated with the differing climatic conditions between the North and the South of Vietnam. The overall increasing trend in the incidence of non-BI associated CNSI is likely influence by the outbreak of non-BI towards the end of the study period (2015).
Our study contains limitations. CNSI are heterogeneous with respect to the causative agent, mode of transmission, disease presentation, and the corresponding treatment. Each CNSI associated pathogen or group of pathogens is likely to have differences in their spatiotemporal distribution and association with climatic factors, other population-based covariates and thus is likely to have differences in corresponding epidemiological models. Whereas, the ICD10 codes for CNSI from the hospital electronic databases may not accurately reflect specific details of the diagnoses; there were very limited data regarding specific pathogens and as a result, stratifying hospital data with ‘microbiological sub-classes’ or pathogens is not possible. Acknowledging this limitation, we stratified our analyses by two large groups of particular clinical relevance (BI and non-BI) since the main effort for CNSI diagnosis clinically is to differentiate between bacterial and non-bacterial etiologies and thus the ICD10 codes might better reflect this. We were additionally unable to identify specific spatial risk factors for paediatric CNSI. This may be due to the fact that BI and non-BI groups are generalized and the information on various characteristics of the districts was limited. Lastly, we were unable to obtain the data on vaccine deployment by district per year to provide a detailed description of the spatiotemporal effect of immunization on the incidence of paediatric CNSI. Therefore, more detailed prospective studies examining the different population factors associated with information regarding specific CNSI pathogens, such as JE and pneumococcal meningitis, and the interplay with their spatiotemporal risk factors are recommended. Furthermore, surveillance systems for specific CNSI pathogens as well as CNSI-related vaccine deployment should be improved in this setting.
In conclusion, we found that BIs are the most common form of CNSI in newborns and infants aged <1 year in HCMC. We additionally found that the urban districts surrounding the referral hospitals and two districts on the outskirts of the city had the highest incidences of paediatric CNSI. Notably, the incidence of CNSI was higher in the dry season and significantly associated with temperature, rainfall and water level of the rivers within the city. Our findings suggest that further prospective studies and better surveillance are needed in this setting to add further guidance into where and when the resources for the prevention, control, and treatment of paediatric CNSI should be directed. This is particularly important, as the vaccines against the bacterial and viral pathogens of CNSI are costly and not routinely available in this location.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S095026881700228X
ACKNOWLEDGEMENTS
The authors are grateful to the staff of Children's Hospital 1, Children's Hospital 2 and The Hospital for Tropical Diseases for providing the databases used in this study. This work was funded by the Wellcome Trust. SB is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). NTH is currently funded by a Mervyn Susser Fellowship at Columbia University Medical Center, USA. The funders had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONS
This study was conceptualized by NTH, HMTV, LNTN, NTD and SB. Data were provided by LNTN, HMTV, NTD, TMP, VMQ, NNQM, TAT, NTH, HMT, NVVC. NTH, AT and DK analyzed the data. NTH, KTT, DH and SB wrote the manuscript. All authors read and approved the final version of the manuscript.
DECLARATION OF INTEREST
None.
ETHICS STATEMENT
Ethical approval was granted from all of the three study hospitals: Children Hospital 1, Children Hospital 2 and the Hospital for Tropical Diseases. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









