Several human studies have shown that ingesting long-chain n-3 PUFA decreases some markers of immune function including lymphocyte proliferative responses and cytokine production(Reference Calder1). This immunosuppressive effect was especially observed with aged people(Reference Meydani, Endres, Woods, Goldin, Soo, Morrill-Labrode, Dinarello and Gorbach2, Reference Bechoua, Dubois, Vericel, Chapuy, Lagarde and Prigent3). However, studies conducted with younger people suggest that healthy adults are relatively insensitive to immunomodulation with long-chain n-3 PUFA(Reference Sijben and Calder4). Another concern is that most studies examined the combined effects of DHA and EPA both present in different proportions depending on the source of fish oil administered to the volunteers. Only a few human studies examined the effects of either EPA or DHA separately on immune function. In the study of Kew et al. (Reference Kew, Mesa, Tricon, Buckley, Minihane and Yaqoob5) examining the effect of dietary supplementation of healthy middle-age subjects with 4·7 g EPA/d or 4·9 g DHA/d, it was shown that only DHA, but not EPA, decreased the expression of CD69, an early marker of lymphocyte activation. These results are at variance with those of Thies et al. (Reference Thies, Nebe-von-Caron, Powell, Yaqoob, Newsholme and Calder6) showing that fish oil, but not highly purified DHA, suppressed lymphocyte proliferation, or with those of Kelley et al. (Reference Kelley, Taylor, Nelson and Mackey7) showing that consuming 6 g DHA/d for 3 months did not alter lymphocyte proliferation. Other studies have even reported quite opposite results. Thus, Schauder et al. (Reference Schauder, Röhn, Schäfer, Korff and Schenk8) reported that patients fed on parenteral nutrition supplemented with DHA-enriched fish oil during the postoperative period produced higher levels of IL-2 than those supplemented with soyabean oil or unsupplemented patients. The supplementation of healthy volunteers' diet with DHA-enriched oil providing 1·62 g DHA/d and 0·78 g EPA/d for 2 months has recently been shown to increase concanavalin A-dependent lymphocyte proliferative responses and IL-10, interferon-γ and TNFα production as compared with levels measured before supplementation(Reference Gorjão, Verlengia and Lima9).
The reported effects of DHA and EPA on apoptosis have ranged from inhibition to stimulation, depending on the cell model used. It is generally believed that long-chain n-3 PUFA increase the rate of apoptosis of tumour cells, both in vitro when added to cell culture medium, and in tumour-bearing rodents fed DHA- or EPA-enriched diets(Reference Field and Schley10). However, a large variability has been observed in normal cell models including monocytes or monocytic cell lines. Thus, Sweeney et al. (Reference Sweeney, Puri and Reen11) have recently shown that the treatment of monocytes purified from human umbilical cord blood with DHA or EPA induced a rapid and dose-dependent cell death, due to a loss of mitochondrial membrane potential. In contrast, the preincubation of U937 monocytic cells with DHA has been shown to attenuate apoptosis induced by stimulation with TNFα in the presence or absence of cycloheximide, this antiapoptotic effect being accompanied by an enrichment of DHA in membrane phospholipids(Reference Yano, Kishida, Iwasaki, Kojo and Masuzawa12). However, to our knowledge, the influence of DHA supplementation of the human diet on ex vivo monocyte susceptibility to proapoptotic stimuli has never been investigated.
In the present study we sought to examine the effects of increasing doses of DHA added to the regular diet of human healthy volunteers on some aspects of immune function. We chose to evaluate IL-2 mRNA expression in response to phorbol ester and ionomycin activation as a marker of lymphocyte ex vivo activation and to measure changes in mitochondrial membrane potential induced by oxidized LDL (oxLDL) as a marker of monocyte susceptibility to apoptosis. Results were examined in regard to changes in the fatty acid composition of mononuclear cell phospholipids.
Subjects and methods
Subjects and experimental design
The eight subjects recruited for the study were healthy male volunteers aged between 53 and 65 years (mean 58·7 years). Each of them gave informed consent and the study was approved by the ‘Comité consultatif de protection des personnes dans la recherche biomédicale de Lyon A’. At the beginning of the study each of them had normal blood cell counts, cholesterol (5·47 (se 0·20) mmol/l), TAG (1·76 (se 0·4) mmol/l) and glucose (5·25 (se 0·2) mmol/l) blood levels. They ingested successively 200, 400, 800, 1600 mg DHA in a TAG form per day, for 2 weeks each dose without interruption. DHA supplement was administered in soft gelatine capsules that each contained 200 mg DHA and 0·125 mg dl-tocopherol plus 0·125 mg ascorbyl palmitate (Pro-Mind Forte; Decola Neutraceutics, Maldegem, Belgium). The fatty acid composition of the TAG was: 22 : 6n-3, 40 %; 18 : 2n-6, 1·2 %; total MUFA, 22·2 % (including 18 : 1n-9, 20·2 %+16 : 1n-7, 2 %); total SFA, 36·6 % (including 16 : 0, 14 %+14 : 0, 16 %+12 : 0, 6·6 %). Blood samples were collected after overnight fasting before DHA supplementation and after each DHA dose, and mononuclear cells were isolated. Blood samples were also collected 5 weeks after supplementation was arrested. The fatty acid composition of cell phospholipids was analysed and cell functions were evaluated. Each individual was asked not to deviate from regular habits during the study period and not to take any drugs at least 10 d before the initial blood sampling and during the test period.
Peripheral blood mononuclear cell and monocyte isolation
Venous blood was drawn into ACD anticoagulant. Peripheral blood mononuclear cells (PBMC) were separated by dextran sedimentation and density gradient centrifugation through Histopaque 1077 and then washed three times with RPMI 1640 by low-speed centrifugation in order to more thoroughly eliminate the contaminating platelets. PBMC were then adjusted to a concentration of 2 × 107cells/ml in RPMI 1640 (with HEPES and bicarbonate) medium. All steps were carried out at room temperature. Under such conditions cell viability, established by the trypan blue exclusion test, was always greater than 95 %. Mononuclear cell suspensions were used to evaluate IL-2 expression and to determine the fatty acid composition of phospholipids. An aliquot of this cell suspension was used for monocyte isolation. Peripheral blood monocytes (PBM) were isolated from mononuclear cell suspensions by adherence (1 h). After non-adherent cells were discarded, PBM (106/ml) were incubated in RPMI medium supplemented as previously described(Reference Xu, Meisel, Ong, Kaul, Cercek, Rajavashisth, Sharifi and Shah13) and were cultured in the presence of native LDL or oxLDL for 4 h. PBM preparations were analysed by flow cytometry using CD14 monoclonal antibody (BD Biosciences, Heildelberg, Germany) and isotype control IgG1 (Serotec, Düsseldorf, Germany) as a negative control. At least 70 % of PBM were CD14+ (not shown).
Peripheral blood mononuclear cell treatment, RNA isolation and semi-quantitative RT–PCR analyses
Mononuclear cells suspended at a concentration of 106 cells/ml in RPMI were incubated with 200 nmol/l tetradecanoylphorbol acetate and 500 nmol/l ionomycin for 2 h at 37°C in an air–CO2 (95:5) atmosphere. At the end of the incubation period, total RNA was isolated from control and tetradecanoylphorbol acetate-treated cells using Tri Reagent according to the manufacturer's instructions. The sense and antisense primers for the amplification of IL-2 were 5′-CACTAAGTCTTGCACTTGTCAC-3′ and 5′-CCTTCTTGGGCATGTAAAACT-3′, respectively (expected size of amplified fragment 186 bp). β-Actin primers were used as internal control to normalize the data. RT–PCR were performed on 2 μg RNA for IL-2 and 1 μg RNA for β-actin using QIAGEN® OneStep RT–PCR kit (Courtaboeuf, France) according to the manufacturer's instructions. Reaction products were resolved by electrophoresis on 1 % agarose gel impregnated with ethidium bromide, and visualized by UV transillumination.
Fatty acid composition of peripheral blood mononuclear cell phospholipids
Diheptadecanoyl phosphatidylcholine (20 μg) as an internal standard was added to 1 ml mononuclear cell suspension. The cell suspensions were then extracted according to Bligh & Dyer(Reference Bligh and Dyer14) and cell lipid extracts were separated on silica gel G60 plates (Merck, Darmstadt, Germany) with the solvent system hexane–diethyl ether–acetic acid (60:40:1, by vol.). The silica gel areas corresponding to phospholipids were scraped off and transmethylated according to Bowyer et al. (Reference Bowyer, Leat, Howard and Gresham15) as previously described(Reference Bechoua, Dubois, Vericel, Chapuy, Lagarde and Prigent3). Briefly, one volume of 5 % H2SO4 in methanol was added to the scraped silica gel and transmethylation was carried out at 100°C for 90 min in screw-capped tubes. The reaction was terminated by the addition of 1·5 volumes of ice-cold 5 % (w/v) K2CO3, and the fatty acid methyl esters were extracted with isooctane and resolved by GC using a Hewlett Packard chromatograph HP 6890 model, equipped with a capillary column (60 m × 0·25 mm, BPx70 SGE; Bellfonte, USA) and flame ionization detection. The column was two-step programmed from 135 to 160°C at 2°C/min and from 160 to 205°C at 1·5°C/min; the detection temperature was maintained at 250°C. The vector gas was helium at a pressure of 0·8 psi (5·52 kPa). Fatty acid methyl esters were identified by their retention time relative to standards.
LDL isolation and oxidation
LDL fraction was isolated from human plasma by sequential ultracentrifugation(Reference Havel, Eder and Bragdon16). LDL (1 mg/ml) oxidation was induced for 30 min at 37°C with 4 mmol/l HOCl (corresponding to oxidant:protein molar ratio of 2000:1). Untreated LDL and oxLDL were dialysed overnight against isotonic PBS. Native LDL and oxLDL were used at a cholesterol concentration of 200 μg/ml in the incubation medium. The lipid peroxide content of native LDL and oxLDL was determined by analysing thiobarbituric acid-reactive substances and expressed as malondialdehyde equivalents(Reference Conti, Morand, Levillain and Lemonnier17). As compared to previous results obtained after copper treatment of native LDL, malondialdehyde was generated at a lower extent in HOCl–oxLDL than in copper–oxLDL (0·68 nmol malondialdehyde/mg protein for HOCl–oxLDL v. 0·10 for native LDL and 4·20 for copper–oxLDL(Reference Ermak, Lacour, Drüeke and Vicca18)).
The degree of oxidation was quantified by an increased relative mobility on 0·6 % agarose gels(Reference Lin, Pan, Yang, Huang, Chang, Ding and Chiang19), indicating an enhanced negative charge of HOCl–oxLDL. The relative mobility of HOCl–oxLDL on agarose gels as an index for lipoprotein oxidation was 2·5–3·0 compared with that of native LDL.
Analysis of mitochondrial membrane potential
Following individual incubations, cells were loaded with the fluorochrome 3,3′-dihexyloxacarbocyanine iodide (Molecular Probes Inc., Eugene, OR, USA), used at 40 nmol/l final concentration for 30 min. The dye accumulates in mitochondria that contain an intact mitochondrial membrane potential (Δψm), and the fluorescence of 3,3′-dihexyloxacarbocyanine iodide can therefore be considered as an indicator of the relative mitochondrial membrane polarization state(Reference Chen20). Relative fluorescence intensities were measured on a FACScan flow cytometer.
Statistical analysis
Data expressed as means and their standard errors were analysed by ANOVA and means were compared by a protected t test. Correlations between changes in parameters induced by DHA were tested by linear regression. P < 0·05 was considered significant.
Results and discussion
DHA supplement was well tolerated throughout the study. No significant variations in systolic and diastolic blood pressure, glucose and TAG blood concentrations were observed before and after DHA supplementation. DHA treatment was associated with a slight but significant increase in HDL-cholesterol (1·43 (se 0·090) v. 1·60 (se 0·12) mmol/l, +11·9 %, P < 0·05) and a trend of LDL-cholesterol to increase (3·23 (se 0·24) v. 3·56 (se 0·27) mmol/l, +10·2 %, NS) after ingestion of the highest dose of DHA (1600 mg/d). Interestingly, the HDL-cholesterol to LDL-cholesterol ratio remained unchanged throughout the study. The present results are in partial agreement with those of a recent study(Reference Kelley, Siegel, Vemuri and Mackey21) reporting an increase of both HDL- and LDL-cholesterol after ingestion of 3 g/d DHA for 90 d. However, at variance with the present results, in the study of Kelley et al. (Reference Kelley, Siegel, Vemuri and Mackey21) only LDL-cholesterol changes were significant.
DHA intake significantly increased the proportion of DHA in mononuclear cell phospholipids (Table 1; Fig. 1), starting from the second dose of 400 mg/d (+25 % as compared with level measured before DHA intake). DHA incorporation linearly increased (R 2 0·99, P < 0·003) as a function of DHA doses up to 800 mg/d dose, and slowed down thereafter. The increase in DHA proportion reached +76 % with respect to baseline (5·67 (se 0·26) v. 3·22 (se 0·28) at baseline) after the highest dose of 1600 mg/d. Although several studies have reported that supplementation of the diet with n-3 fatty acids results in an increased n-3 fatty acid content of PBMC(Reference Bechoua, Dubois, Vericel, Chapuy, Lagarde and Prigent3, Reference Thies, Nebe-von-Caron, Powell, Yaqoob, Newsholme and Calder6), only a few studies have examined n-3 fatty acid incorporation in PBMC phospholipids as a function of the ingested dose. In a recent study, Rees et al. (Reference Rees, Miles, Banerjee, Wells, Roynette, Wahle and Calder22) have shown that EPA was incorporated in a linear dose–response fashion into PBMC phospholipids following ingestion of EPA doses ranging from 1·35 to 4·05 g/d provided as EPA-rich oil. However, to our knowledge the present study is the first one to describe a dose–response relationship between increased DHA intake and increased DHA incorporation in mononuclear cell phospholipids. After a 5-week washout period, DHA proportion no longer differed from that measured before DHA supplementation (Table 1). Although the algal oil used in the present study was totally devoid of EPA, the proportion of EPA in mononuclear cell phospholipids was also increased, the rise being significant only after ingestion of the third and fourth DHA doses (0·38 (se 0·06) at baseline v. 0·60 (se 0·07) and 0·61 (se 0·07) after 800 and 1600 mg DHA/d, respectively). The present result suggests that DHA was efficiently retroconverted to EPA and increased in blood mononuclear cells as previously observed(Reference Joulain, Guichardant, Lagarde and Prigent23, Reference Stark and Holub24). These modifications were accompanied by decreased proportions of long-chain n-6 fatty acids, specifically arachidonic acid (20 : 4n-6) and adrenic acid (22 : 4n-6). Surprisingly, the proportion of 22 : 5n-3 was also significantly lowered by DHA treatment, the decrease being significant from the dose of 200 mg/d. Such a decrease in 22 : 5n-3 concentration has already been reported in serum phospholipids of women supplemented with 2·8 g DHA/d for 28 d(Reference Stark and Holub24) or in erythrocyte phospholipids of middle-aged people supplemented with 0·7 g DHA/d for 3 months(Reference Theobald, Goodall, Sattar, Talbot, Chowienczyk and Sanders25). This is likely a result of DHA competition for esterification at the sn-2 position of glycerophospholipids.
Table 1 Fatty acid composition of mononuclear cell phospholipids (mol/100 mol total fatty acids) at baseline (0) and after supplementation with increasing doses of DHA*
(Mean values with their standard errors for eight subjects)

a–d Mean values within a row with unlike superscript letters were significantly different (P < 0·05). Data were analysed by ANOVA and means were compared by a protected t test.
* For details of procedures, see Subjects and methods.
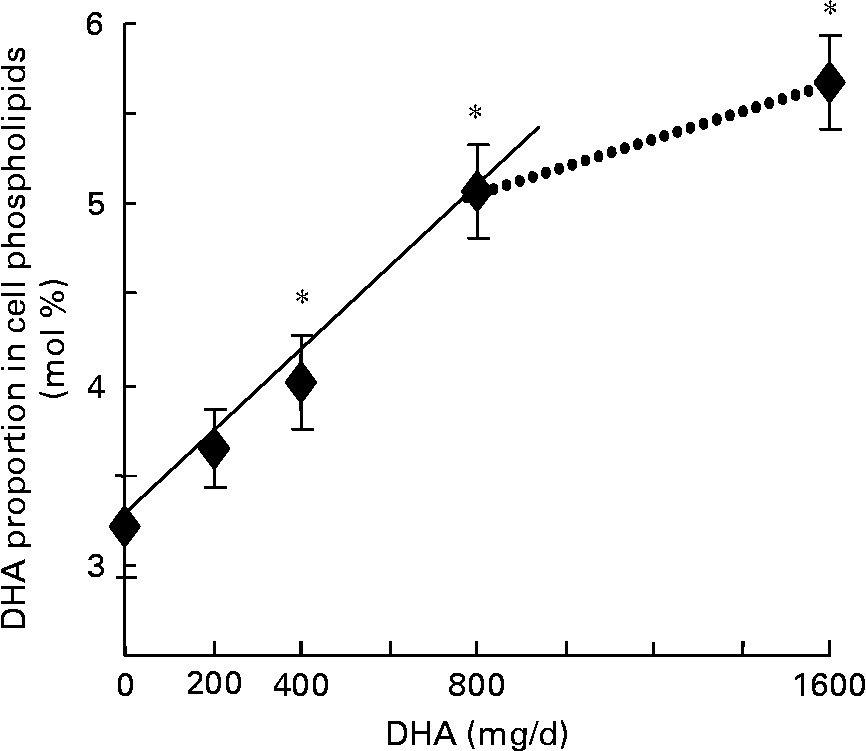
Fig. 1 Change in DHA proportion in mononuclear cell phospholipids during supplementation with different doses of DHA-rich oil (—, R 2 0·99; P < 0·003). ……, Non-linear. Values are means with their standard errors depicted by vertical bars (n 8). *Mean values were significantly different from those at baseline (P < 0·05).
An early response of lymphocytes to activation by phorbol ester and ionomycin is the expression of IL-2 mRNA, which starts after 1 h of treatment and reaches a maximum after 2 h of stimulation(Reference Gaffen and Liu26, Reference Diaz, Mébarek-Azzam, Benzaria, Dubois, Lagarde, Némoz and Prigent27). Thus, we examined by RT–PCR IL-2 mRNA expression after 2 h of tetradecanoylphorbol acetate and ionomycin treatment of mononuclear cells before DHA ingestion and after each dose intake. In the absence of activators, the basal IL-2 mRNA expression remained very low and unaffected by DHA supplementation (Fig. 2). In contrast, the stimulated mRNA level started to increase after ingestion of 400 mg DHA/d, peaked after the dose of 800 mg/d and remained elevated after ingestion of the highest DHA dose. After the 5-week washout period, the stimulated level of mRNA expression returned to values observed before the supplementation period, as did DHA proportion in cell phospholipids. Interestingly, the stimulated mRNA level of IL-2 was positively correlated with DHA enrichment in cell phospholipids (Fig. 3). The present results indicate that a moderate DHA intake does not decrease, but rather enhances, IL-2 mRNA expression in lymphocytes from healthy middle-aged volunteers, thus suggesting an improvement of their activability. This is in agreement with recent reports showing that n-3 fatty acids may have different effects on immune function depending on the age and health status of the subjects(Reference Sijben and Calder4, Reference Miles, Banerjee, Wells and Calder28). Because tetradecanoylphorbol acetate plus ionomycin-induced signals bypass cell membrane receptors, modifications of membrane fluidity or lipid rafts are probably not involved. An alternative mechanism might be a specific effect of DHA or metabolites on the regulation of expression of key lymphocyte genes(Reference Gorjão, Verlengia and Lima9).
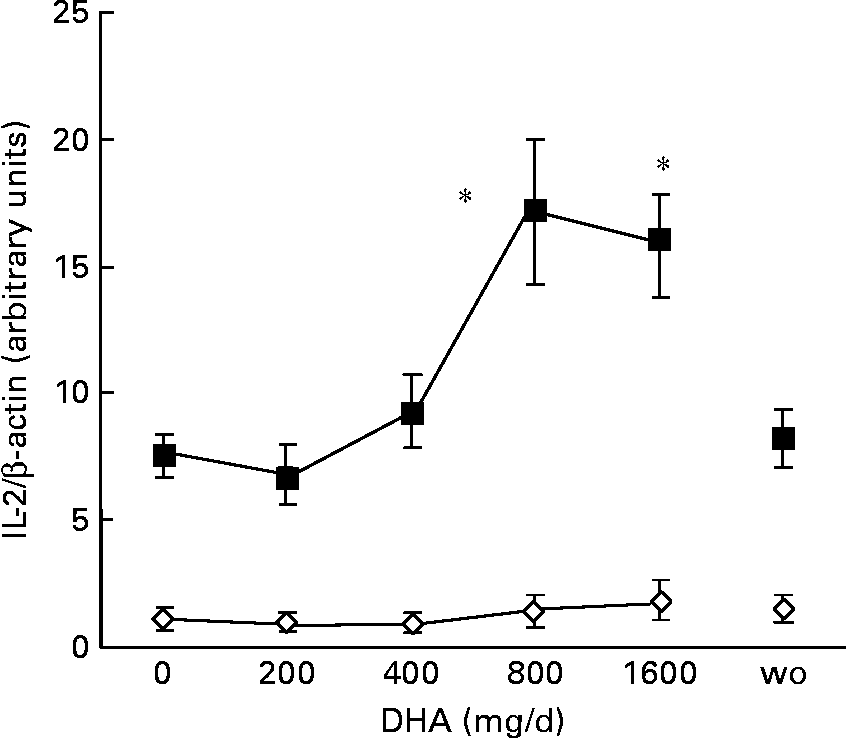
Fig. 2 Effect of increasing DHA intake on IL-2 mRNA expression in mononuclear cells. Peripheral blood mononuclear cells suspended at a concentration of 106 cells/ml in RPMI were incubated with 200 nmol/l tetradecanoylphorbol acetate (TPA) and 500 nmol/l ionomycin for 2 h at 37°C. At the end of the incubation period, total RNA was isolated from control (⋄) and TPA-treated cells (■), and submitted to RT–PCR. Amounts of IL-2 amplification products were normalized by the amounts of β-actin amplification products. Ratio values are expressed relative to IL-2/β-actin ratio of unstimulated cells at baseline taken as 1. Values are means with their standard errors depicted by vertical bars (n 8). Mean values were significantly different from those at baseline: *P < 0·05. wo, Washout.
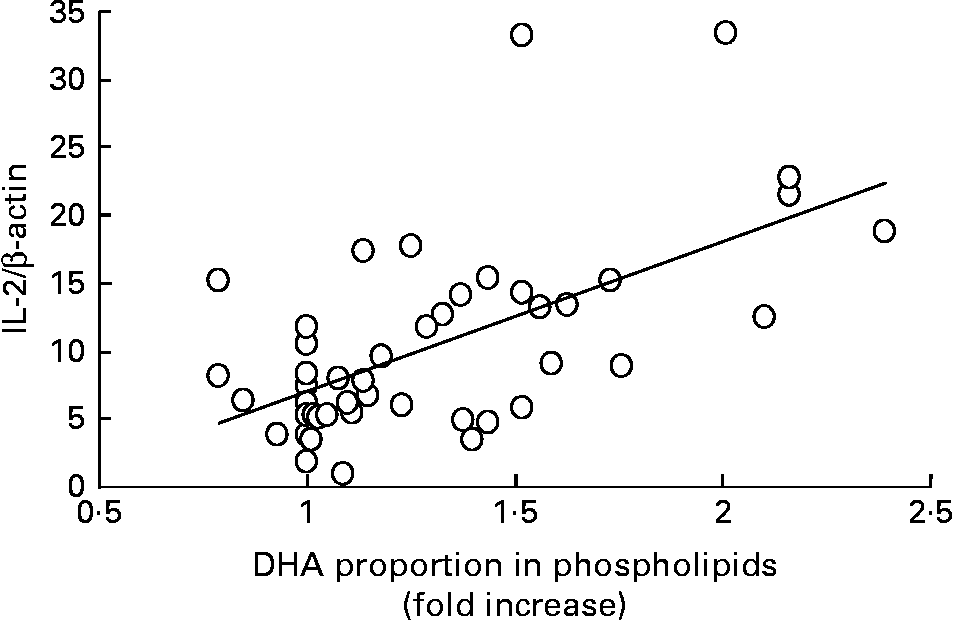
Fig. 3 Positive relationship between DHA enrichment in mononuclear cell phospholipids and IL-2 mRNA expression (R 2 0·36; P < 0·001). For each subject normalized IL-2/β-actin ratio is plotted as a function of the relative DHA enrichment (with respect to baseline value taken as 1) in cell phospholipids.
In a previous study(Reference Ermak, Lacour, Drüeke and Vicca18), we showed that HOCl–oxLDL was able to induce apoptosis of the human cultured U937 monocytic cell line in a concentration-dependent manner, via the mitochondrial apoptotic pathway. Based on this, we chose to expose human primary monocytes to a HOCl–oxLDL concentration of 200 μg/ml. As shown in Fig. 4, the treatment of monocytes freshly isolated from volunteers before DHA supplementation by oxLDL drastically reduced mitochondrial membrane potential, as compared with native LDL treatment (negative control). After DHA intake monocytes became more resistant to oxLDL toxic effect starting from the second DHA dose of 400 mg/d (62·8 (se 1·8) % of cells with Δψm disruption for oxLDL treatment and DHA 400 mg/d v. 77·1 (se 1·2) % for oxLDL treatment before DHA supplementation). The protective effect of DHA supplementation was maintained throughout the experiment although it tended to decline after ingestion of the two highest DHA doses (68·3 (se 2·0) and 69·3 (se 1·8) % of cells with low Δψm after 800 and 1600 mg DHA/d). However, whatever the DHA doses ingested, oxLDL still induced monocyte mitochondrial membrane depolarization, which may promote apoptosis. At the present time, it is unclear whether apoptosis induced by various stimuli is harmful or beneficial in various physiological and pathological circumstances. Indeed, apoptosis of mature macrophage is thought to promote plaque destabilization and vessel occlusion in the late stages of atherosclerosis, whereas monocyte apoptosis may be beneficial in the initial stages of the atheromatous process(Reference Ermak, Lacour, Drüeke and Vicca18). After the washout period, the susceptibility of monocytes to oxLDL apoptosis was no longer different from that observed at baseline (oxLDL alone before DHA ingestion). Some nutritional studies have shown that LDL isolated from fish oil-supplemented volunteers, thus enriched in n-3 fatty acids, induced less apoptosis in U937 cells after oxidation compared to LDL isolated from sunflower oil-supplemented subjects(Reference Wu, Geigerman, Lee and Wander29, Reference Lee and Wander30). On the other hand, the effects of n-3 fatty acid enrichment of monocyte phospholipids on susceptibility to apoptosis seem to be largely dependent on experimental conditions(Reference Sweeney, Puri and Reen11, Reference Yano, Kishida, Iwasaki, Kojo and Masuzawa12). Thus, in umbilical cord monocytes the pro-apoptotic effect of DHA involved a loss of mitochondrial membrane potential accompanied by caspase-3 activation(Reference Sweeney, Puri and Reen11), whereas in the U937 monocytic cell line the anti-apoptotic effect of DHA was attributed to inhibition of cytosolic phospholipase A2 through esterification in cell phospholipids(Reference Yano, Kishida, Iwasaki, Kojo and Masuzawa12).
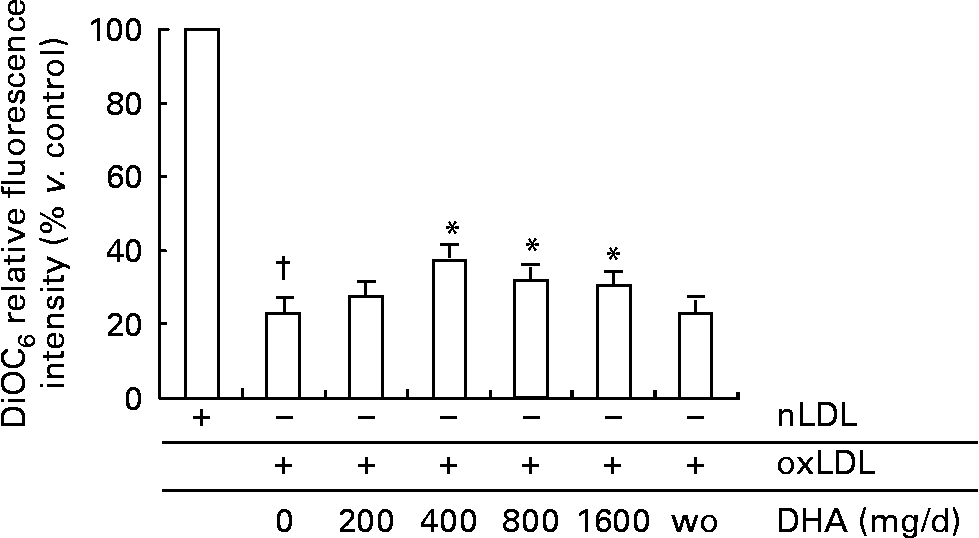
Fig. 4 Effect of increasing DHA intake on oxidized LDL (oxLDL)-treated monocyte mitochondrial membrane potential. Human monocytes were treated with 200 μg/ml native LDL (nLDL) or 200 μg/ml oxLDL for 4 h at 37°C. Cells were then incubated for 30 min with 40 nmol/l 3,3′-dihexyloxacarbocyanine iodide (DiOC6) at 37°C, and analysed by flow cytometry. Values are means with their standard errors depicted by vertical bars (n 4). Mean values for oxLDL and DHA were significantly different from those of oxLDL only: *P < 0·05. Mean values were significantly different from those of nLDL only: †P < 0·0001. wo, Washout.
Hypochlorous acid is known to preferentially modify the protein moiety of human LDL, but HOCl-modified LDL can in turn induce lipid peroxidation and antioxidant depletion as secondary events subsequent to amino acid modifications(Reference Malle, Marsche, Arnhold and Davies31). Thus, we have recently shown that HOCl-modified oxLDL induced the generation of reactive oxygen species in U937 monocytic cells(Reference Ermak, Lacour, Drüeke and Vicca18). Although highly susceptible to peroxidation, DHA may have antioxidant properties especially when used at low concentrations. This proposal is supported both by nutritional studies in man, and by studies involving in vitro DHA enrichment of different cell models. Indeed, Véricel et al. (Reference Véricel, Calzada, Chapuy and Lagarde32) have shown a significant increase of α- and γ-tocopherol accompanied by a decrease in malondialdehyde in platelets of elderly people supplemented for 6 weeks with low doses of marine oil. Bechoua et al. (Reference Bechoua, Dubois, Dominguez, Goncalves, Némoz, Lagarde and Prigent33) have reported that low concentrations of DHA decrease malondialdheyde production induced by H2O2 in human mononuclear cells. Similarly, the pretreatment of RAW264 macrophages with DHA has been shown to prevent the accumulation of intracellular peroxides induced by interferon-γ plus lipopolysaccharide in a dose-dependent manner by a mechanism involving up-regulation of intracellular glutathione level(Reference Komatsu, Ishihara, Murata, Saito and Shinohara34). Finally, low doses of DHA have been shown to strengthen cellular antioxidant defences by increasing glutathione peroxidase activity/expression in various types of cells including platelets(Reference Lemaitre, Véricel, Polette and Lagarde35), mononuclear cells(Reference Joulain, Prigent, Némoz and Lagarde36) or neuronal cells(Reference Leonardi, Attorri, Benedetto, Biase, Sanchez, Tregno, Nardini and Salvati37). Thus, the antioxidant properties of low DHA doses might explain the antiapoptotic effects observed in monocytes. Furthermore, they could also explain the stimulating effects on lymphocytes as both prooxidant and antioxidant states are required sequentially during lymphocyte activation(Reference Williams and Kwon38).
Acknowledgements
This work was supported by INSERM and a grant from the Groupe Lipides et Nutrition (GLN) (2005). The contribution of each author was as follows: S. M., N. E., A. B., M. D., performing experiments; S. V., G. N., B. L., A.-F. P., writing the manuscript; M. Laville, promotor, investigator, coordinator; E. V., M. Lagarde, A.-F. P., designing the study. All the authors have seen and approved the contents of the paper. All the authors state that there are no conflicts of interest and all adhere to the Committee on Publication Ethics guidelines on research and publication ethics.







